Abstract
The localized scale of most fisheries management does not account for potential regional connectivity, particularly for fish species with prolonged planktonic larval durations (PLD). Although bonefish (Albula vulpes) inhabits shallow coastal habitats from juvenile through adult life stages, it is a strong candidate for population connectivity via larval dispersal with a PLD of 41–71 days. To address this knowledge gap, surface trajectories of particles (“virtual larvae”) released from 26 known and predicted spawning sites of bonefish around the Caribbean Sea, Florida, and Bahamas were simulated for 2009–2015 using a realistic ocean circulation hindcast model coupled with an online particle tracking simulator to study larval transport variations. At each site, 100 surface particles were released twice per month (at full and new moons) from October to April in each year and tracked for 53 days. We then estimated the likelihood that management regions would rely upon larval retention versus larval dispersal from other management zones. Overall, separately managed areas are likely to be connected via larval dispersal rather than entirely self-recruiting. Significant temporal differences in particle dispersal found for new and full moon phases, and between winter and spring, highlight that it is vital to resolve multiscale temporal and spatial variability in circulation transport when studying larval transport and connectivity. Results underscore the need to include the likelihood of population connectivity in fisheries management and conservation strategies, and to ensure that the ontogenetic habitat requirements of bonefish are properly managed at a regional scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of marine species have a dispersive pelagic larval stage, whereby larvae with limited swimming abilities are transported by ocean currents (Roberts 1997; Kim et al. 2007). Some of these larvae are carried tens of thousands of kilometers during just a few months, whereas others are retained relatively close to their source (McCain and Hardy 2010). During the planktonic stage, some larvae may also go through complex vertical migrations (e.g., Kudo 2001). Depending on location and corresponding hydrodynamic environment, local marine population composition may be determined by processes occurring locally, at distant locations, or a combination of local and distant locations (Roberts 1997). Despite improved methods to estimate connectivity, most fisheries management remains local, whereby stocks are managed as isolated units and the connectivity to other stocks is not incorporated (Kerr et al. 2017). Whereas for many fisheries data on connectivity are not yet available, even for fisheries with sufficient data these data are not incorporated into management (Kough et al. 2013). Given that a major challenge in marine resource management is to understand connections between locations and populations, methods that illuminate potential larval dispersal trajectories can be helpful for marine ecosystem management (Kough et al. 2016). Because of the difficulty in deriving potential larval dispersal pathways through sampling larvae due to the large potential geographical dispersal range, multi-scale ocean modeling techniques provide a powerful tool to address this problem (Kough et al. 2013).
This study focuses on bonefish, Albula vulpes, which is an economically important tropical and subtropical species that inhabits shallow habitats throughout the Caribbean Sea and northwest Atlantic (Colton and Alevizon 1983a, 1983b; Fig. 1). Bonefish supports economically important, catch-and-release, recreational fisheries throughout its range. For example, the annual economic impact of the recreational bonefish fishery exceeds $141 million in the Bahamas (Fedler 2010). Bonefish are part of the recreational flats fishery with an annual economic impact exceeding $465 million in the Florida Keys (Fedler 2013) and $55 million in Belize (Fedler 2014). The economic value of the recreational fishery has influenced regulators to impose strict regulations to protect the fishery; bonefish are regulated as catch and release only in Florida and Belize, for example. Conservation efforts are increasingly focusing on protecting essential habitats for adults and juveniles and for spawning (Adams 2016; Adams et al. this issue; Boucek et al. this issue), such as recent national park designations in the Bahamas to protect important bonefish habitats. Despite increasing knowledge of bonefish benthic habitats and the need for protection, little is known about the connectivity among bonefish population centers that results from their planktonic larval period. This makes prioritization of habitat protection locations difficult, and furthers the status quo of localized management that is unlikely to be sufficient for a species with a prolonged planktonic larval duration.
