Abstract
Striped Bass naturally inhabit a wide range of temperatures, yet little is known about the processes that control their acute and chronic temperature limits. The objective of this study was to determine the effect of temperature acclimation on acute thermal maxima and physiology of juvenile Striped Bass. Juvenile fish were acclimated to 15, 25 or 30 °C for 4 weeks, then split into two sampling groups: post-acclimation and post-critical thermal maximum trials. We found that fish survived in all acclimation temperatures with little change to underlying hematology, and that critical thermal maximum (CTmax) increased with increasing acclimation temperature. At CTmax, fish acclimated to 30 °C had elevated plasma cortisol, lactate and potassium levels. These results suggest that, while 30 °C is likely to be outside their thermal optima, Striped Bass can survive at high temperatures. This ability to cope with warm temperatures may provide an advantage with increasing global temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temperature has profound effects on the biology of poikilothermic fishes (Fry 1947, 1967; Brett 1971). As ectothermic aquatic animals, fish must be able to cope with the physiological ramifications of varying temperatures in their environment. The effects of temperature are wide-ranging and can been seen from changes in whole-animal metabolism to specific molecular responses (Bœuf and Payan 2001; Hochachka and Somero 2002; Evans 2008; Currie and Schulte 2013; Schulte 2015). For example, temperature has been shown to affect rates of enzymatic activities (Hochachka and Somero 2002) through to behavioral effects of determining optimal habitats (Bettoli 2005). The response to temperature may not be consistent across fish species, as species may have evolved different mechanisms to counter the same problem. Even within a species, populations may be locally adapted to different thermal conditions and therefore have different physiological responses (Fangue et al. 2006; Losos 2011; Schulte et al. 2011; Conte et al. 2012; Whitehead 2012). For example, different populations of Striped Bass have been shown to have different growth rates at the same temperature (Secor et al. 2000). Additionally, within populations of a single species, individuals may exhibit different life history strategies and react differently to temperatures (Morgan and Iwama 1991; Pavey et al. 2015).
The ubiquitous effects of temperature on fish biology are well documented, with temperature being considered the “master ecological factor” for ectothermic organisms, including fish (e.g., Brett 1971). Methods for quantifying temperature tolerance in fishes in an ecologically relevant manner has been widely discussed (Fry 1967; Brett 1971; Becker and Genoway 1979; Lutterschmidt and Hutchison 1997a, b; Beitinger and Bennett 2000). One such standard method is the Critical Thermal Maximum (“CTmax”) test, whereby temperature tolerance limits are estimated by exposing a fish to a linear increase of temperature, up to the point of loss of equilibrium (LOE) (Lutterschmidt and Hutchison 1997a; Beitinger and Bennett 2000). While fishes in the natural environment rarely experience such an acute change in temperature, extreme events are more likely to offer more selection pressure than “normal” temperatures (Lutterschmidt and Hutchison 1997b). This method also allows for a quantitative comparison of acute temperature tolerance between different groups of fishes and across various acclimation temperatures (see Beitinger and Bennett (2000) for an extensive comparison across North American fishes), which can facilitate understanding of the evolution of temperature tolerance across geography and species groups.
Geographically wide-ranging fishes provide an interesting model for exploring temperature biology. Owing to their life history, these fishes may inhabit rivers with drastically different temperature regimes. The Striped Bass, Morone saxatilis (Walbaum, 1792), is a large anadromous fish, that occupies a large putative geographical range along the east coast of North America. Following a population collapse in the 1980’s, stocks have widely recovered, yet the continued loss of multiple historical Canadian spawning locations recently led to classification of the Bay of Fundy population of Striped Bass as endangered (COSEWIC 2012). Given the implications for Striped Bass aquaculture to supplement wild populations, the effects of temperature on growth has long been of interest (Otwell and Merriner 1975; Cox and Coutant 1981; Cook 2003; Cook et al. 2010). Other temperature research has demonstrated effects of temperature on disease (Carlson et al. 1995; Lapointe 2014), angling or handling stresses (Bettoli and Osborne 1998; Wilde 2000; Bradley et al. 2018), and movements of wild fish (Coutant et al. 1984; van den Avyle and Evans 1990; Secor and Houde 1995; Bettoli 2005; Secor and Piccoli 2007; Duston et al. 2018), with adverse effects typically occurring above and/or below more moderate/optimum temperatures. Less is known about the limits and underlying processes of temperature tolerance in Striped Bass.
While temperature change is inherent and common in fishes’ natural environments, it still can be a stressful stimulus. The cascade of effects of stressors on fishes have been well documented and may span many levels of biological organization ranging from hormonal changes through to whole-animal or even population-level changes (Barton 2002). Primary responses to stress in fishes may be seen through elevation in stress hormones (e.g. cortisol), followed by secondary changes to metabolism (Kir et al. 2017; Kır and Demirci 2018; Kır 2020) and other disturbances to homeostasis (e.g. ability to osmoregulation) before tertiary responses are seen in the animal as a whole. To date, the data on Striped Bass temperature biology has focused on whole-animal endpoints (e.g. CTmax or growth (Otwell and Merriner 1975; Cook 2003; Cook et al. 2006, 2010)). While whole-animal studies offer practical insight into absolute limits, investigation into the primary and secondary stress responses (e.g. cortisol, metabolism, changes in plasma ions) may indicate disturbances not captured in whole-animal approaches.
