Abstract
In this review we compare the sensitivity of a range of aquatic invertebrate and fish species to gamma-cyhalothrin (GCH), the insecticidally active enantiomer of the synthetic pyrethroid lambda-cyhalothrin (LCH), in single-species laboratory tests and outdoor multi-species ecosystem tests. Species sensitivity distribution curves for GCH gave median HC5 values of 0.47 ng/L for invertebrates, and 23.7 ng/L for fish, while curves for LCH gave median HC5 values of 1.05 ng/L and 40.9 ng/L for invertebrates and fish, respectively. A model ecosystem test with GCH gave a community-level no observed effect concentration (NOECcommunity) of 5 ng/L, while model ecosystem tests with LCH gave a NOECcommunity of 10 ng/L. These comparisons between GCH and LCH indicate that the single active enantiomer causes effects at approximately one-half the concentration at which the racemate causes similar effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concerns have been raised about the reliability of environmental risk assessments for mixed enantiomer pyrethroid and organophosphate insecticides (Ali et al. 2003; Cai et al. 2008; Liu et al. 2004, 2005a, b). For example, Liu et al. (2005a) reported differences in toxicity to the aquatic invertebrates Daphnia magna and Ceriodaphnia dubia of 15- to 38-fold between enantiomers for bifenthrin and permethrin, and for cypermethrin and cyfluthrin only two of the eight isomers were toxic. In addition, for bifenthrin and permethrin Liu et al. (2005b) reported differences in degradation rates in sediments for different enantiomers and noted that such differences could significantly impact risk assessments. If toxicity is associated with a single enantiomer, ecotoxicological effects of enantiomer mixtures will depend upon the fate, behavior and bioavailability of only that enantiomer. To increase the accuracy and reliability of risk assessments for chiral pesticides, the toxicity and behavior of the component enantiomers need to be fully understood.
In this paper we will compare the extensive amount of aquatic toxicity data available for invertebrate and fish species for the synthetic pyrethroid lambda-cyhalothrin (LCH, CAS No. 91465-08-6) and its insecticidally active enantiomer gamma-cyhalothrin (GCH, CAS No. 76703-62-3). Lambda-cyhalothrin consists of two of the four enantiomers of the cyhalothrin molecule. Gamma-cyhalothrin is the insecticidally active enantiomer, 1R-cis, αS cyhalothrin. This enantiomer has been reported to be >162 times more toxic to zebrafish (Danio rerio) than 1R-cis, αR cyhalothrin, the other component of LCH (Xu et al. 2008). In this paper we compare data generated in laboratory studies with several fish and aquatic invertebrates to quantify the toxicological relationship between GCH and LCH. The significance of these toxicological differences and of differences in environmental fate are further evaluated using data generated in multi-species outdoor micro/mesocosm studies under environmentally relevant conditions.
Single species laboratory studies
Data selection
Acute toxicity data for GCH (Table 1) were gleaned from studies meeting regulatory guidelines and using standard test species (D. magna, Lepomis macrochirus, Oncorhynchus mykiss, and Selenastrum capricornutum), guideline studies with additional fish species (Poecilia reticulata, Brachydanio rerio, and Pimephales promelas), a special study with three life stages of the amphipod Gammarus pseudolimnaeus, and another special study with nine additional aquatic invertebrate species (van Wijngaarden et al. 2008). Results of acute toxicity tests with LCH (Table 2) were found in the open literature (Maund et al. 1998; Schroer et al. 2004), the EPA Pesticide Toxicity Database (U.S. EPA 2007), and three unpublished guideline studies. Most of these studies used static or static-renewal exposures.
Many of the results for arthropods were reported as both immobilization (EC50s) and mortality (LC50s) after 48 and 96 h of exposure. In some of these tests immobilization was equal to mortality; that is, EC50s were the same as LC50s. In other tests, immobility did not lead to mortality, resulting in EC50s much lower than LC50s. Moreover, in many of the tests, some individuals that were immobile at 48 h had recovered when the 96-h observations were made. In such cases, 48-h EC50s were lower than 96-h EC50s, contrary to the general expectation that longer exposures lead to greater effects.
