Abstract
Since the first reported sighting of Indo-Pacific lionfish (Pterois volitans and P. miles) in the Atlantic Ocean over 30 years ago, growing evidence suggests they may have a marked negative impact upon ecosystems and demersal communities. While lionfish populations expanded rapidly in most locations following their initial establishment, the population in Bermuda, the first location outside of the United States to be invaded, appears to be growing at a slower pace. This study investigated the life history characteristics of the invasive lionfish population in Bermuda to help understand population dynamics and thus potential impacts on Bermuda’s coral reef ecosystem. Annual growth rings in lionfish otoliths were counted to describe population structure and establish size-at-age, which was then utilized to estimate growth parameters using von Bertalanffy models. Macroscopic and histological staging of ovaries, calculations of gonadosomatic and hepatosomatic indices, and enumerations of mature oocytes were used to describe reproductive seasonality and capacity. Our findings show that lionfish in Bermuda appear to grow faster and attain larger mean sizes than they do in their native range or elsewhere in the invaded range. Lionfish in Bermuda, however, appear to reach maturity at larger sizes and have a shorter spawning season, a likely result of the cool winter seawater temperatures in the region. It is possible that the combination of these life history characteristics could mitigate or delay the ecological impact that invasive lionfish may have on this marine ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Presumably introduced via the aquarium trade, two species of Indo-Pacific lionfishes, Pterois volitans (Linnaeus, 1758) and P. miles (Bennet, 1828), hereafter referred to as “lionfish”, have been recognized as the first successful invasive teleost fishes in the Atlantic Ocean (Whitfield et al. 2002). First officially sighted off Florida in 1985, lionfish dispersed northward along the U.S. coast, reached Bermuda by 2000, then expanded southwards through the Caribbean Sea and west into the Gulf of Mexico, reaching Brazil by 2014 (Morris 2009; Schofield 2010; Ferreira et al. 2015). The ecological impact and invasive character of these introduced species have since been demonstrated by numerous researchers in varied locations (as reviewed by Arndt et al. 2018).
Following the original introduction, lionfish quickly became one of the most common mesopredators (i.e., medium-sized, mid-trophic level predator) in the northwestern Atlantic, even outnumbering native predators in some locations (Whitfield et al. 2007; Green et al. 2012a). Predation by lionfish can markedly reduce the abundance and species richness of juvenile reef fish (Albins and Hixon 2008, 2013; Green et al. 2012a), and has been implicated in the widespread decline of herbivorous reef fish at mesophotic depths around the Bahamas that induced a phase shift from a healthy coral ecosystem to one dominated by algae (Lesser and Slattery 2011). Considering their broad diet (Morris and Akins 2009; Eddy et al. 2016; Peake et al. 2018) and apparent lack of natural predators (Albins and Hixon 2013), there is concern that invasive lionfish could cause significant ecological disruption through both predation and resource competition.
Although Bermuda was the first location outside of United States waters to report non-indigenous lionfish, the local population appears to be expanding at a slower pace when compared to other locations in the region (e.g., Bahamas and Little Cayman, Eddy 2016). It therefore appears that Bermuda may still be in the early stages of the lionfish invasion, as characterized by slow population growth (Sakai et al. 2001), with a period of exponential growth perhaps yet to come. Indeed, mounting evidence (i.e., the number of lionfish culled annually or caught as bycatch in the commercial lobster trap fishery; Bermuda Department of Environment and Natural Resources, unpubl. data) suggests that the population continues to grow, and that the impacts observed elsewhere have merely been delayed in Bermuda (Eddy 2016). This slower trajectory could be attributed to 1) the effects that seasonal temperature changes (a range of ~14 °C in Bermuda) may have on reproduction (i.e., a relatively short spawning season constrained by cold winter seawater temperatures; Morris 2009), 2) limited recruitment from other regions as a result of Bermuda’s geographic isolation (Schultz and Cowen 1994), and 3) the possible advection of spawned eggs and larvae away from the islands and into the open ocean by off-shore currents (R. Johnson, Bermuda Institute of Ocean Sciences, pers. comm.).
Effective management of marine species, and any attempt to model the impact of an invader, requires an understanding of that species’ location-specific life history characteristics, which is currently lacking for Bermuda. Therefore, the objectives of this study were to investigate the growth rate, life span, age and size at maturity, spawning seasonality, and fecundity of the invasive lionfish in Bermuda. This information will assist Bermuda’s Department of Environment and Natural Resources in the development of strategic plans to control the invasive lionfish population in hopes of limiting its impact.
Methods
Fish collection
Lionfish were collected from multiple locations around the Bermuda platform from 2012 to 2016 (Fig. 1). Specimens were collected by fishermen, snorkelers, SCUBA divers, researchers, and environmental management professionals, using a variety of methods including pole spear, collecting nets, experimental lionfish traps, and as bycatch in commercial lobster traps. Prior to dissection, total length (TL) and body mass were recorded to the nearest 1 mm and 1 g, respectively. Lionfish were placed on ice and dissected the same day as captured. If dissections had to be delayed, lionfish were frozen for the duration after being weighed and measured.