To date, few studies have assessed critical thermal limits and temperature physiology of Striped Bass. Cook et al. (2006) published a thorough investigation into the thermal limits of Shubenacadie Striped Bass across a range of acclimations temperatures, using both incipient and critical thermal tests. Cook et al. (2006) also suggest that thermal tolerance may be inversely related to body size in Striped Bass, not directly related, as is common for many fishes. CTmax values for Oklahoma Striped Bass are also reported by Lutterschmidt and Hutchison (1997a) at a single acclimation temperature (10 °C) with a small sample size (N = 3). Some thermal limits (CTmax or incipient) have also been published for hybrid Striped x White Bass hybrids (Woiwode and Adelman 1992; Kelly and Kohler 1999; Davis and Parker 1990) demonstrated variations in various hematological endpoints (e.g. cortisol, plasma chloride) following a stressor in southern Striped Bass acclimated to different temperatures. It has also been suggested that Northern populations of Striped Bass possess a broader temperature tolerance range than Southern counterparts (Cook et al. 2006). While these studies provide some insight into the range and variation of temperature tolerance in Striped Bass, there is an absence of studies exploring underlying physiological processes with multiple acclimation temperatures and at extreme thermal limits, such as CTmax. Understanding these processes could shed light on the limiting factors which limit the thermal tolerance ranges of Striped Bass and help define traits important for selection.
The objective of this study is to determine the effect of acclimation temperature on the upper thermal limit (CTmax) of juvenile Striped Bass from Nova Scotia, Canada. Furthermore, we aim to determine whether acclimation and CTmax trials also cause changes to ion regulation (plasma ions), indicators of anaerobic metabolism (lactate) and stress (cortisol). Lastly, we test the putative inverse relationship of thermal tolerance and body weight. We hypothesize that CTmax will increase with increasing acclimation temperature and that increased temperature will result in significant changes in plasma hematology.
Materials and methods
General husbandry
All fish were juvenile Striped Bass (mass = 74.5 ± 3.4 g; fork length = 19 ± 0.4 cm; mean ± standard error), raised from wild-caught eggs, spawned naturally in the Shubenacadie River (Nova Scotia, Canada). Fish were maintained in circular, fiberglass tanks (1 m in diameter; ~750 L) with a constant flow through of fresh (0 ‰), dechlorinated municipal water and prior to any temperature trials, all fish were maintained at 15 °C. Water quality was routinely monitored for constant pH, chlorine and salinity. The photoperiod was kept constant at 14 h light: 10 h dark throughout acclimation and experiments. Fish were fed daily to satiation on commercial Trout pellets (3mm Vita Pellets; EWOS). Prior to trials, food was withheld for 24 h. All procedures were approved by the University of New Brunswick Saint John’s Animal Care Committee according to Canadian Council of Animal Care standards.
Experimental design
In their rearing tanks, fish were acclimated to one of three temperatures: 15 °C, 25 or 30 °C. Three replicate tanks were used to acclimate fish to each of these temperatures. Temperatures were maintained by the use of multiple 800-watt submersible heaters (Aquatop Titanium 800 W; TH800W) with external temperature controllers (Inkbird; ITC-308). Fish were acclimated to the respective temperatures at a rate of 1-1.5 °C per day. Once at the acclimation temperature, fish were maintained at this temperature for 4 weeks prior to thermal maxima trials and/or blood sampling.
Fish were sampled as part of one of two treatment groups: (1) acclimation control or (2) at critical thermal maximum (CTmax). Acclimation control fish underwent no further temperature change nor manipulation other than acclimation to one of the three temperatures (15, 25 or 30 °C) and were removed directly from acclimation tanks for sampling. CTmax group fish were sampled immediately following the CTmax trial (described below). For each treatment and temperature combination, three fish from the three replicate acclimation tanks were selected to account for possible tank variation (N = 3 fish x 3 tanks = 9 per temperature & treatment). More than three fish were housed per holding tank and the three fish were randomly selected at the sampling time The 30 °C acclimation group was not originally planned for in the study design but was added after we noted the relatively small disturbances at 25 °C; therefore only 7 fish from the same cohort remained to be used for the 30 °C acclimation/CTmax group. New fish were not added to the experiment to avoid confounding source variation.
Critical temperature maximum (CTmax) chamber
All CTmax trials were performed on individual fish, in the same experimental system and monitored by the same observer, to avoid inter-observer bias. The experimental system was comprised of an insulated test tank (56 × 28 × 17.75 cm; 27.7 L) and heater tank (48.25 × 43 × 24 cm; 50.3 L). The water in the test tank was pumped to the heater tank at ~ 1100 L · h− 1 (Marineland Maxi-Jet Pro 1200; ML90509), where it was heated before being returned to the holding tank via passive overflow from the heater tank. The water in the heater tank was heated by two 800-watt submersible heaters (Aquatop Titanium 800 W; TH800W) with external temperature controllers (Inkbird; ITC-308) and circulated with a powerhead (Hydor Koralia Evolution Powerhead; HY-KOR03A) for efficient heating. The temperature in both chambers were monitored and recorded electronically to ensure consistent heating rates (MyDevices IoT Cayenne via Raspberry Pi 3 Model B with DS18b20 temperature probes). Both the header and experimental tanks were well aerated using pressurized air and ceramic air stones. Dissolved oxygen was periodically monitored (YSI Model 85) to ensure levels did not fall with increasing temperatures and stayed above 80 % saturation.
Critical temperature maximum (CTmax) trials
An individual fish was removed from the acclimation tanks and quickly moved to the previously described experimental holding tank, containing water of the respective acclimation temperature. The fish was left to adjust to the experimental tank for 1 h prior to any experimentation. Following this 1 h period, the temperature in the system was increased at a rate of rate of 0.3 °C · min− 1 (18 °C · hour− 1), which is commonly used for CTmax tests in fishes (Woiwode and Adelman 1992; Lutterschmidt and Hutchison 1997a, b; Beitinger and Bennett 2000; Cook et al. 2006). Loss of equilibrium (LOE) was used as the endpoint for CTmax, as defined as the point where the fish could not maintain a dorso-ventral position for 10 s (Becker and Genoway 1979; Lutterschmidt and Hutchison 1997b; Zhang and Kieffer 2014). When LOE was reached, the temperature was recorded, then the fish was immediately removed from the chamber and sampled. To ensure any changes found in the CTmax treatment groups were not artefacts of the experimental design, a pilot study was run using an additional control group of fishes which underwent a sham CTmax trial. These sham pilot trials included transferring fish to the CTmax chamber, and holding them for a similar length of time of a typical CTmax trial but without any further temperature increases before sampling. We did not find any significant changes to plasma endpoints when compared to the acclimated control fish.