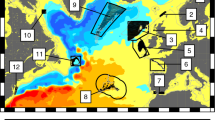
Long-term (2009–2015) mean surface velocity (vectors) and temperature (color shading). Velocity is in m/s, and temperature is in oC. Cyan squares with numbers indicate the location of 26 sites for particle releasing. Management regions: Bahamas – sites 1–5; Cuba – sites 6, 7, 12–15; Florida Keys – sites 8–11; Mexico – sites 16, 18; Belize – sites 17, 19; Puerto Rico – sites 20, 21; British Virgin Islands – site 22; U.S. Virgin Islands – site 23; Los Roques, Venezuela – sites – 24 – 26
There is limited but growing information on bonefish biology. Tag–recapture and acoustic telemetry research suggests that adult bonefish occupy relatively small home ranges (e.g., Boucek et al. this issue) and undergo long-distance migrations to spawning locations (Haley 2009; Danylchuk et al. 2011; Boucek et al. this issue). Only recently have pre-spawning sites been identified and associated behaviors documented: spawning occurs offshore, at the edge of the continental shelf, near full and new moons between October and April (Danylchuk et al. 2011). Planktonic larval duration ranges from 41 to 71 d (Mojica et al. 1995). Bonefish have leptocephalus larvae for which behaviors and swimming abilities are unknown. Settlement and early juvenile habitats are protected, shallow, open bottoms of sand or sandy mud in bays adjacent to deeper water channels that provide larval access (Haak et al. this issue). In addition, there have been no reported metapopulation genetic studies on bonefish to understand genetic stock structure if any differentiation has occurred. Studying the likely dispersal patters of the leptocephali is expected to yield fundamental information related to regional management.
Planktonic larval dispersal models in conjunction with ocean circulation simulation have become a powerful tool to simulate marine organism (e.g., larvae) transport and illustrate long-distance migration patterns. In the pioneer study by Roberts (1997), Caribbean sea surface current patterns were used to map dispersal routes of pelagic larvae from 18 coral reef sites within one and two months, which showed that larvae could be imported from upstream and exported to downstream areas. Subsequently, numerous particle tracking models were developed, which can be categorized as online or offline. Online particle tracking models generally simulate particle trajectories by embedding advection-diffusion schemes (e.g., fourth-order Runge–Kutta) into circulation models with “random walk” schemes added for subgrid scale dynamics. Particle position is calculated every model time step. For example, Cowen et al. (2006) applied a biophysical model for the Caribbean region to calculate the scaling of connectivity of a variety of reef fish species. Xue et al. (2008) embedded a particle tracking subroutine into a circulation model to study the connectivity of lobster populations in the coastal Gulf of Maine. Moon et al. (2010) used a particle tracking module to explore jellyfish behaviors in the East China Sea and Japan Sea. Qian et al. (2014) explored the connectivity in the Intra-American Seas using similar tools.
Alternately, instead of embedding a subroutine into a circulation model, offline particle tracking models typically use existing circulation model results to calculate particle position. For example, North et al. (2008) quantified the vertical swimming behavior influence on oyster larval dispersal in Chesapeake Bay using the larval transport model (LTRANS) and ocean circulation model results. Young et al. (2012) described deep-sea larval dispersal in the Intra-American Seas based on LTRANS and ocean simulations. Li et al. (2014) explored coastal connectivity in the Gulf of Maine in spring and summer of 2004–2009. In addition to LTRANS, another offline particle tracking model, the Connectivity Modeling System (CMS) was developed by Paris et al. (2013). This probabilistic modeling tool for multi-scale tracking of biotic and abiotic variability in the ocean is based on different circulation models with different spatial resolutions. Kough et al. (2013) predicted the Caribbean-wide pattern of larval connectivity for the Caribbean spiny lobster using the CMS. Kough et al. (2016) also using the CMS, analyzed the decadal larval connectivity of Cuban snapper spawning aggregations based on Hybrid Coordinate Ocean Model (HYCOM) circulation model results. Using similar HYCOM model results, Putman et al. (2013) predicted the distribution of oceanic-stage Kemp’s ridley sea turtles with ichthyoplankton particle-tracking software (Lett et al. 2008).
Although many recent studies take advantage of existing circulation model results (e.g., HYCOM), the models’ outputs have been averaged to a coarse time resolution (e.g., weekly or monthly) to simulate marine organism movement (Putman and He 2013). By comparing simulated particle trajectories with in situ near-surface drifter trajectories, Putman and He (2013) pointed out that the “over averaging” models yield predictions inconsistent with observations and that the simulated trajectories heavily depend on the resolution of model outputs.