It can be argued that reversible sublethal effects are not of concern for acute risk assessments, and that lethal and sublethal endpoints should not be combined for analysis. Risk assessments for acute effects generally use toxicity data with mortality as the measured endpoint. For small invertebrates such as daphnids in which mortality can be difficult to confirm, immobilization is generally used as a surrogate for mortality. For example, the endpoints of the G. pseudolimnaeus tests were reported as “mortality/immobility” implying that immobilization was assumed equivalent to mortality. Use of sublethal EC50s for an acute risk assessment is a conservative (protective) approach.
Therefore, the invertebrate endpoints summarized in Tables 1 and 2 include EC50 and LC50 values, and in some cases for both 48 and 96 h. For fish, only the 96-h LC50 has been summarized and taken for the purpose of comparisons between GCH and LCH.
Comparison of toxicity data for individual species
The reported toxicity endpoints for GCH are presented in Table 1, and those for LCH in Table 2. Six arthropod species, five fish species, and one algal species were tested with both GCH and LCH (Table 3).
Of all the species tested only Chaoborus obscuripes was more sensitive to LCH than GCH. For the other arthropod species tested, the LCH/GCH ratio ranged from 1.05 (Asellus aquaticus) to 3.77 (Cloeon dipterum). The average ratio for the six arthropod species alone was 1.98. For fish, the LCH/GCH ratio ranged from 1.93 (O. mykiss) to 3.15 (L. macrochirus) for four species, consistent with GCH as a refined form of LCH, but was 13.5 for P. reticulata. The difference for P. reticulata was much greater than for any other species, a discrepancy that cannot be explained from the available information.
In addition to these exact comparisons, four pairs of closely related species were tested with GCH and LCH (Table 3). The LCH/GCH ratios for these four species were more variable than for the exact taxonomic matches, ranging from 0.40 to 1.53 for damselflies to 16.55 for chironomids.
Comparison of species sensitivity distributions
Invertebrates
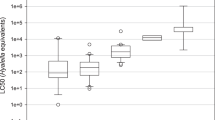
Species sensitivity distributions (SSDs; see Posthuma et al. 2002) for GCH and LCH were generated based on 48-h EC50s and LC50s, 96-h EC50s and LC50s, and the lowest EC50s and LC50s for each invertebrate species (Fig. 1). The SSD describes the relationship between the concentration of GCH or LCH and the fraction of species whose EC50 or LC50 is exceeded at that concentration (the Potentially Affected Fraction). The SSD for the most sensitive endpoint, lowest EC50, is presented in Fig. 2 (upper left panel) with the relative positions of those species tested with both GCH and LCH identified.
To develop the SSDs, toxicity values (sample size = n) were first sorted from the lowest value to the highest value. Each value was assigned a rank and an associated probability of occurrence of the form y i = rank i / n + 1. A cumulative logistic regression function of the following form was fit to the data using the iteratively reweighted least squares maximum likelihood method shown in Eq. 1.
Confidence intervals on y i (95%) were calculated from the nonlinear model fit as a function of the parameter covariance matrix. The 50th percentile of the cumulative logistic function was estimated as exp (−α/β). The 5th percentile of the cumulative logistic function (the HC5) was calculated as exp [(−2.94 − α)/β]. The associated EC50 concentration units associated with the upper and lower confidence interval for any specific y i were estimated through the equation by calculating the log (EC50 i ) associated with each bound.
The SSDs for GCH were approximately parallel to those for LCH, and always shifted slightly toward lower concentrations (Fig. 1). The differences in SSDs generated from different endpoints were small and mainly affected the SSD slopes. The HC5 based on each species’ lowest EC50 was 0.47 ng/L (95% confidence interval 0.11–2.00 ng/L) for GCH and 1.05 ng/L (0.47–2.34 ng/L) for LCH (Table 4).
The SSD comparison based on the lowest EC50 values (Fig. 2, Table 4) supports two conclusions: (a) the rank order of species sensitivities is very similar for GCH and LCH, and (b) the entire SSD for GCH is shifted to the left of the SSD for LCH by a factor of approximately 2.2 at the HC5 and 3.3 at the HC50, similar to both the ratio of EC50s for individual arthropod species (Table 3) and the value expected based on isomeric composition.