Sampling and hematological analysis
At the appropriate sampling period (acclimation or CTmax), fish were anesthetized in 100 mg · ml− 1 of tricaine methanesulfonate (TMS; Sigma-Aldrich E10521) buffered with sodium bicarbonate (200 mg · ml− 1). Once the fish was anesthetized, a ~ 0.7mL blood sample was collected via caudal puncture using a 23 g needle and 1 mL syringe. The fish was then euthanized, weighed and measured (total length; TL). The blood sample was spun in a mini centrifuge at 8500 g for 5 min. The plasma was separated into duplicate 1.5mL tubes then frozen in a metal rack on dry ice, and stored at -80 °C for future analyses.
From one duplicate tube of plasma, plasma ions and lactate were measured. Concentrations of plasma sodium, chloride and potassium (Na+, Cl+ and K+) were quantified using an electrolyte analyzer (Smartlyte; Diamond Diagnostics) and plasma lactate was quantified using a medical lactate meter (Lactate Statstrip; Nova Biomedical). From the remaining duplicate vial, plasma cortisol levels were measured via enzyme-linked immunosorbent assay (“ELISA”; Neogen kit # 402,710). To expedite the solvent evaporation step, 40 samples were extracted at once, and a stream of nitrogen was provided to each sample with a length of PE50 catheter tubing connected to a 23 g needle (barrel removed) which was pierced into silicone tubing header containing pressurized nitrogen gas (T. McCormack, Mount Allison University, Canada; pers comm.). ELISAs were run according to manufacturer’s directions (using the ethyl acetate and HCl stop solution options), aside from the following modification: to allow for smaller volume samples, starting volume of samples and all reagents were decreased by half up to the point of the 1:100 dilution. Samples that were below the detection limit were re-analyzed at a lower dilution (1:10) as available. ELISA plates were read using a plate reader (Syngery HTX with Gen5 software; Biotek); sample concentrations were calculated with the reader’s software, using the plate standards, blank adjustment and accounting for background absorbance. All standards, controls and samples were run in duplicate and averaged (duplicate variation was 16 % ± 2 % (mean ± SEM)).
Statistical analyses
The effect of temperature CTmax was assessed using a linear mixed effect model with originating rearing tank as a random factor to avoid pseudoreplication (e.g. CTmax ~ Acclimation_Temperature + (1|Tank)) (Bates et al. 2015). Similarly, the effects and interaction of acclimation temperature and treatment (i.e. acclimation control or at CTmax) on plasma ions, lactate and cortisol were also assessed using linear mixed effects models with originating tank as a random factor (e.g. Sodium ~ Acclimation_Temperature*Treatment + (1|Tank)). For all tests where interactions were significant, the effects of each fixed factor were then assessed in isolation (Logan 2010). Where significant effects of a factor were found, differences across the levels of each factor (e.g. 15 vs. 25 vs. 30 °C for acclimation temperature) were determined post-hoc with Tukeys tests (Hothorn et al. 2009). The effects of acclimation temperature and body weight on CTmax were tested using an ANCOVA (acclimation temperature = categorical factor; body weight = continuous factor)(Logan 2010). The effect of body weight on CTmax within each temperature treatment was also assessed using linear regressions. The acclimation response ratio (ARR) was also calculated for CTmax differences as ARR = Δ CTmax / Δ Acclimation Temperature according to Claussen (1977). Furthermore, to determine the relationship of acclimation temp and thermal tolerance, linear (CTmax = Acclimation Temperature) and hyperbolic (CTmax = I(Acclimation Temperature ^2)) regressions were run and then compared with an ANOVA for best fit. All data were assessed for normality and outliers (Grubbs test). All statistical tests were performed using R (Version 4.0.1) (R 2008) and an alpha of 0.05.
Results
Critical thermal maximum
Critical thermal maxima were significantly different at each acclimation temperature, with higher CTmax occurring at warm acclimation temperatures (Fig. 1; p < 0.001 for linear mixed effects model). Overall, the mean CTmax in 25 and 30 °C acclimated fish were 2.7 and 5.6 °C higher than CTmax at 15 °C. Mean difference between observed CTmax and respective acclimation temperatures were 16.7, 9.4 and 7.25 °C for the 15, 25 and 30 °C acclimation groups, respectively. The acclimation response ratio was highest when comparing 25 and 30 °C acclimated fish (Table 1). The relationship between CTmax and acclimation temperature was determined to be non-linear (CTmax = -0.55*Acc_temp + 0.021* Acc_temp^2 + 35.35; p < 0.001; Adjusted R2 = 0.8714). When assessed together with acclimation temperature, body weight did not affect CTmax (Fig. 2; p = 0.5 for body weight; p = 0.015 for acclimation temperature). Linear regressions of CTmax vs. body weight within each temperature group were also non-significant (p = 0.97, 0.16 and 0.53 for 15, 25 and 30 °C, respectively; Fig. 2).