In this study, we developed a northwest Atlantic online particle tracking system to simulate larval transport trajectories in the northwest Atlantic Ocean and Caribbean Sea, to eliminate the time resolution problems of offline particle tracking methods and produce more realistic particle trajectories. We address several questions relevant to management strategy for bonefish, with applications to other species with long planktonic larval durations, and thus high potential population connectivity: 1) What are the potential larval dispersal paths for different sites of origin? 2) How far can the larvae be carried within a defined pelagic larval duration? 3) What is the temporal variation in larval dispersal distance? and 4) Which management zones are most likely to be connected by larval dispersal, and which are more likely to be self-recruiting?
Materials and methods
Circulation model setting
We used the Regional Ocean Modeling System (ROMS) to simulate hydrodynamics in the study region from 2009 to 2015. ROMS is a terrain-following coordinate, primitive equation model developed specifically for regional applications (Haidvogel et al. 2008). Its computational kernel uses high-order time stepping and advection schemes, and a carefully designed temporal averaging filter to guarantee exact conservation for tracers and momentum (Shchepetkin and McWilliams 2005; Zhang et al. 2009).
The model domain shown in Fig. 1 spans the northwest Atlantic with ~7 km horizontal grid spacing and 36 vertical layers. The bathymetry was generated using 1-min gridded GEBCO data and smoothed with a linear programming procedure (Sikirić et al. 2009) to remove overly large gradients that may lead to unwanted numerical pressure gradient errors for the model. The model uses fourth-order centered advection with the generic length scale vertical mixing scheme (Warner et al. 2005) using k-kl mixing coefficients (corresponding to Mellor-Yamada Level 2.5).
At its only open boundary to the east, the model is configured to conserve volume with a free-surface Chapman condition (Chapman 1985), a Flather condition for 2D momentum (Flather 1976), and the Orlanski-type radiation condition (Orlanski 1976) for 3D momentum and tracers (Marchesiello et al. 2001; Zeng et al. 2015a). Boundary values of ocean states are derived from the daily global HYCOM product (https://hycom.org).
Surface forcing used in the ROMS simulation is derived from the European Center for Medium Range Weather Forecast (ECMWF) reanalysis product (http://apps.ecmwf.int/datasets/data/interim-full-daily) with 0.125-degree grid spacing every three hours. Air temperature, surface pressure, humidity, wind speed and direction, short- and long-wave radiation, and precipitation from ECMWF are used to compute the ROMS surface momentum and buoyancy forcing with the bulk flux formulation of Fairall et al. (1996).
The model was initialized with daily HYCOM output and ran from October 1, 2008 to December 31, 2015. The results from 2009 to 2015 were used for further analysis. Similar model settings were used to study Gulf Stream meanders in the South Atlantic Bight (Zeng and He 2016).
Particle tracking model setting
The benthic (i.e., non-larval) portion of the bonefish life cycle is confined to shallow benthic habitats, with the exception of spawning. Bonefish migrate from their home ranges to pre-spawning sites that are typically shallow bays near deep water (Danylchuk et al. 2011). Bonefish make these migrations near full and new moons between October and April each year (Danylchuk et al. 2011; Adams et al. this issue). At dusk, schools of bonefish ready to spawn migrate offshore off the shelf edge (water depth exceeds 1000 m), where they descend to depths exceeding 40 m for hours before rushing toward the surface; it is assumed that bonefish release eggs and sperm during this ascent as part of broadcast spawning (Danylchuk et al. 2011; Adams et al. this issue). This activity was accounted for in the model particle release times used in the numerical particle tracking analysis detailed below.
For the particle release site selection, because the focus of this study was to determine the extent that local management is a valid strategy for bonefish, we first defined management regions based on national boundaries. Thus, each country was defined as a unique management region, which is the manner that bonefish are being managed presently. To emphasize the focus on incorporating connectivity into management, we included only countries in which there are active recreational bonefish fisheries: Florida Keys (USA), Bahamas, Cuba, Mexico (Caribbean Yucatan Peninsula), Belize, Puerto Rico, U.S. Virgin Islands, British Virgin Islands, Los Roques (Venezuela). These are all the regions with bonefish populations large enough to support recreational fisheries. We recognize that many of these locations also support subsistence and commercial bonefish fisheries. For Cuba, we followed Paris et al. (2005) and Kough et al. (2016) and identified four zones (ecoregions) based on shelf width and associated habitats. Since the benthic portion of the bonefish life cycle depends upon shallow habitats, portions of Cuba without such habitats were considered barriers to benthic connectivity, similar to the justification for ecoregions by Paris et al. (2005) and Kough et al. (2016). Countries with suitable habitat but with insufficient habitat coverage for a bonefish population large enough to support a recreational fishery (e.g., Cayman Islands) or where bonefish were overfished to the point of local extirpation (e.g., Jamaica, Dominican Republic) were not considered in this study. Based on these considerations, 26 locations were selected as spawning sites in this study (Fig. 1). Sites 01, 02, 03, 04, 05, 07, 12, and 17 are known sites based on observations (site 03, Danylchuk et al. 2011; site 04, Haley 2009; all others, Adams unpubl. data). Based on the topographical characteristics of the known sites, additional spawning sites were selected for areas with known bonefish populations and fisheries.