The HC5 of 0.47 ng/L for GCH differs from the value of 2.12 ng/L estimated by Van Wijngaarden et al. (2008). This difference can be ascribed to several factors. Most importantly, our SSD analysis included three species not included by those authors, notably G. pseudolimnaeus with an EC50 more than eight times lower than the next most sensitive species, C. obscuripes. Second, our SSD analysis was based on the lower of the 48- and 96-h EC50 values for each species, while van Wijngaarden et al. used only 96-h EC50 values. Third, the methods of HC5 calculation differed between the two analyses. While this may raise important questions that need to be considered when generating and interpreting absolute HC5 values derived from SSD curves, these questions are outside the scope of this work. For the purpose of this assessment, it was considered more important to ensure that consistent criteria were applied to the selection of endpoints for both GCH and LCH to allow a comparative analysis of the SSD curves to be conducted.
Fish
SSD curves for fish for GCH and LCH were constructed using the same methods as described above. The resulting SSD curves for GCH and LCH are shown in Fig. 2 (upper right panel). The fish HC5 values for GCH and LCH differed by a factor of 1.72 (Table 4), which is consistent with GCH as a refined form of LCH.
Compared to the SSD for arthropods, the SSD for fish was shifted considerably to the right (less sensitive) for both GCH (Fig. 2, lower left panel) and LCH (Fig. 2, lower right panel). Regression parameters are shown in Table 4. The toxicity data clearly demonstrate that fish are less sensitive to LCH than are arthropods.
The fish HC5 for LCH is 41 ng/L, nearly 40 times greater than the HC5 for arthropods (Table 4) and 5 times greater than the community-level no observed effect concentration (NOECcommunity) for arthropods (see below). For GCH, the fish HC5 is 24 ng/L, 50 times greater than the HC5 for arthropods (Table 4) and 5 times greater than the arthropod NOECcommunity. The finding of greater sensitivity of arthropods than fish to GCH and LCH based on HC5 values is consistent with the conclusions of Maltby et al. (2005) for 16 insecticides, including LCH. For both LCH and GCH, a safe exposure level for arthropods (i.e., the NOECcommunity) will also be safe for fish.
Field studies
The effects of LCH on aquatic communities under natural conditions have been extensively studied (Farmer et al. 1995; Hill et al. 1994a, b; Schroer et al. 2004; Roessink et al. 2005; Van Wijngaarden et al. 2006). Since LCH consists of ca. 50% GCH, and the toxicity profiles of LCH and GCH illustrate that GCH is the ecotoxicologically relevant component of LCH, information derived from field studies with LCH can also be directly applied to GCH. To confirm this expectation, a microcosm study was conducted with GF-317, a capsule suspension formulation of GCH (van Wijngaarden et al. 2008). The study used macrophyte-dominated enclosures in the same set of experimental ditches as were used previously for LCH (Schroer et al. 2004; Roessink et al. 2005; van Wijngaarden et al. 2006). GF-317 was added three times at weekly intervals to give nominal GCH concentrations of 5, 10, 25, 50, and 100 ng/L in the water column. GCH dissipated rapidly from the water column, with only 40% remaining 24 h after application (similar to LCH, of which 30% remained after 24 h; Roessink et al. 2005). Zooplankton and macroinvertebrates were monitored closely; phytoplankton chlorophyll, macrophyte biomass, litter decomposition, and community metabolism were also measured.
The ecological responses observed in the enclosures treated with GCH were very similar to those observed in previous studies with LCH (Table 5). Based on multivariate principal response curve (PRC) analysis (Van den Brink and ter Braak 1998, 1999), the NOEC for the macroinvertebrate community was 5 ng/L in the GCH study, and less than 10 ng/L in the LCH study. PRC indicated consistent differences at 50 ng/L in the GCH study and at 100 ng/L in the LCH study. In both studies, the macroinvertebrate communities recovered from effects at the highest treatment level. PRC analysis indicated that the macroinvertebrates most affected by GCH were Chaoborus sp., Caenis sp., and G. pulex; the macroinvertebrates most affected by LCH were G. pulex and C. obscuripes. (Caenis, though not abundant in macrophyte-dominated enclosures in the LCH study, were among the most sensitive macroinvertebrates in plankton-dominated enclosures, consistent with the GCH enclosures). Based on univariate analysis in the GCH study, the abundance of the mayfly Caenis sp. was briefly reduced at 5 ng/L, and the midge subfamily Orthocladiinae may also have been affected at 5 ng/L. The phantom midge Chaoborus was reduced at 10 ng/L, the midge family Ceratopogonidae at 25 ng/L, and the amphipod G. pulex and isopod Proasellus spp. at 50 ng/L. Where comparisons could be made, the effects of GCH on macroinvertebrate taxa were similar to or slightly greater than effects of similar concentrations of LCH. For example, the NOEC for Chaoborus was 5 ng/L for GCH and 10 ng/L for LCH; for G. pulex, the NOEC was 25 ng/L in both studies.