Critical thermal maxima (CTmax) of juvenile Striped Bass, Morone saxatilis, acclimated to various temperatures according to body weight. Fish were acclimated to 15 (○), 25 (■), or 30 °C (▽) for 4 + weeks prior to undergoing CTmax trials. Lines reflect linear regressions within each temperature, though none of these regressions were found to be significant. Effects of acclimation temperature (“Temp”), Body Weight and their interaction (“Int”) were determined via ANCOVA
Plasma analyses
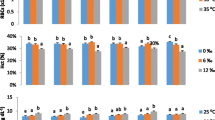
Overall, the effects of acclimation temperature and treatment (acclimation control vs. CTmax) varied across plasma variables (Figs. 3 and 4). No interactions between acclimation temperature and treatment group were found for plasma sodium and chloride (p = 0.56 and 0.24, respectively), though interactions were found for potassium, lactate and cortisol (p = 0.03, < 0.001 and 0.04, respectively). Acclimation temperature was found to have significant effects on plasma sodium (Na+), potassium (K+), cortisol and lactate (p = 0.0013, 0.0016, for sodium and potassium and p < 0.001 for lactate & cortisol) whereas temperature did not affect chloride (Cl− ) (p = 0.37).
Mean (± SEM) plasma ion concentrations (sodium, chloride & potassium in i, ii, & iii, respectively) in juvenile Striped Bass, Morone saxatilis, acclimated to various temperatures. Fish were sampled as following: after 4 weeks acclimated to appropriate temperature (▲) or immediately following critical thermal maxima (CTmax) trial (☐). Lowercase letter designations denote significant differences across temperature within the CTmax trial group. Uppercase letter designations designate differences across temperature, regardless of CTmax treatment (no interaction). An asterisk (*) denotes a significant difference in CTmax from respective acclimation control. Statistical differences were determined via linear mixed effects models followed by pairwise comparisons determined via Tukey’s post hoc tests as appropriate
Mean (± SEM) plasma lactate and cortisol (i & ii, respectively) in juvenile Striped Bass, Morone saxatilis, acclimated to various temperatures. Fish were sampled as following: after 4 weeks acclimated to appropriate temperature (▲) or immediately following critical thermal maxima (CTmax) trial (☐). Lowercase letter designations denote significant differences across temperature within the CTmax trial group. Uppercase letter designations denote significant differences across temperature within the acclimation trial group. An asterisk (*) denotes a significant difference in CTmax from respective acclimation control. Statistical differences were determined via linear mixed effects models followed by pairwise comparisons determined via Tukey’s post hoc tests as appropriate
Within each plasma endpoint, the patterns of change across temperature and treatment also differed. Plasma sodium (Fig. 3i) was lower in 25 °C acclimated fish compared to 15 and 30 °C fish (p < 0.001). Plasma chloride (Fig. 3ii) did not vary significantly by temperature nor treatment group. In fish acclimated to 15 and 30 °C, plasma potassium levels were significantly elevated at CTmax (p = 0.002 and p < 0.001, 25 vs. 15 and 30 °C respectively). Furthermore, plasma potassium was highest in fish acclimated to 30 °C at CTmax (p = 0.006 and p < 0.001, 30°c vs. 15 and 25 °C respectively). Plasma lactate (Fig. 4i) increased with each temperature increase within both the acclimated and post-CTmax fish (p < 0.001 for all except p = 0.002 for 25 vs. 30 °C acclimated fish). Lactate was significantly increased following CTmax at both 25 and 30 °C compared to acclimated control fish (p < 0.001 for both). Cortisol was increased at 25 °C (Fig. 4ii) and elevated in all temperature groups following CTmax (p = 0.02 at 15 °C, p < 0.001 at 25 and 30 °C). Cortisol was highly variable in fish acclimated to 25 °C at CTmax; this pattern persisted when analyses were re-run removing one outlier (p < 0.001), however removing the outlier did not change the pattern of significant changes. The fold change in cortisol levels at CTmax from acclimated values was greatest at 25 °C (Fold changes = 1.1, 5.71, 2.16 for 15, 25 and 30 °C acclimation groups, respectively).
Discussion
Juvenile Striped Bass survived in warm temperatures up to four weeks, without disruption to stress hormones and plasma ions. Qualitatively, fish acclimated to 30 °C did not have any changes to behaviour during acclimation and holding, nor were there mortalities in any acclimation groups. Furthermore, no change in resting hematology was found across acclimation temperatures, even following a four week acclimation period to 30 °C. In contrast, Davis and Parker (1990) found increases in plasma chloride and cortisol when juvenile Southern Striped Bass were acclimated to 30 °C for 8 days. Such changes in cortisol and plasma ions are typical in fish following stressful events (Barton 2002). The lack of stress response found at this high temperature may suggest that Northern Striped Bass used in this study are better equipped to adapt to higher temperatures. Sunday et al. (2011) demonstrated that while thermal tolerance breadth increases, absolute upper thermal tolerance levels tend to decrease in more Northern population, which may be contrary to our findings. Future research should aim to investigate potential differences in tolerance limits between North and South Striped Bass. It should be noted that plasma lactate was elevated in 25 and 30 °C fish (both acclimated and at CTmax), and thus there was a possible switch to anaerobic processes, suggesting these temperatures may be outside the ideal temperature range for these fish as they were not able to meet energy demands with aerobic processes. This aligns with Cox and Coutant (1981) who found ideal growth temperatures to be more moderate (14–22 °C), though this optimum may differ if fish were not provided a fully satiating diet.