Age estimation and growth modelling
Age estimates were made by examining transverse sections of sagittal otoliths, prepared following Epperly et al. (1991) and Secor et al. (1992). Otoliths were embedded in resin, cured for 48 h, and sectioned through the core by a dual-blade low-speed diamond saw, providing ~0.3 mm sections. The sections were mounted on glass slides, polished with lapping film (3–10 μm), and viewed under both compound and dissecting microscopes (40-100x magnification). Both reflected and transmitted light were used to provide alternate views of otoliths to clarify growth rings. Each combination of opaque and translucent zones was considered an annulus (i.e., annual growth ring) and these were counted to determine the age of each lionfish. A second reader counted the annuli from 125 otoliths in order to estimate precision and bias of readers. Following Beamish and Fournier (1981), Average Percent Error (APE), a method used to measure the precision of age determinations between readers, was calculated for each lionfish as
where Xij is the ith age reading of the jth lionfish, Xj is the mean age of the jth fish, and R is the number of times each fish was aged. APEj was averaged across all fish whose otoliths were aged twice to provide the Index of APE. Bowker’s test (X2; Bowker 1948; Hoenig et al. 1995) was used to test whether bias existed between readers and was calculated as
where nij is the number of specimens aged i by the first reader and j by the second, nji is the number of lionfish aged j by the first reader and i by the second, and m is the maximum age.
Length-at-age data were used to model growth by deriving von Bertalanffy growth parameters (von Bertalanffy 1957):
where Lt is the length at age (t), L∞ is asymptotic maximum length, K is the growth coefficient, and t0 is the theoretical age at which lionfish would be 0 mm TL. Growth parameters were derived for the Bermuda lionfish population as a whole, and for male and female lionfish separately, using the clus.vb.fit script available in the vegan package for R. These parameters were then used to plot sex-specific growth curves for male and female lionfish. For comparison, sex-specific size-at-age curves for lionfish in other regions of the Atlantic were generated using growth parameters available from the literature (Edwards et al. 2014; Fogg et al. 2015). These estimates were used to make statistical comparisons of lionfish growth curves using likelihood ratio tests (Kimura 1980) performed with the vblrt function in the R package “fishmethods”. The von Bertalanffy growth equation was then manipulated to provide an equation from which the age (t) was calculated of all lionfish sampled:
Validation of annulus growth
The periodicity of annulus formation was examined by giving one individual lionfishFootnote 1 an intraperitoneal injection of oxytetracycline (OTC) (75 mg per kg body mass). The lionfish was held in captivity for 14 months in an outside aquarium to ensure its exposure to a natural light cycle. The aquarium received a constant flow of seawater, collected at approximately 3 m depth, to ensure the captive fish was exposed to a natural seasonal temperature regime. Every 24–48 h, the captive lionfish was fed quantities of prey (i.e., Bermuda anchovy, Anchoa choerostoma) calculated using the consumption equation from the bioenergetics model of Cerino et al. (2013) that allows for modification based upon sea surface temperature and body mass. Uneaten food was removed after 20 min, assuming that satiation had been reached. It was assumed that the formation of one annulus distal to the fluorescent OTC mark would indicate an annual period of formation. Otoliths from the lionfish marked with OTC were processed as noted above and examined under fluorescent light in order to visually identify the OTC mark, while counting annuli.
Reproduction
Ovaries were excised, blotted dry, weighed to the nearest 0.001 g, and the reproductive stage of each was assessed macroscopically, following guidelines provided in Green et al. (2012b). Ovaries were immediately fixed in 10% buffered formalin for a minimum of 48 h and transferred to 70% ethanol for long term storage (i.e., 4–6 month). For processing, ovaries were removed from the 70% ethanol, blotted dry, and prepared following standard histological methods and stained with hematoxylin and eosin. Stained sections (~0.3 mm thick) were examined under a compound microscope (40-100x magnification) using transmitted light to determine the overall reproductive stage according to the terminology provided in Brown-Peterson et al. (2011; Table 1) and characteristics described by Morris et al. (2011; Table 2). As lionfish are presumed asynchronous batch spawners (Morris 2009), when multiple stages of maturity were observed, the most advanced was used to represent reproductive stage.
To assess spawning periodicity, reproductive stages (both macroscopically and histologically determined) and the mean monthly values of the Gonadosomatic Index (GSI) and Hepatosomatic Index (HSI) were plotted against time (i.e., month). The GSI was calculated as:
where Mg is gonad mass (g) and M is fish mass (g). Similarly, the HSI was calculated as:
where Ml is liver mass (g). The statistical significance of monthly variation in both the GSI and the HSI was examined using One-Way analysis of variance (ANOVA) using arcsine transformed data. We consider spawning to likely occur in months when GSI was highest, HSI lowest, and when a large proportion of females were macroscopically determined to be ripe, and histologically recognized as undergoing oocyte maturation. The seasonal relationship between reproductive state and ambient seawater temperature was examined using monthly sea temperature data from a site at 15 m depth on the south shore of Bermuda, representative of where many of the samples were collected (MEP 2012). Spawning condition of lionfish from mesophotic (>30 m; n = 49) and shallow (<30 m; n = 38) reefs was compared for the month of July (the only month where a sufficient number of specimens were captured from both depth ranges), in order to investigate whether dense lionfish aggregations observed at 60 m (Eddy 2016) might be associated with spawning.