For the zooplankton community, PRC analysis indicated a NOEC of 50 ng/L for GCH, twice as great as in macrophyte-dominated LCH enclosures (Table 5). The cladocerans Daphnia longispina and Ceriodaphnia quadrangula showed the greatest negative responses to GCH treatment, while the rotifers Cephalodella gibba and Colurella uncinata showed the greatest positive responses. Univariate analysis of the GCH data indicated that the NOEC for C. gibba and C. uncinata (increased abundance) was 50 ng/L. NOECs (decreased abundance) were 50 ng/L for D. longispina and 25 ng/L for C. quadrangula. There were no effects on calanoid copepods, cyclopoid copepods, or copepod nauplii at 100 ng/L GCH, the highest treatment level. Some of these changes corresponded to observations in the LCH study, while others did not. For example, the rotifers Lecane lunaris and Anureopsis fissa increased in response to LCH, similar to the responses of other rotifer species to GCH. On the other hand, copepod nauplii decreased at 50 ng/L LCH, a response not observed with GCH, and cladocerans increased at 10 ng/L LCH but two cladoceran species decreased at 50 and 100 ng/L GCH. Overall, zooplankton were less affected than macroinvertebrates in both studies.
Neither GCH nor LCH affected phytoplankton chlorophyll concentrations, macrophyte biomass, or community metabolism. A small, transitory, non-dose-related reduction in litter decomposition rate was observed at 10 ng/L GCH, whereas LCH had no observed effect on litter decomposition.
When the results for each microcosm endpoint were expressed using the effect classes described by Brock et al. (2006), the Class 3 effects (short-term effects) were first noted at 10 ng/L for GCH (van Wijngaarden et al. 2008) and at 25 ng/L for LCH (Roessink et al. 2005). Long-term effects (Classes 4 and 5) were first noted at 25 ng/L for GCH and 100 ng/L for LCH. Taking all the ecological responses into account, the NOECcommunity was 5 ng/L for GCH, 10 ng/L for LCH. The difference between the NOECcommunity for GCH and LCH was consistent with the observed differences in single-species toxicity (Table 3) and in SSDs (Table 4). Like the laboratory toxicity results, the GCH enclosure results were consistent with GCH as a refined form of LCH.
Conclusions
Results for GCH toxicity to algae, invertebrates, and fish supported the inferred correspondence between the aquatic toxicity of GCH and LCH. Microcosm results with both substances were consistent with the single-species toxicity data. The data from toxicity tests and field studies indicated that GCH causes effects at approximately one-half the concentration at which LCH causes similar effects. These results are overwhelmingly consistent with GCH as a refined form of LCH. The difference in toxicity is small, within the range of variation generally expected from repeated studies with a single species and a single test substance in a single laboratory. Because GCH has approximately twice the biological activity of LCH, application rates needed for insect control are correspondingly lower, so the risks to aquatic species posed by LCH and GCH are essentially the same.
In light of the consistencies, and the extensive database available for GCH and LCH, there is a high degree of certainty about the likely effects of these pyrethroids on aquatic organisms. The high degree of certainty implies that the NOECcommunity based on the microcosm studies can be used to directly infer a “regulatory acceptable concentration” (RAC) for both GCH and LCH (Giddings et al. 2002).