One potential confounding aspect of comparing tolerance limits within and across species is the length of acclimation. In the present study, fish were acclimated for at least 28 days, while other studies on Striped Bass used shorter acclimations, ranging from 8 days (Davis and Parker 1990) to 2 weeks (Cook et al. 2006). In their discussion and critique of utilizing CTmax, Lutterschmidt and Hutchison (1997b) acknowledge that rate and length of acclimation is important to the resultant CTmax but suggest only that in ecotherms, thermal acclimation is rapid, occurring with hours to a few days. Beitinger and Bennett (2000) suggest acclimation of new thermal tolerance limits suggest this may range from 1 to 20 days. While the absolute definition of what precisely defines acclimation has long been discussed (Lagerspetz 2006), it generally thought of as physiological or behavioural changes related to a new environment (e.g. higher temperature tolerance), but the time course to acclimation may depend on which system is being investigated. There is no “gold standard” of how long fishes should be acclimated for thermal tolerance testing. Still, many studies in other fish species use longer acclimation times more similar to our period than shorter times used in other aforementioned Striped Bass papers (for example see acclimation time in Table 1). The difference acclimation time may explain some of the differences seen between the present study and others. For example, the increased cortisol by Davis and Parker (1990), may be an artefact of the shorter (8 day) acclimation period if this was insufficient time to acclimate. Although it should be noted that similar tolerance limits to ours were found by Cook et al. 2006, suggesting that the 2 weeks used by Cooke et al. was sufficient acclimation to adjust thermal tolerance in these Northern fish. Without further information on the timeline of thermal acclimation in Striped Bass, it is difficult to determine whether the differences between Northern and Southern fish are valid or an artefact of methodology.
In addition to their ability to acclimate to warm waters, the upper acute thermal tolerance of juvenile Striped Bass increased with increasing acclimation temperatures. While CTmax is widely used in fishes, few other studies have used it to test thermal limits in Striped Bass, though our values generally coincide with these previously published values. While the authors did not report mean CTmax directly, our CTmax values are close to the estimated values from regressions published by Cook et al. (2006). As the fish used by Cook et al. (2006) are from the same population and rearing facility, similar thermal tolerances were expected. The slightly lower values at the higher temperatures may be due to experimental set-up or an artefact of differing statistical methods (regression values vs. means). The pattern of change in CTmax across acclimation temperatures found in this study is surprising. Typically, the upper thermal limit will increase linearly as acclimation temperature increases, and then plateau as acclimation temperature approaches thermal maxima (Beitinger and Bennett 2000). Thus, we would expect the acclimation response ratio to decrease as the acclimation temperature passes the linear portion and approaches thermal maximum (see Table 1 for ARR values from other fishes). The fish species selected in this table represent a wide range of taxonomic and ecological diversity, from ancient lineages of temperate sturgeon (Acipenser brevirostrum), to tropical modern Perciformes (Therapon jarbua), to a similar temperate sea-bass (Dicentrarchus labrax). Yet only the Striped Bass and Striped Bass hybrid show increased ARR at the highest temperature pairs. Our data show that the ARR is largest between the two highest temperatures, suggesting the scope for increase in acute maxima is still increasing. The ARR’s found at higher temperatures in this study are also on the high end for a mid-latitude fish (Morley et al. 2019). Other studies examining Morone spp. thermal tolerance across similar temperatures used in this study have found linear relationships of CTmax to acclimation temperature, thus implying a constant ARR (Table 1), unlike our data. Given that CTmax continued to increase with each increase in acclimation temperature (i.e. the relationship has not plateaued/nonlinear), it is possible that further increases to acute thermal maximum may be possible at even higher acclimation temperatures.
While upper thermal tolerance did increase with acclimation, indicators of physiological stress and acid-base changes at CTmax were present in fish acclimated to warmer temperatures. During a stressful event, such as a CTmax trial, disturbances to ion regulation following the cascade of stress hormones are common (Barton 2002; Farrell 2011). Thus, the increases in cortisol found at CTmax are to be expected, given the stressful nature of such an acute test. The modest fold change in cortisol pre/post CTmax found at 25 °C was within the range of fold changes reported before/after confinement stress (~ 3 to 5 fold change) (Davis and Parker 1990; Secor et al. 2000), but lower fold-changes were found at the other two acclimation temperatures. When compared to other types of stressors, much higher fold changes (~ 86-fold) in cortisol have been documented in Striped Bass following acute salinity transfer (Madsen et al. 1994), though this may be related to the additional role of cortisol in seawater readiness in addition to stress response (Evans et al. 2005). It is surprising that the highest CTmax cortisol values were found in 25 °C acclimated fish, not the highest acclimation temperature, which we would expect to be the most stressful. This may be an artefact of the variable nature of cortisol response found, especially at 25 °C, or there may be a depression of cortisol response occurring at the highest temperatures combined with an acute stress. Furthermore, the highly variable cortisol at 25 °C coincides with the highest variability in CTmax, which could suggest increased variation across individuals at this moderate temperature treatment. The cortisol response following stress at high temperatures may be tied to an overall reduction to metabolism, which has been seen in other fishes (Kir et al. 2017; Kır and Demirci 2018; Kır 2020), should be investigated further.
Evidence of plasma ion disruption was also illustrated in the increase seen in plasma potassium at CTmax, particularly in fish acclimated to 30 °C. The apparent hyperkalemia at 30 °C is mirrored by the increased lactate at CTmax at the same acclimation temperature. This paring of changes is common in fishes following stressful or strenuous activities, in which the increase in lactate production from anerobic metabolism results in plasma acidosis, which in turn leads potassium to migrate into the plasma in order to close the anion gap (Wood et al. 1983; Kieffer et al. 1994). This was not seen at CTmax in the lower temperature groups, which again suggests that while fish may survive at these elevated temperatures for many weeks, it is likely to be less ideal than moderate temperatures. While the other CTmax studies in striped bass did not measure plasma ions (Lutterschmidt and Hutchison 1997a; Cook et al. 2006), chloride fluctuation has been documented during recovery from confinement stress in Striped Bass and their hybrids (Davis and Parker 1990; Davis 2004). We did not find chloride fluctuations immediately following CTmax, though ion disruption may not occur until many hours after the stressor (Davis and Parker 1990; Davis 2004), thus it is possible changes in chloride may have been seen if fish were allowed to recover and monitored. Future studies should aim to investigate the time course of ion perturbation following temperature stress.