Size-at-maturity was determined in two ways, following methods outlined by Gardner et al. (2015). First, a linear relationship between batch fecundity and TL was used to estimate the length at which egg production was theoretically zero (i.e., the size at maturity; McBride et al. 2002). Second, histologic and macroscopic stages were used to determine size at maturity following the logistic fit method (McBride et al. 2002). Mature fish were assigned a value of 1, while immature fish were assigned a 0. Using these binomial data, a curve showing the percent maturity of increasing size-classes was then fit to a multi-parameter logistic function using the sizeMat package in R:
where P(mat) is the probability of maturity, TL is the total length, L50mat is the length at which 50% of the females were mature, and σ was a parameter characterizing the slope of the fitted curve.
Fecundity was determined following methods outlined by Gardner et al. (2015), using a subsample of ovaries (n = 19) that had been macroscopically identified as spawning capable and preserved as described above. To release the eggs for analysis, whole ovaries were agitated with a stir bar and the connective tissue and oocytes were separated with forceps. After the ethanol volume was measured, three 1 ml samples were removed by pipette and examined under 4-6x magnification using a dissecting microscope. Using image analysis software (Image Pro Digital), digital images were taken of each sample and the diameter of all oocytes was measured. All oocytes larger than 0.5 mm in diameter were considered to be mature and belonging to the next batch (Morris et al. 2011), so were therefore included in the count. Counts from these subsamples were scaled to estimate batch fecundity using:
where Nt is batch fecundity, Ns is the oocyte count in the sub-sample, Vt is the volume of material from one ovary, and Vs is the volume of a subsample. Batch fecundity for each lionfish was plotted against TL to investigate changes in fecundity with increased size.
Results
Age and growth
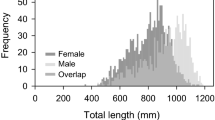
A total of 1507 lionfish were collected from waters around the Bermuda platform. However, the sex of 149 lionfish could not be identified as they had been eviscerated prior to delivery to the researchers. Females (n = 541) ranged in size from 157 mm to 412 mm TL (mean ± SE: 302 ± 2 mm; Fig. 2a) and males (n = 817) ranged in size from 144 mm to 467 mm TL (mean ± SE: 367 ± 2 mm; Fig. 2a). As male TL was not normally distributed (χ2 (5, n = 809) = 115.7, p < 0.05), mean male and female TL were compared using the Mann-Whitney test. The mean TL for males was significantly larger than females (Mann-Whitney test, U = 63,884, p < 0.05). Female weight ranged from 60 to 900 g (mean ± SE: 370 ± 7 g) and male weight ranged from 10 to 1370 g (mean ± SE: 660 ± 10 g; Fig. 2b). As female weight was not normally distributed (χ2 (7, n = 425) = 15.8, p < 0.05), mean male and female weight female weight were compared using the Mann-Whitney test. The mean weight for males was significantly larger than females (Mann-Whitney test, U = 41,120, p < 0.05).
In total, 587 otoliths were processed and read to determine length-at-age (females, n = 228; males, n = 323, unidentified, n = 34). Overall, the APE (4.56%) indicated a high level of precision between otolith readers and the Bowker’s Index (X2 = 10.2; p = 0.33) suggested no bias between readers, thus counts were considered consistent. Separate growth parameters were derived for males, females, and the entire population (males and females combined; Table 1, Fig. 3).
a von Bertalanffy growth curves (VBGCs) for male and female lionfish derived from this study’s estimates of length-at-age and b VBGCs for Bermuda lionfish compared to those from Little Cayman (Edwards et al. 2014) and the northern Gulf of Mexico (GOM; Fogg et al. 2015). Refer to Table 1 for VBGC parameters
Males and females ranged from 0 to 9 years in age (Fig. 4). The mean (± SE) age for males and females was 3.1 (±0.1) and 2.5 (±0.1) years, respectively.
Validation of annuli growth frequency
Examination of the otoliths collected from a single lionfish injected with OTC, and then held in captivity for 14 months prior to sampling, supports the hypothesis that opaque zones are laid down annually. Under fluorescent light, an OTC mark was clearly visible within an opaque zone and there was one additional opaque zone distal to that mark (Supp. Fig. 1).
Reproductive staging and seasonality
Gonadosomatic and hepatosomatic indices were calculated for 362 and 205 female lionfish, respectively (Fig. 5). Both GSI and HSI values varied significantly by month (ANOVA: F(11,323) = 14.1, p < 0.001 and F(10, 213) = 5.8, p < 0.001, respectively). Median GSI values for immature, developing, spawning capable, and actively spawning lionfish were 0.33, 1.9, 3.0, and 6.2, respectively. GSI values remained low (mean = 0.9) through the winter (January–March), while HSI values remained high (mean = 1.4). Conversely, GSI values were greater than 2.5 between July and October, with a peak of 4.1 in September, and HSI values were lowest in October (mean = 0.8). GSI values dipped in August, being 12% lower than in July, and a contrasting short-term peak was noted in HSI values in September (Fig. 5).
a Mean monthly Gonadosomatic Index (GSI) and Hepatosomatic Index (HSI) for female lionfish caught in Bermuda, with mean temperature (°C) calculated from data recorded every 30 min at a site 15 m deep off John Smith’s Bay between 2004 and 2011 (TEMP; MEP 2012). b GSI values versus total length, color-coded by reproductive stage. Grey line indicates the median GSI of lionfish in the developing stage (1.9)
Reproductive development of females was staged macroscopically for 405 individuals and histologically for 253 individuals, including lionfish captured during every month of the year (Fig. 6). Macroscopic staging accurately determined whether a female lionfish was mature ~97% of the time. Agreement between macroscopic and histological staging was 87% for immature lionfish (n = 15), 84% for developing lionfish (n = 44), and 84% for spawning capable lionfish (n = 32). Immature fish, as determined by histological assessment, accounted for 1.24% of all lionfish captured throughout this study.