To study bonefish larval transport, 2600 virtual surface particles (100 particles per site) were released within the model twice a month (at new and full moons) from October 2009 to May 2015 (Table 1; 218,400 particles total). The particles were designed to stay at a constant water depth (0.5 m) without vertical migration. This setting is based on the fact that during the pelagic stage most larvae have limited swimming ability relative to ocean currents (Roberts 1997), and because bonefish larval behavior is undocumented. The pelagic larval duration of bonefish ranges from 41 to 71 days (Mojica et al. 1995), and for this study we used a PLD of 53 days.
Circulation model validation
General circulation patterns in the study domain can be well illustrated by the mean circulation fields generated by the temporal averages (over 2009–2015) of circulation hindcast output. The most pronounced circulation feature is the North Atlantic Ocean’s western boundary current (WBC), which originates from the equatorial north Atlantic, and moves through our study domain (Fig. 1). Its Caribbean Current-Loop Current- Florida Current- Gulf Stream flow system is a powerful conduit that transports larvae and other material properties in the regional ocean.
To assess the model’s skill, numerous model-data comparisons were performed. We first applied AVISO gridded, quarter degree resolution, daily Absolute Dynamic Topography data, which are measured by satellite altimetry and have been widely used to study ocean circulation variation (e.g., Yin et al. 2014; Zeng et al. 2015b, 2015c; Zeng and He 2016). Model-simulated sea surface height (SSH) resembles AVISO observations well, capturing all major circulations features in the study domain. Simulated and satellite-observed mean SSH and eddy kinetic energy (EKE) from 2009 to 2015 had spatial correlation coefficients of 0.95 and 0.77, respectively (Fig. 2).
Simulated and observed mean sea surface height (SSH) and eddy kinetic energy (EKE) from 2009 to 2015. (a) Model mean SSH; (b) AVISO (observed) mean SSH; (c) model mean EKE; (d) AVISO mean EKE. SSH is in meters, and EKE is in m2/s2. The spatial correlation coefficients between simulated and observed SSH and EKE are 0.95 and 0.77, respectively. To account for the different reference level between AVISO and model results, the spatial mean AVISO SSH is deducted in (b). Pink triangles represent sea level stations for model validation
Eight sea level stations were used to compare daily observed and simulated sea level variation along the U.S. east and south coasts from January 1, 2009 to December 31, 2015 (Fig. 3). The sea level observation is extracted from the Research Quality dataset archived by the NOAA National Centers for Environmental Information (Caldwell et al. 2015). Because of the different sea level reference values between observation and simulation, both were normalized (subtract mean then divide by standard deviation) before the comparison. Simulated sea level (red) generally followed the observations (blue), and the correlation coefficients are mostly greater than 0.6 at 5% significance level, suggesting the model is capable of capturing coastal sea level and circulation variation during the study period.
Simulated (red) and observed (blue) sea level comparisons (2009–2014) at eight stations. Station locations are indicated as pink triangles in Fig. 2a. Sea level is normalized to account for the different reference level between observation and model. Correlation coefficients (r) are shown at the bottom left corner of each figure
Estimates of connectivity
We used three approaches to examine potential connectivity between spawning locations and larval settlement locations. First, we combined data across years to estimate overall levels of connectivity among management regions. We used the combined data to examine Particle Dispersal Trajectory by charting transport paths of virtual larvae from 0 to 53 d, and location of virtual larvae at 53d. We also used the combined data to estimate Particle Occurrence Distribution by quantifying the number of occurrences of particles for each representative site by dividing the model domain into a 0.1 × 0.1 degree grid, and noted the presence or absence of a particle in each grid cell at 53 d. This approach created probability maps (also referred as “heat maps”) showing where virtual larvae are located at 53 d.