References
Ali I, Gupta VK, Aboul-Enein HY (2003) Chirality: a challenge for the environmental scientists. Curr Sci 84(2):152–156
Brock TCM, Arts GHP, Maltby L, van den Brink P (2006) Aquatic risks of pesticides, ecological protection goals, and common aims in European Union legislation. Integr Environ Assess Manag 2:e20–e46. doi:10.1897/1551-3793(2006)2[e20:AROPEP]2.0.CO;2
Cai X, Liu W, Sheng G (2008) Enantioselective degradation and ecotoxicity of the chiral herbicide diclofop in three freshwater algal cultures. J Agric Food Chem 2008:2139–2146. doi:10.1021/jf0728855
Farmer D, Hill IR, Maund SJ (1995) A comparison of the fate and effects of two pyrethroid insecticides (lambda-cyhalothrin and cypermethrin) in pond mesocosms. Ecotoxicol 4:219–244. doi:10.1007/BF00116342
Giddings JM, Brock TCM, Heger W, Heimbach F, Maund SJ, Norman SM, Ratte HT, Schäfers C, Streloke M (2002) Community level aquatic system studies—interpretation and criteria (CLASSIC). Society of Environmental Toxicology and Chemistry, Pensacola
Henry KS, McClymont EL, Najar JR (2003a) XDE-225: an acute toxicity study with the amphipod, Gammarus pseudolimnaeus. Report No. 021059. Dow Chemical Company, Midland
Henry KS, McClymont EL, Najar JR (2003b) XDE-225: toxicity testing with various life stages of the amphipod Gammarus pseudolimnaeus. Study No. 021119. Dow Chemical Company, Midland
Hill IR, Runalls JK, Kennedy JH, Ekoniak P (1994a) Effects of lambda-cyhalothrin on aquatic organisms in large-scale mesocosms. In: Hill IR, Heimbach F, Leeuwangh P, Matthiessen P (eds) Freshwater field tests for hazard assessment of chemicals. Lewis Pubs, Boca Raton, pp 345–360
Hill IR, Runalls JK, Kennedy JH, Ekoniak P (1994b) Lambda-cyhalothrin: a mesocosm study of its effects on aquatic organisms. In: Graney RL, Kennedy JH, Rodgers JH (eds) Aquatic mesocosm studies in ecological risk assessment. Lewis Pub, Boca Raton, pp 403–468
Kirk HD, Giles MM, McClymont EL, McFadden LG (2000) XR-225: growth inhibition test with the freshwater green alga, Selenastrum capricornutum Printz. Study No. 001093. Dow Chemical Company, Midland
Kirk HD, Henry KS, Staley JL, McClymont EL, McFadden LG (2001) XDE-225: evaluation of the chronic toxicity with the daphnid, Daphnia magna Straus. Study No. 011051. Dow Chemical Company, Midland
Liu W, Gan J, Lee S, Werner I (2004) Isomer selectivity in aquatic toxicity and biodegradation of cypermethrin. J Agric Food Chem 52:6233–6238. doi:10.1021/jf0490910
Liu W, Gan J, Lee S, Werner I (2005a) Isomer selectivity in aquatic toxicity and biodegradation of bifenthrin and permethrin. Environ Toxicol Chem 24:1861–1866. doi:10.1897/04-457R.1
Liu W, Gan J, Schlenk D, Jury WA (2005b) Enantioselectivity in environmental safety of current chiral insecticides. Proc Natl Acad Sci USA 102:701–706. doi:10.1073/pnas.0408847102
Machado MW (2001a) XDE-225 and lambda-cyhalothrin: comparative toxicity to rainbow trout (Oncorhynchus mykiss) under flow-through conditions. Study No. 011056. Dow Chemical Company, Midland
Machado MW (2001b) XDE-225 and lambda-cyhalothrin: comparative toxicity to daphnids (Daphnia magna) under static-renewal conditions. Study No. 011050. Dow Chemical Company, Midland
Maltby L, Blake N, Brock TCM, van den Brink PJ (2005) Insecticide species sensitivity distributions: importance of test species selection and relevance to aquatic ecosystems. Environ Toxicol Chem 24:379–388. doi:10.1897/04-025R.1
Marino TA, Rick DL (2000a) XR-225: an acute toxicity study with the rainbow trout, Oncorhynchus mykiss Walbaum. Study No. 001073. Dow Chemical Company, Midland