Histologic staging of ovaries taken from lionfish captured in Bermuda, showing mean temperature (black line) calculated from data recorded every 30 min at a site 15 m deep off John Smith’s Bay between 2004 and 2011 (MEP 2012)
Histological staging showed that the active spawning subphase was only encountered between June and November, but spawning capable fish were found from June through January (Fig. 6). Approximately 60% of the ovaries in lionfish caught in December, 90% in January, 100% in February, and 50% in March were in the regenerating phase.
Approximately half of all lionfish sampled from both mesophotic (deeper than 30 m) and shallow (shallower than 30 m) reefs in July were categorized as actively spawning (49.0% and 47.4% respectively), with 93.8% of lionfish on mesophotic reefs and 79.0% of lionfish on shallow reefs classified as spawning capable (which includes those in the active spawning subphase). Only 4.1% of lionfish on mesophotic reefs and 10.5% of lionfish on shallow reefs were developing, and 2.0% of lionfish on mesophotic reefs were immature, while 10.5% of lionfish on shallow reefs were immature.
Female length at maturity
The smallest mature female lionfish, using histological stage as an indicator, was 189 mm TL and the largest immature lionfish was 226 mm TL. The logistic fit method suggests 50% of female lionfish in Bermuda are sexually mature at 203 mm TL with 95% confidence intervals ranging from 194 to 213 mm TL. Using the linear relationship between batch fecundity and TL (Fig. 7), the theoretical size for zero egg production was 193 mm, providing an estimate of length at first maturity. The largest age-0 lionfish (i.e, 1st year of life) measured 245 mm TL, while the smallest age-1 (i.e., 2nd year) lionfish measured 215 mm TL. All age-1 and older lionfish sampled were longer than the lengths estimated for first-maturity and 50% maturity. All lionfish less than 225 mm TL had GSI values less than the median reported for the developing stage (1.98; Fig. 5b).
Batch fecundity as a function of total length (mm). Black circles and solid black regression line indicate data from this study. Dashed gray line indicates regression from Gardner et al. (2015: BF = 308.67TL - 58265). BF = batch fecundity (#eggs). TL = total length (mm)
Batch fecundity
Batch fecundity for female lionfish was measured for 19 individuals (223–309 mm TL), all captured during the summer (July–September), and ranged from 7000 to 48,240 eggs per fish (Fig. 7). Batch fecundity increased significantly with TL (ANOVA: F(1,15) = 4.54, p < 0.05; Fig. 7).
Discussion
Lionfish age and growth in Bermuda
Our study is the first to provide life history data for lionfish in Bermuda and offers the first direct, albeit preliminary, evidence that lionfish form a single pair of translucent and opaque growth zones (i.e., one growth ring) yearly. Our confidence in this conclusion is supported by the previous use of marginal increment analysis to demonstrate annuli formation in lionfish (Edwards et al. 2014) and the general idea that annual periodicity in otolith banding in most fish species can be safely assumed without validation (Choat et al. 2009).
The broad range of ages (0–9 years, Fig. 4) and sizes (157–476 mm TL, Fig. 2) included in this study provides a strong basis for describing the extended asymptotic growth phase of lionfish. Compared to lionfish from other locations in both the invaded and native ranges, lionfish in Bermuda appear to attain larger sizes (i.e., mean TL and mean body mass; Table 2), and previous work on other teleosts (e.g., red hind, Epinephelus guttatus) in Bermuda has also suggested that fish in this area may reach larger average sizes when compared to Caribbean locations (Luckhurst et al. 1992; Robertson et al. 2005). It is possible that this Bermuda size-effect may be a product of the colder winters and markedly lower mean seawater temperatures (Smith et al. 2013). In fish, exposure to a relatively colder environment for long periods of time may result in a decreased energetic investment for both gonadal growth and reproduction, leading to an increase in the rate of somatic growth and longevity (Smith-Vaniz et al. 1999; Robertson et al. 2005; Smith et al. 2013). Indeed, the parameters derived from the von Bertalanffy population growth model (i.e., males and females combined; Table 1), show that maximum length is reached faster in Bermuda when compared to other locations in the Atlantic (e.g., Little Cayman; Edwards et al. 2014). Although Pusack et al. (2016) showed a larger growth coefficient for lionfish from both the Atlantic and Pacific Oceans, this is most likely a result of their focus on juvenile lionfish, which may have led to over-estimation of this parameter.
The foregoing is not meant to suggest that individual lionfish in Bermuda reach a larger maximum size compared to those found elsewhere in the region, but that on average, the Bermuda population may have a greater proportion of large individuals. This may be explained by the fact that lionfish arrived in Bermuda much earlier than other locations. It is important to consider whether the collection methods in our study (e.g., depth of capture, type of gear used, an individual’s ability to capture lionfish) or sampling design (i.e., randomized or opportunistic) may have under-sampled small lionfish, resulting in a size-bias towards larger individuals. However, our collection methods are the same as other lionfish studies and it seems unlikely that they would bias our sample in a way that has not occurred in the studies being used for comparison.