To provide a quantitative metric of whether a spawning site is more dispersive or retentive, we calculated Particle Dispersal Distance for all sites, with two distances defined as: D1, the entire distance the larvae travels with ocean currents from spawning site to location at 53d; and D2, the straight-line distance between the release location and location at 53 d. D1 is typical larger than D2, but a significant disparity between the two is indicative of a high likelihood that larvae are entrained in a retentive local ocean current system, and remain close to the point of origin (i.e., within the management region). We also examined D1 and D2 to estimate temporal variability of larval dispersal using a paired t-test for lunar phase (full vs. new moon) and season (winter – November-January; spring – February-April), and an ANOVA for year.
Results
Particle dispersal trajectory
The model estimated high larval retention within the Bahamas management region (sites 1–5), with likely connectivity to the northern Cuba zones as well (Fig. 4a). In addition, substantial portions of larvae were lost to the Gulf Stream and open ocean – a “graveyard” for most larvae (Kough et al. 2016). In general, the more southeast the location, the greater the likelihood of connectivity to north Cuba. In fact, a small chance of connectivity to the southeast Cuba region is possible. The northern Cuba sites (6 and 7) showed similar patterns -high likelihood of retention, with connectivity to the Bahamas. (For sites not shown in Fig. 4, see Appendix.)
Overall (2009–2015) particle dispersal trajectory (red) and location at day 53 (blue). Cyan squares with numbers indicate the site location. Trajectories (red lines) are simulation results from day 0 to 53 for all releasing dates in Table 1 from 2009 to 2015
Within the Florida Keys, there was a wide disparity between sites as the results show very little estimated local retention (site 8, Fig. 4b), moderate retention (site 9), and high likely larval retention (sites 10, 11). However, even sites with some retention also exhibited a high estimated level of loss to the Gulf Stream and open ocean, as well as to latitudes north of present day suitable environmental conditions for bonefish (bonefish are limited to the Florida Keys, south of Key Biscayne in the continental US). (For sites not shown in Fig. 4b, see Appendix).
Larvae from the spawning sites in Cuba’s southern regions (sites 12–15) had a high likelihood of retention. The westernmost site (12) (Fig. 4c), however, exhibits moderate connectivity to the Florida Keys and loss of larvae to the open ocean. (For sites not shown in Fig. 4c, see Appendix).
The spawning sites in Belize (17, 19) and the Mexican Yucatan (16, 18) exhibit a similar mix of dispersal to the Florida Keys and retention locally (Fig. 4d). Site 16, the farthest north, exhibits almost full dispersal, with highest likelihoods of larvae ending the 53 d period in the Florida Keys region or the open ocean. Sites 17 and 18, each near the border between Belize and Mexico, show high estimated retention within both management regions (but especially in Mexico), as well as the likelihood of contributing larvae to the Florida Keys. Site 19, in southern Belize exhibits almost entirely local retention. (For sites not shown in Fig. 4d, see Appendix).
Most larvae originating from the spawning sites in the northeastern Caribbean (Puerto Rico, site 20 – Culebra, site 21 – Vieques; site 22 – Anegada, British Virgin Islands; site 23 – St. Croix, U.S. Virgin Islands) were lost northward to the open ocean, though there was estimated retention in each management zone and in the northeastern Caribbean area (i.e., in adjacent management zones) (Fig. 4e). (For sites not shown in Fig. 4e, see Appendix).
For the three sites in Los Roques, Venezuela (sites 24–26), there appears to be little local retention with broad-scale dispersal to the Caribbean, with larvae ending the 53 day period in the open sea as well as in other management zones in the central and western Caribbean including the Florida Keys (Fig. 4f). (For sites not shown in Fig. 4f, see Appendix). (Figs. 5 and 6)
Particle dispersal distance
For many sites, particle dispersal distances (D1 and D2) differed by lunar phase (Table 2), season (Table 3), and year (Supplemental Table 1), highlighting the variability inherent in oceanographic currents affecting larval dispersal. The largest difference between D1 and D2 can reach ~1000 km (site 17), because parcels are moving with retentive eddies and meander associated with the Loop Current and Gulf Stream, making the difference between D1 and D2 even larger.