Marino TA, Rick DL (2000b) XR-225: an acute toxicity study with the daphnia, Daphnia magna Strauss. Study No. 001075. Dow Chemical Company, Midland
Marino TA, Rick DL (2001a) XR-225: an acute toxicity study with the bluegill sunfish, Lepomis macrochirus Rafinesque. Study No. 001074(A). Dow Chemical Company, Midland
Marino TA, Rick DL (2001b) XR-225 and lambda-cyhalothrin: an acute toxicity comparison study with the bluegill sunfish, Lepomis macrochirus Rafinesque. Study No. 001074. Dow Chemical Company, Midland
Maund SJ, Hamer MJ, Warinton JS, Kedwards TJ (1998) Aquatic ecotoxicology of the pyrethroid insecticide lambda-cyhalothrin: considerations for higher-tier aquatic risk assessment. Pestic Sci 54:408–417. doi:10.1002/(SICI)1096-9063(199812)54:4<408::AID-PS843>3.0.CO;2-T
Posthuma L, Suter GW II, Traas TP (2002) Species sensitivity distributions in ecotoxicology. Lewis Publishers, Boca Raton
Roessink I, Arts GHP, Belgers JDM, Bransen F, Maund SJ, Brock TCM (2005) Effects of lambda-cyhalothrin in two ditch microcosm systems of different trophic status. Environ Toxicol Chem 24:1684–1696. doi:10.1897/04-130R.1
Schroer A, Belgers J, Brock T, Matser A, Maund S, van Den Brink P (2004) Comparison of laboratory single species and field population-level effects of the pyrethroid insecticide λ-cyhalothrin on freshwater invertebrates. Arch Environ Contam Toxicol 46:324–335. doi:10.1007/s00244-003-2315-3
Sewell IG, McKenzie J (2006a) Gamma-cyhalothrin: acute toxicity to fathead minnow (Pimephales promelas). SafePharm laboratories project No. 2119/0014. The Dow Chemical Company report 050607. April, 2006
Sewell IG, McKenzie J (2006b) Gamma-cyhalothrin: acute toxicity to guppy (Poecilia reticulata). SafePharm laboratories project No. 2119/0013. The Dow Chemical Company report 050606. April, 2006
Sewell IG, McKenzie J (2006c) Gamma-cyhalothrin: acute toxicity to zebra fish (Brachydanio rerio). SafePharm laboratories project No. 2119/0015. The Dow Chemical Company report 050608. April, 2006
U.S. EPA (United States Environmental Protection Agency) (2007) Office of pesticide programs (OPP) pesticide toxicity database. http://www.ipmcenters.org/Ecotox/index.cfm. Accessed Dec 2007
Van den Brink PJ, ter Braak CJF (1998) Multivariate analysis of stress in experimental ecosystems by principal response curves and multivariate analysis. Aquat Ecol 32:163–178. doi:10.1023/A:1009944004756
Van den Brink PJ, ter Braak CJF (1999) Principal response curves: analysis of time-dependent multivariate responses of a biological community to stress. Environ Toxicol Chem 18:138–148. doi:10.1897/1551-5028(1999)018<0138:PRCAOT>2.3.CO;2
Van Wijngaarden RPA, Barber I, Brock TCM (2008) Effects of the pyrethroid insecticide gamma-cyhalothrin on aquatic invertebrates in laboratory and outdoor microcosm tests. Ecotoxicology. doi:10.1007/s10646-008-0274-1
Van Wijngaarden RPA, Brock TCM, van den Brink PJ, Gylstra R, Maund SJ (2006) Ecological effects of spring and late summer applications of lambda-cyhalothrin on freshwater microcosms. Arch Environ Contam Toxicol 50:220–239. doi:10.1007/s00244-004-0249-z
Xu C, Wang J, Liu W, Sheng G, Tu Y, Ma Y (2008) Separation and aquatic toxicity of enantiomers of the pyrethroid insecticide lambda-cyhalothrin. Environ Toxicol Chem 27:174–181. doi:10.1897/07-134.1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giddings, J.M., Barber, I. & Warren-Hicks, W. Comparative aquatic toxicity of the pyrethroid insecticide lambda-cyhalothrin and its resolved isomer gamma-cyhalothrin. Ecotoxicology 18, 239–249 (2009). https://doi.org/10.1007/s10646-008-0277-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0277-y