In contrast to Cook et al. (2006), we did not find any indication that thermal tolerance was inversely related to body size in juvenile Striped Bass. Generally, CTmax would be expected to increase with body mass, likely owing to the lag of temperature transfer to the interior of a larger bodied animal (Beitinger and Bennett 2000; Ziegeweid et al. 2008; Cook et al. 2006) has suggested an inverse relationship of body weight to thermal tolerance. We found no indication that temperature tolerance is related to body size. Compared to Cook et al. (2006), our paper assessed the patterns of thermal tolerance change on a continuous basis across a wider range of sizes and generally larger size juveniles (52–102 g in the present study; small = 21.8 g vs. large = 34.4 g by Cook et al. (2006)). Zhang and Kieffer (2014), found a positive relationship between thermal tolerance and body weight within small and large groups of Shortnose Sturgeons, Acipenser brevirostrum (Lesueur 1818 non Heckel 1836), yet the smaller fish as a group had higher tolerance when compared to large fish categorically. It is possible that the inverse relationship exists in smaller juvenile Striped Bass as previously described, but the direction changes in larger juveniles. As previously mentioned, our CTmax values were slightly lower than those found by Cook et al. (2006), which may support this notion. Notwithstanding, both the current study and Cook’s previous work on body size assess these trends over a small subsection of juvenile Striped Bass sizes; future investigation into this phenomenon should assess trends across a larger size range.
As a wide-ranging, migratory fish, the natural thermal regimes of Striped Bass vary greatly across their range. In addition to changes in thermal regimes, Northern Striped Bass are more migratory and typically utilize saline stretches of their environment more than their Southern counterparts (Secor et al. 2000; Conroy et al. 2015). The Shubenacadie Striped Bass used in the present study are one of the most Northern populations of the species. Furthermore, the Shubenacadie River is a tidal bore system (COSEWIC 2012) and is connected to the Bay of Fundy, which boasts the highest tides in the world (Desplanque and Mossman 2001). As such, this population faces extremes in both temperature and salinity fluctuations on and hourly and seasonal scale. The temperatures used in this study span a range of temperatures that may be found naturally in this system: spring spawning occurs around 15 °C (COSEWIC 2012) and summer temperatures in the estuary are expected to be around 22 °C (Duston et al. 2004). In 2020, this population experienced an abnormal summer fish kill, purportedly related to unusually warm temperatures (Withers 2020). With rising global temperatures, understanding the processes with limit tolerance to these fluctuations will become increasingly important.
The ability of juvenile Striped Bass to acclimate to increased temperatures is demonstrated in this paper. Few physiological disruptions were found at acclimation, although increased stress hormones and likely anerobic metabolism occurred at CTmax at the highest temperatures. Nonetheless, juveniles were able to live at 30 °C for multiple weeks, which is only 1.7 °C away from the CTmax measured of fish acclimated to 15 °C. This impressive ability for a temperate fish to withstand warm temperatures may have ecological ramifications as it provides a competitive edge in the face of global temperature change. Better understanding is needed to determine the mechanisms which underpin temperature tolerance, including the potential of heritable genetic traits. Furthermore, as a species which inhabits a wide range of temperature regimes, future research should aim to explore the difference in temperature tolerance across their range.
Data availability
Data from this manuscript will be archived on the University of New Brunswick’s Dataverse Research Data Repository.
References
Barton BA (2002) Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42:517–525. https://doi.org/10.1093/icb/42.3.517
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. 2015 67:48. https://doi.org/10.18637/jss.v067.i01
Becker CD, Genoway RG (1979) Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ Biol Fishes 4:245–256. https://doi.org/10.1007/BF00005481
Beitinger TL, Bennett WA (2000) Quantification of the role of acclimation temperature in temperature tolerance of fishes. Environ Biol Fishes 58:277–288. https://doi.org/10.1023/a:1007618927527
Bettoli PW (2005) The fundamental thermal niche of adult landlocked Striped Bass. Trans Am Fish Soc 134:305–314. https://doi.org/10.1577/T03-204.1
Bettoli PW, Osborne RS (1998) Hooking mortality and behavior of Striped Bass following catch and release angling. N Am J Fish Manage 18:609–615.
Bœuf G, Payan P (2001) How should salinity influence fish growth? . Comp Biochem Physiol C Toxicol Pharmacol 130:411–423. https://doi.org/10.1016/S1532-0456(01)00268-X
Bradley CE, Rice JA, Aday DD, Hightower JE, Rock J, Lincoln KL (2018) Juvenile and adult Striped Bass mortality and distribution in an unrecovered coastal population. N Am J Fish Manag 38:104–119. https://doi.org/10.1002/nafm.10036
Brett JR (1971) Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerka). Am Zool 11:99–113. https://doi.org/10.1093/icb/11.1.99
Carlson RE, Baker EP, Fuller RE (1995) Immunological assessment of hybrid Striped Bass at three culture temperatures. Fish Shellfish Immunol 5:359–373. https://doi.org/10.1006/fsim.1995.0034
Claussen D (1977) Thermal acclimation in ambystomatid salamanders. Comp Biochem Physiol A: Mol Integr Physiol 58:333–340. https://doi.org/10.1016/0300-9629(77)90150-5
Conroy CW, Piccoli PM, Secor DH (2015) Carryover effects of early growth and river flow on partial migration in Striped Bass Morone saxatilis. Mar Ecol Prog Ser 541. https://doi.org/10.3354/meps11474
Conte GL, Arnegard ME, Peichel CL, Schluter D (2012) The probability of genetic parallelism and convergence in natural populations. Proc R Soc B 279:5039–5047. https://doi.org/10.1098/rspb.2012.2146
Cook AM (2003) Growth and survival of age 0 + Shubenacadie River Striped Bass (Morone saxatilis) in relation to temperature and salinity. MSc Dissertation, Nova Scotia Agricultural College
Cook A, Duston J, Bradford R (2006) Thermal tolerance of a northern population of Striped Bass Morone saxatilis. J Fish Biol 69:1482–1490. https://doi.org/10.1111/j.1095-8649.2006.01211.x
Cook A, Duston J, Bradford R (2010) Temperature and salinity effects on survival and growth of early life stage Shubenacadie River Striped Bass. Trans Am Fish Soc 139:749–757. https://doi.org/10.1577/T08-173.1
COSEWIC (2012) COSEWIC assessment and status report on the Striped Bass (Morone saxatilis) in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa
Coutant CC, Zachman KL, Cox DK, Pearman BL (1984) Temperature selection by suvenile Striped Bass in laboratory and field. Trans Am Fish Soc 113:666–671.