Our findings show strong sexual dimorphism in the lionfish population in Bermuda, with females achieving a smaller asymptotic size when compared to males, which has also been documented for lionfish in other areas (Fig. 3b). In addition, males have a higher estimated instantaneous growth rate and thus reach their maximum size faster than females (Fig. 3b, Table 1). The relatively slower growth rate of females may arise from the large investment of energy that may be diverted from somatic growth to reproduction (i.e., gonadal growth and maturation) as they reach the size of sexual maturity.
For fish, slower rates of somatic growth are typically associated with species that occupy higher latitudes and are thus exposed to relatively lower temperatures (Atkinson 1994; Berrigan and Charnov 1994; Ruttenberg et al. 2005; Trip et al. 2014). Surprisingly, when lionfish (i.e., male and female) in Bermuda are compared to other areas in the Caribbean (i.e., Little Cayman) they appear to have higher instantaneous growth rates during the first year of life (Fig. 3b). The unexpected fast rates of somatic growth for lionfish in Bermuda may arise from their feeding habits (Peake et al. 2018) and the availability of diverse prey (Eddy 2016), allowing them to avoid seasonal energy deficits, thereby reducing any temperature-dependent physiological impediment to growth (Present and Conover 1992; Billerbeck et al. 2000; Robertson et al. 2005).
Lionfish reproduction in Bermuda
Previous work in Little Cayman, North Carolina, and the Bahamas has shown that lionfish have a year-round reproductive season (Gardner et al. 2015; Morris 2009). By contrast, our findings show an apparent absence of winter reproductive activity (Figs. 5a and 6). The GSI, HSI, and histological staging data suggest a short active spawning period (June – November), while those lionfish captured during the coldest months (December–May) predominantly had developing or regenerating ovaries (i.e., reproductively mature, but not active; Fig. 6). A similar seasonal spawning pattern has also been documented for lionfish in the northern Gulf of Mexico, with peaks of activity in May and October. (Fogg et al. 2017). In species with a broad geographic range, individuals inhabiting higher latitudes often have shorter spawning seasons when compared to their conspecifics at lower latitudes (Robertson 1991; Kokita 2004; Srinivasan and Jones 2006), and studies suggest this pattern is directly correlated to decreasing ambient temperatures (Danilowicz 1995; Ruttenberg et al. 2005). As sea surface temperature drops substantially around Bermuda through the winter, reaching a minimum of 16.8 °C in 2013 (Bermuda Weather Service) for example, it appears likely that these cold winter seawater temperatures may contribute to the abbreviated spawning season observed in lionfish. Previous studies have already shown that the reproductive seasons of many teleosts in Bermuda are shorter than elsewhere in the wider Caribbean and primarily occur through the warmer months (Smith et al. 2013). Although lionfish are known to spawn year-round in North Carolina, which is located at a higher latitude than Bermuda, the water at depth in North Carolina is warmed significantly by the Gulf Stream (Atkinson et al. 1983) and work by Whitfield et al. (2014) showed that the median temperature did not fall below ~18 °C when lionfish occupied these waters during the winter months.
Although our findings suggest that lionfish off Bermuda appear to have an abbreviated reproductive season, batch fecundity estimates for lionfish in Bermuda are similar to those reported from Little Cayman (Gardner et al. 2015) and North Carolina (Morris 2009) and overlap with those from the northern Gulf of Mexico (Fogg et al. 2017; Table 3). With similar seawater temperatures during the reproductive seasons in these three regions and because lionfish productivity is not likely limited by food availability, it seems reasonable to assume that fecundity would be similar across the three locations. However, the maximum batch fecundity observed in the northern Gulf of Mexico was more than twice that recorded in Bermuda (Table 3), while estimates of batch fecundity for lionfish in North Carolina waters (Morris 2009) are somewhat lower than suggested by our regression (Fig. 7). It is possible that the variability in the batch fecundity may reflect the different methodologies used: estimated egg counts from spawned egg masses (Morris 2009) vs. unspawned egg masses physically removed from ovaries that were identified as spawning capable (this study). All hydrated eggs in a spawned egg mass are mature by definition (Morris 2009) and can therefore be assumed to accurately represent batch fecundity, but there is no way to guarantee that all hydrated oocytes in an unspawned egg mass that was physically separated from an ovary were going to be ovulated and spawned, thus potentially leading to an overestimate in batch fecundity.
In general, fish that are found at higher latitudes and colder temperatures often show a slower growth rate, a delay in the onset of sexual maturity, are larger when they reach sexual maturity, and have an increased maximum length, a situation referred to as the Temperature-Size Rule (Atkinson 1994; Trip et al. 2014). Although our finding that lionfish in Bermuda mature at slightly larger sizes when compared to conspecifics in the other regions (e.g., Bahamas, North Carolina, and Little Cayman) seems to follow this rule (Table 3), the difference between our estimates of length-at-maturity and those for Little Cayman are small (~10 mm, Gardner et al. 2015). However, considering the growth rate of lionfish, this may not have a particularly large influence on the age at which lionfish begin to reproduce or the overall reproductive output of Bermuda’s lionfish population.
While larger lionfish are quite common in Bermuda, immature individuals (<~200 mm TL) were rarely encountered and the smallest lionfish captured was 124 mm TL, although that individual was not sexed as it had been eviscerated during capture. Only 1.1% of all lionfish captured in Bermuda were less than 180 mm TL, the length of 50% female maturity determined by Morris (2009), and only 2.1% were less than 203 mm TL, the length of 50% female maturity noted by this study. While small, immature lionfish are likely present in Bermuda, the abundance of these smaller individuals may be under-represented due to 1) the difficulty in finding small lionfish in structurally complex habitats during the underwater surveys (Green et al. 2013), 2) a lack of surveys from potential juvenile habitats, such as mangroves and seagrass beds, and 3) the bias in conducting surveys primarily during midday (daylight) hours. Alternatively, the relative absence of immature lionfish could suggest some proportion of Bermuda’s population arrives as mature adults from other parts of the northwest Atlantic, as is common for some species found in Bermuda (Glasspool 1994). This may also explain how a lionfish was observed swimming at the sea’s surface 150 miles south of Bermuda in 2004 (R. Johnson, Bermuda Institute of Ocean Sciences, pers. comm.).
While large aggregations of lionfish have been reported at two mesophotic reefs on the Bermuda platform in midsummer (>1000 lionfish/ha; Eddy 2016), the reason for such aggregations remains unknown. Recognizing that previous work has shown that lionfish spawn in pairs (Fishelson 1975; Morris 2009; Supp. Fig. 2), this study provided an interesting opportunity to investigate whether reproduction might play a role in the formation of these aggregations. Although future research will be necessary to answer this question definitively, this study found equal proportions of lionfish were in active spawning condition on mesophotic (49.0%) and shallow reefs (47.4%) in July, when the densest aggregations had previously been noted and when lionfish reproduction appears to peak. With that in mind, it does not immediately appear that reproduction is a factor in lionfish aggregation.
Conclusion
In summary, it appears that latitudinal variation in life history characteristics of lionfish, in particular the abbreviated spawning season, likely caused by seasonal variation in sea temperature, may explain the apparently slower population growth rates and resulting smaller population size of Bermuda’s lionfish. Although lionfish may initially grow faster in Bermuda compared to some other regions, females reach maturity at larger sizes, potentially limiting their overall reproductive output. While batch fecundity estimates are similar to those determined in Little Cayman and elsewhere, Bermuda’s lionfish have a spawning season that is approximately half the length of those seen in the Caribbean and along the southeastern United States coast. The abbreviated spawning season would certainly limit the overall productivity of lionfish and, in addition to explaining the slower increase in population size, also suggests that potential ecological impacts could be delayed. Depending upon the rate at which global climate change warms the ocean around Bermuda, this could provide Bermuda’s Department of Environment and Natural Resources with a window of opportunity to develop an effective management strategy to reduce lionfish numbers and potentially avoid an impact overall.
Notes
It was only possible to inject one lionfish with OTC because, although others were available and held in captivity, they were part of a controlled feeding experiment.
References
Albins MA, Hixon MA (2008) Invasive indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reef fishes. Mar Ecol Prog Ser 367:233–238
Albins MA, Hixon MA (2013) Worst case scenario: potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ Biol Fish 96:1151–1157
Arndt E, Marchetti MP, Schembri PJ (2018) Ecological impact of alien marine fishes: insights from freshwater systems based on a comparative review. Hydrobiologia 817(1):457–474
Atkinson D (1994) Temperature and organism size: a biological law for ectotherms? Adv Ecol Res 25:1–1
Atkinson LP, Lee TN, Blanton JO, Chandler WS (1983) Climatology of the southeastern United States continental shelf waters. J Geophys Res 88:4705–4718
Beamish RJ, Fournier DA (1981) A method for comparing the precision of a set of age determinations. Can J Fish Aquat Sci 38:982–983
Berrigan D, Charnov EL (1994) Reaction norms for age and size at maturity in response to temperature: a puzzle for life historians. Oikos 70:474–478
Billerbeck JM, Schultz ET, Conover DO (2000) Adaptive variation in energy acquisition and allocation among latitudinal populations of the Atlantic silverside. Oecologia 122:210–219
Bowker AH (1948) A test for symmetry in contingency tables. J Am Stat Assoc 43:572–574
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK (2011) A standardized terminology for describing reproductive development in fishes. Mar Coast Fish 3:52–70