Cox DK, Coutant CC (1981) Growth dynamics of juvenile Striped Bass as functions of temperature and ration. Trans Am Fish Soc 110:226–238.
Currie S, Schulte PM (2013) Thermal stress. In: Evans DH, Claiborne JB, Currie S (eds) The physiology of fishes, 4th edn. CRC Press, Boca Raton, pp 257–287
Davis KB (2004) Temperature affects physiological stress responses to acute confinement in sunshine bass (Morone chrysops× Morone saxatilis). Comp Biochem Physiol A: Mol Integr Physiol 139:433–440. https://doi.org/10.1016/j.cbpb.2004.09.012
Davis KB, Parker NC (1990) Physiological stress in Striped Bass: effect of acclimation temperature. Aquaculture 91:349–358. https://doi.org/10.1016/0044-8486(90)90199-W
Desplanque C, Mossman DJ (2001) Bay of Fundy tides. Geoscience Canada
Dülger N, Kumlu M, Türkmen S, Ölçülü A, Eroldoğan OT, Yılmaz HA, Öçal N (2012) Thermal tolerance of European Sea Bass (Dicentrarchus labrax) juveniles acclimated to three temperature levels. J Therm Biol 37:79–82. https://doi.org/10.1016/j.jtherbio.2011.11.003
Duston J, Astatkie T, MacIsaac P (2004) Effect of body size on growth and food conversion of juvenile Striped Bass reared at 16–28 C in freshwater and seawater. Aquaculture 234:589–600
Duston J, Manríquez-Hernández J, MacInnis GM, Reesor CM, Astatkie T (2018) Striped bass early life history in the macrotidal Shubenacadie river. Trans Am Fish Soc 147:919–938. https://doi.org/10.1002/tafs.10076
Evans DH (2008) Teleost fish osmoregulation: what have we learned since August Krogh, Homer Smith, and Ancel Keys. Am J Physiol Regul Integr Comp Physiol 295:R704–R713. https://doi.org/10.1152/ajpregu.90337.2008
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177. https://doi.org/10.1152/physrev.00050.2003
Fangue NA, Hofmeister M, Schulte PM (2006) Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J Exp Biol 209:2859–2872. https://doi.org/10.1242/jeb.02260
Farrell AP (2011) Encyclopedia of fish physiology: from genome to environment. Academic, London
Fry FEJ (1947) Effects of the environment on animal activity. University of Toronto Press, Toronto
Fry F (1967) Responses of vertebrate poikilotherms to temperature. In: Rose AH (ed) Thermobiology. Academic Press, New York, NY, pp 375–409
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, New York
Hothorn T, Bretz F, Westfall P (2009) Multcomp: Simultaneous inference for general linear hypotheses. Biom J 50:346–363
Kelly AM, Kohler CC (1999) Cold tolerance and fatty acid composition of Striped Bass, white bass, and their hybrids. N Am J Aquacult 61:278–285.
Kieffer JD, Currie S, Tufts BL (1994) Effects of environmental-temperature on the metabolic and acid-base responses of rainbow-trout to exhaustive exercise. J Exp Biol 194:299–317
Kir M, Sunar MC, Altindag BC (2017) Thermal tolerance and preferred temperature range of juvenile meagre acclimated to four temperatures. J Therm Biol 65:125–129. https://doi.org/10.1016/j.jtherbio.2017.02.018
Kır M (2020) Thermal tolerance and standard metabolic rate of juvenile gilthead seabream (Sparus aurata) acclimated to four temperatures. J Therm Biol 93:102739. https://doi.org/10.1016/j.jtherbio.2020.102739
Kır M, Demirci Ö (2018) Thermal tolerance and standard metabolic rate of juvenile European sea bass (Dicentrarchus labrax, Linnaeus, 1758) acclimated to four temperatures. J Therm Biol 78:209–213. https://doi.org/10.1016/j.jtherbio.2018.10.008
Lagerspetz KY (2006) What is thermal acclimation? J Therm Biol 31:332–336
Lapointe DDD (2014) Temperature, hypoxia, and mycobacteriosis: Effects on adult Striped Bass Morone saxatilis metabolic performance. Dis Aquat Org 108:113–127. https://doi.org/10.3354/dao02693
Logan M (2010) Biostatistical design and analysis using R. Wiley-Blackwell, West Sussex
Losos JB (2011) Convergence, adaptation, and constraint. Evolution 65:1827–1840. https://doi.org/10.1111/j.1558-5646.2011.01289.x
Lutterschmidt WI, Hutchison VH (1997a) The critical thermal maximum: data to support the onset of spasms as the definitive end point. Can J Zool/Rev Can Zool 75:1553–1560. https://doi.org/10.1139/z97-782
Lutterschmidt WI, Hutchison VH (1997b) The critical thermal maximum: history and critique. Can J Zool/Rev Can Zool 75:1561–1574. https://doi.org/10.1139/z97-783
Madsen SS, McCormick SD, Young G, Endersen JS, Nishioka RS, Bern HA (1994) Physiology of seawater acclimation in the striped bass, Morone saxatilis (Walbaum). Fish Physiol Biochem 13:1–11. https://doi.org/10.1007/BF00004114
Morgan JD, Iwama GK (1991) Effects of salinity on growth, metabolism, and ion regulation in juvenile rainbow and steelhead trout (Oncorhynchus mykiss) and fall chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 48:2083–2094. https://doi.org/10.1139/f91-247
Morley SA, Peck LS, Sunday JM, Heiser S, Bates AE (2019) Physiological acclimation and persistence of ectothermic species under extreme heat events. Global Ecol Biogeogr 28:1018–1037. https://doi.org/10.1111/geb.12911
Otwell WS, Merriner JV (1975) Survival and growth of juvenile Striped Bass, Morone saxatilis, in a factorial experiment with temperature, salinity and age. Trans Am Fish Soc 104:560–566.