Cerino D, Overton AS, Rice JA, Morris JA (2013) Bioenergetics and trophic impacts of the invasive indo-Pacific lionfish. Trans Am Fish Soc 142:1522–1534
Choat JH, Kritzer JP, Ackerman JL (2009) Ageing in coral reef fishes: do we need to validate the periodicity of increment formation for every species of fish for which we collect age-based demographic data? In: Green BS, Mapstone BD, Carlos G, Begg GA (eds) Tropical fish otoliths: information for assessment, management and ecology. Reviews: methods and technologies in fish biology and fisheries, vol 11. Springer, Dordrecht, pp 23–54
Dahl KA, Patterson WF III (2014) Habitat-specific density and diet of rapidly expanding invasive red lionfish, Pterois volitans, populations in the northern Gulf of Mexico. PLoS One 9(8):e105852
Danilowicz BS (1995) The role of temperature in spawning of the damselfish Dascyllus albisella. Bull Mar Sci 57:624–636
Darling ES, Green SJ, O’Leary JK, Côté IM (2011) Indo-Pacific lionfish are larger and more abundant on invaded reefs: a comparison of Kenyan and Bahamian lionfish populations. Biol Invasions 13:2045–2051
Eddy CE (2016) An investigation of the biology and ecology of the invasive lionfish (Pterois volitans and P. miles) to explore their ecological impact and inform management in Bermuda’s marine ecosystem. PhD Dissertation, University of Massachusetts Dartmouth, Dartmouth, Massachusetts
Eddy C, Pitt J, Morris JA, Smith S, Goodbody-Gringley G, Bernal D (2016) Diet of invasive lionfish (Pterois volitans and P. miles) in Bermuda. Mar Ecol Prog Ser 558:193–206
Edwards MA, Frazer TK, Jacoby CA (2014) Age and growth of invasive lionfish (Pterois spp.) in the Caribbean Sea, with implications for management. Bull Mar Sci 90:953–966
Epperly SP, Ahrenholz DW, Tester P (1991) A universal method for preparing, sectioning, and polishing fish otoliths for daily ageing. NOAA Technical Memorandum NMFS-SEFSC. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Southeast Fisheries Science Center, Beaufort Laboratory, Beaufort
Ferreira CE, Luiz OJ, Floeter SR, Lucena MB, Barbosa MC, Rocha CR, Rocha LA (2015) First record of invasive lionfish (Pterois volitans) for the Brazilian coast. PLoS One 10:e0123002
Fishelson L (1975) Ethology and reproduction of pteroid fishes found in the Gulf of Aqaba (Red Sea) especially Dendrochirus brachypterus (Cuvier) (Pteroidae, Teleostei). Publ Staz Zool Napoli 39:635–656
Fogg AQ, Brown-Peterson NJ, Peterson MS (2015) Northern Gulf of Mexico lionfish: insights into their reproductive life history. 67th meeting of the Gulf and Caribbean Fisheries Institute, Panama City, Panama
Fogg AQ, Brown-Peterson NJ, Peterson MS (2017) Reproductive life history characteristics of invasive red lionfish (Pterois volitans) in the northern Gulf of Mexico. Bull Mar Sci 93:791–813
Gardner PG, Frazer TK, Jacoby CA, Yanong RP (2015) Reproductive biology of invasive lionfish (Pterois spp.). Front Mar Sci 2:1–10
Glasspool AF (1994) Larval distribution, population structure and gene flow in Bermuda’s reef fish. Doctoral dissertation, University of Wales. Cardiff
Green SJ, Akins JLM, Maljkovic A, Côté IM (2012a) Invasive lionfish drive Atlantic coral reef fish declines. PLoS One 7:1–3
Green SJ, Akins JL, Morris Jr JA (2012b) Lionfish dissection: Techniques and applications. NOAA Technical Memorandum NOS NCCOS: 139. Silver Spring, MD, National Oceanic and Atmospheric Administration/National Ocean Service, 24pp
Green SJ, Tamburello N, Miller SE, Akins JL, Cote IM (2013) Habitat complexity and fish size affect the detection of indo-Pacific lionfish on invaded coral reefs. Coral Reefs 32:413–421
Hoenig JM, Morgan MJ, Brown CA (1995) Analysing differences between two age determination methods by tests of symmetry. Can J Fish Aquat Sci 52:364–368
Kimura DK (1980) Likelihood methods for the von Bertalanffy growth curve. Fish Bull 77:765–776
Kokita T (2004) Latitudinal compensation in female reproductive rate of a geographically widespread reef fish. Environ Biol Fish 71:213–224
Lesser MP, Slattery M (2011) Phase shift to algal dominated communities at mesophotic depths associated with lionfish (Pterois volitans) invasion on a Bahamian coral reef. Biol Invasions 13:1855–1868
Luckhurst BE, Barnes JA, Sadovy Y (1992) Record of an unusually large red hind, Epinephelus guttatus (Pisces: Serranidae) from Bermuda with comments on its age. Bull Mar Sci 51:267–270
McBride R, Stengard F, Mahmoudi B (2002) Maturation and diel reproductive periodicity of round scad (Carangidae: Decapterus punctatus). Mar Biol 140:713–722
MEP (2012) Marine Environmental Program 2004–2011 synthesis report. Unpublished technical report submitted to the Bermuda government Department of Environmental Protection. Bermuda Institute of Ocean Sciences, Bermuda
Morris JA (2009) The biology and ecology of the invasive indo-Pacific lionfish. PhD Dissertation, North Carolina State University, Raleigh, NC
Morris JA, Akins JL (2009) Feeding ecology of invasive lionfish (Pterois volitans) in the Bahamian archipelago. Environ Biol Fish 86:389–398
Morris JA, Sullivan CV, Govoni JJ (2011) Oogenesis and spawn formation in the invasive lionfish, Pterois miles and Pterois volitans. Sci Mar 75:147–154