Pavey SA, Gaudin J, Normandeau E, Dionne M, Castonguay M, Audet C, Bernatchez L (2015) RAD sequencing highlights polygenic discrimination of habitat ecotypes in the panmictic American eel. Curr Biol 25:1666–1671. https://doi.org/10.1016/j.cub.2015.04.062
R (2008) R: A Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rajaguru S (2002) Critical thermal maximum of seven estuarine fishes. J Therm Biol 27:125–128. https://doi.org/10.1016/S0306-4565(01)00026-2
Schulte PM (2015) The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J Exp Biol 218:1856–1866. https://doi.org/10.1242/jeb.118851
Schulte PM, Healy TM, Fangue NA (2011) Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr Comp Biol 51:691–702. https://doi.org/10.1093/icb/icr097
Secor DH, Houde ED (1995) Temperature effects on the timing of Striped Bass egg production, larval viability, and recruitment potential in the Patuxent River (Chesapeake Bay. Estuaries 18:527–544. https://doi.org/10.2307/1352370
Secor DH, Piccoli PM (2007) Oceanic migration rates of Upper Chesapeake Bay striped bass (Morone saxatilis), determined by otolith microchemical analysis. Fish Bull 105:62–73
Secor DH, Gunderson TE, Karlsson K (2000) Effect of temperature and salinity on growth performance in anadromous (Chesapeake Bay) and nonanadromous (Santee-Cooper) strains of Striped Bass Morone saxatilis. Copeia 2000:291–296
Sunday JM, Bates AE, Dulvy NK (2011) Global analysis of thermal tolerance and latitude in ectotherms. Proc R Soc Lond Ser B: Biol Sci 278:1823–1830. https://doi.org/10.1098/rspb.2010.1295
van den Avyle MJ, Evans JW (1990) Temperature selection by Striped Bass in a Gulf of Mexico Coastal River System. N Am J Fish Manag 10:58–66. https://doi.org/10.1577/1548-8675
Whitehead A (2012) Comparative genomics in ecological physiology: toward a more nuanced understanding of acclimation and adaptation. J Exp Biol 215:884–891. https://doi.org/10.1242/jeb.058735
Wilde GRGGR (2000) Bait and temperature effects on Striped Bass hooking mortality in freshwater. N Am J Fish Manage 20:810–815.
Withers P (2020) Warm weather is possible cause for Shubenacadie River fish kill. https://www.cbc.ca/news/canada/nova-scotia/warm-weatherfish-kill-shubenacadie-river-1.5633432. Accessed8 March 2021
Woiwode JG, Adelman IR (1992) Effects of starvation, oscillating temperatures, and photoperiod on the critical thermal maximum of hybrid Striped× White Bass. J Therm Biol 17:271–275. https://doi.org/10.1016/0306-4565(92)90066-O
Wood C, Turner J, Graham M (1983) Why do fish die after severe exercise? J Fish Biol 22:189–201. https://doi.org/10.1111/j.1095-8649.1983.tb04739.x
Zhang Y, Kieffer JD (2014) Critical thermal maximum (CTmax) and hematology of shortnose sturgeons (Acipenser brevirostrum) acclimated to three temperatures. Can J Zool/Rev Can Zool 92:215–221. https://doi.org/10.1139/cjz-2013-0223
Ziegeweid J, Jennings C, Peterson D, Black M (2008) Effects of salinity, temperature, and weight on the survival of young-of-year shortnose sturgeon. Trans Am Fish Soc 137:1490–1499. https://doi.org/10.1577/t07-046.1
Acknowledgements
Funding for this project was provided by Natural Sciences and Engineering Research Council of Canada, Canada Research Chair, University of New Brunswick, New Brunswick Innovation Foundation, and the Canada Foundation for Innovation. The authors would like to thank Dr. J Duston at the Haley Institute at Dalhousie Faculty of Agriculture, Truro for the fish used in this manuscript. Also, Dr. J Keffer for useful insight in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All experiments were assessed and approved by the University of New Brunswick Saint John’s Animal Care Committee as directed by the Canadian Council of Animal Care (Protocols 2017-2 S-08, 2018-2R-01 and 2019-2R-05).
Conflict of interest
The authors are not aware of any conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Penny, F.M., Pavey, S.A. Increased acute thermal tolerance and little change to hematology following acclimation to warm water in juvenile Striped Bass, Morone saxatilis. Environ Biol Fish 104, 489–500 (2021). https://doi.org/10.1007/s10641-021-01088-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01088-6