Peake J, Bogdanoff AK, Layman CA, Castillo B, Reale-Munroe K, Chapman J, Dahl K, Patterson WF, Eddy C, Ellis RD, Faletti M, Higgs N, Johnston MA, Muñoz RC, Sandel V, Villasenor-Derbez JC, Morris JA (2018) Feeding ecology of invasive lionfish (Pterois volitans and Pterois miles) in the temperate and tropical western Atlantic. Biol Invasions 20:2567–2597
Present TMC, Conover DO (1992) Physiological basis of latitudinal growth differences in Menidia menidia: variation in consumption or efficiency? Funct Ecol 6:23–31
Pusack TJ, Benkwitt CE, Cure K, Kindinger TL (2016) Invasive red lionfish (Pterois volitans) grow faster in the Atlantic Ocean than in their native Pacific range. Environ Biol Fish 99:571–579
Robertson DR (1991) The role of adult biology in timing of spawning of tropical reef fishes. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic Press, USA, pp 356–386
Robertson DR, Ackerman JL, Choat JH, Posada JM, Pitt J (2005) Ocean surgeonfish Acanthurus bahianus. I. The geography of demography. Mar Ecol Prog Ser 295:229–244
Ruttenberg BI, Haupt AJ, Chiriboga AI, Warner RR (2005) Patterns, causes and consequences of regional variation in the ecology and life history of a reef fish. Oecologia 145:394–403
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O'Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Sandel V, Martínez-Fernández D, Wangpraseurt D, Sierra L (2015) Ecology and management of the invasive lionfish Pterois volitans/miles complex (Perciformes: Scorpaenidae) in southern Costa Rica. Rev Biol Trop 63:213–221
Schofield PJ (2010) Update on geographic spread of invasive lionfishes (Pterois volitans [Linnaeus, 1758] and P. miles [Bennett, 1828]) in the Western North Atlantic Ocean, Caribbean Sea and Gulf of Mexico. Aquat Invasions 5:S117–S122
Schultz ET, Cowen RK (1994) Recruitment of coral reef fishes to Bermuda: local retention or long-distance transport? Mar Ecol Prog Ser 109:15–28
Secor DH, Dean JM, Curtis TA, Sessions FW (1992) Effect of female size and propagation methods on larval production at a South Carolina striped bass (Morone saxatilis) hatchery. Can J Fish Aquat Sci 49:1778–1787
Smith SR, de Putron S, Murdoch TJ, Pitt JM, Nagelkerken I (2013) Biology and ecology of corals and fishes on the Bermuda platform. In: CRD S (ed) Coral reefs of the United Kingdom overseas territories. Springer, Dordrecht, pp 135–151
Smith-Vaniz WF, Collette BB, Luckhurst BE (1999) Fishes of Bermuda: history, zoogeography, annotated checklist, and identification keys. American Society of Ichthyologists and Herpetologists, Special Publication Number 4, Lawrence
Srinivasan M, Jones GP (2006) Extended breeding and recruitment periods of fishes on a low latitude coral reef. Coral Reefs 25:673–682
Trip L, Elizabeth D, Clements KD, Raubenheimer D, Choat JH (2014) Temperature-related variation in growth rate, size, maturation and life span in a marine herbivorous fish over a latitudinal gradient. J Anim Ecol 83:866–875
von Bertalanffy L (1957) Quantitative laws in metabolism and growth. Q Rev Biol 32:217–231
Whitfield PE, Gardner T, Vives SP, Gilligan MR, Courtenay WR Jr, Ray GC, Hare JA (2002) Biological invasion of the indo-Pacific lionfish Pterois volitans along the Atlantic coast of North America. Mar Ecol Prog Ser 235:289–297
Whitfield PE, Hare JA, David AW, Harter SL, Munoz RC, Addison CM (2007) Abundance estimates of the indo-Pacific lionfish Pterois volitans/miles complex in the Western North Atlantic. Biol Invasions 9:53–64
Whitfield PE, Muñoz RC, Buckel CA, Degan BP, Freshwater DW, Hare JA (2014) Native fish community structure and Indo-Pacific lionfish Pterois volitans densities along a depth-temperature gradient in Onslow Bay, North Carolina, USA. Mar Ecol Prog Ser 509:241–254
Acknowledgements
Funding was provided by the National Science Foundation (DGE-1144241); the United Kingdom Department for Environment, Food, and Rural Affairs’ Darwin Plus Programme (DPLUS001); and the University of Massachusetts Dartmouth. The Bermuda Zoological Society helped administer and manage the DEFRA grant. Logistic support was provided by the National Oceanic and Atmospheric Administration, Bermuda’s Department of Environment and Natural Resources (DENR), and the Ocean Support Foundation. We thank the DENR for allowing us to collect lionfish in Bermuda waters and the NOAA reviewers for their valuable comments as we revised the manuscript. The authors thank Mandy Shailer from the DENR for her assistance with GIS mapping, Alex Fogg for his help with coding in R, and Jirani Welch for spotting the spawning pair of lionfish while reviewing hundreds of mesophotic photos. The authors are especially grateful to all lionfish hunters in the Bermuda Lionfish Culling Program, and those commercial fishermen who provided lionfish for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 6687 kb)
Rights and permissions
About this article
Cite this article
Eddy, C., Pitt, J., Oliveira, K. et al. The life history characteristics of invasive lionfish (Pterois volitans and P. miles) in Bermuda. Environ Biol Fish 102, 887–900 (2019). https://doi.org/10.1007/s10641-019-00877-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-019-00877-4