Both D1 and D2 showed interesting differences between new and full moon releases for most sites (Table 2), suggesting underlying circulation and transport conditions are transient in nature, and that resolving temporal variability is vitally important for quantifying particle dispersal. However, which moon phase had longer dispersal distance varied by site, suggesting possible location-specific factors. For example, total particle dispersal distances (D1) for 15 stations were greater during full moons, while D1 for 11 stations were greater during new moons. However, the magnitude of these differences were generally small – 11 stations have a difference between the two values of <5%, and only one has a value of >15%, thus the implications are unclear. There is a similar lack of a consistent pattern between D2 full and new values.
Dispersal distances for many sites also differed seasonally (Table 3). Estimated D2 distances in spring were longer for four of the five Bahamas sites (sites 2–5), but only between 17 and 91 km (Table 3). In contrast, spring D2 distances were greater for the northern Cuba sites by >200 km. The Florida Keys also exhibited a mix, with spring D2 greater for three of the four sites. The two sites on the central Yucatan Peninsula, near the Mexico-Belize border, had much greater D2 distances in spring. The sites in the northeastern Caribbean showed little seasonal change in D2 distances. D2 distances were shorter for all Los Roques sites.
The seasonal variation in dispersal distance was mainly due to the difference in mean wind forcing and circulation between winter and spring. Mean surface wind is stronger and more southwestward in winter than in spring. This results in strong surface currents and Ekman transport. Further, the Loop Current in winter is in retraction stage after eddy shedding, while in spring it extends further north into the Gulf. The surface velocity field is more spatially uniform in spring than in winter for the region between Cuba and the Bahamas, resulting in a longer spring dispersal distance. In contrast, surface velocity along Cuba’s south coast is stronger and more uniform in winter. This northwest winter flow can carry the Cuba south coast particles farther than the spring circulation. Similarly, surface velocity around the major dispersal trajectories of the Puerto Rico and Venezuela clusters is also stronger in winter than in spring. Particle dispersal distance (both D1 and D2) differed by year for all sites (Supplemental Table 1), exemplified by site 8 (Fig. 7).
Distribution of annual (2010–2014) along-trajectory particle dispersal distances (D1 and D2) of site 08. The central mark (red line) in each box is the median and the edges of the box are the 25th (Q1) and 75th (Q3) percentiles. The whiskers extend to the most extreme data points that are not considered outliers. The extremes correspond to Q2–1.57(Q3 – Q1)/\( \sqrt{\mathrm{n}} \) and Q2 + 1.57(Q3 – Q1)/\( \sqrt{\mathrm{n}} \), where Q2 is the median (50th percentile), Q1 and Q3 are the 25th and 75th percentiles, respectively, and n is the number of observations. Red plus symbols are outliers
Particle occurrence distribution
The estimates of larval retention and dispersal for the Particle Occurrence Distribution maps are similar to those for the Particle Dispersal Trajectory approach, but have the advantage of showing the relative probability of settlement areas. This may make it easier to share with resource managers who are incorporating connectivity into management plans. Particle Occurrence Distribution maps estimate high retention with the Bahamas and the north coast of Cuba, but with a strong likelihood of dispersal between these management zones, as exemplified in site 1 (Fig. 8a). The Particle Occurrence Distribution map for Site 8 in the Florida Keys (Fig. 8b), shows a greater likelihood of larval retention than does the Particle Dispersal Trajectory approach (Fig. 4b), but both approaches estimate that most larvae are lost to the open ocean and northern latitudes. The likelihood of larval retention increases as sites are located farther south and west (higher retention for site 11 and 10 than size 9), similar to the Particle Dispersal Trajectory approach. Estimates of larval retention are high for sites on the south coast of Cuba (sites 12–15), with potential for dispersal to the Florida Keys from site 12 (Fig. 8c). Along the Yucatan Peninsula (sites 16–19), the likelihood of larval retention increases as latitude decreases, with site 17 showing moderate larval retention and high dispersal (Fig. 8d). Larvae from sites in the northeastern Caribbean (sites 20–23), tend to be dispersed into the open ocean, with low retention within each management zone and even among management zones in this area of the Caribbean (e.g., site 20, Fig. 8e). The location of Los Roques adjacent to the WBC results in dispersal of larvae to the central and western Caribbean (Fig. 8f). (For sites not shown in Fig. 8, see Appendix). (Fig. 9)
Particle dispersal distance (km) at 53 days by year and site for D1 (particle trajectory distance) and D2 (straight-line distance between locations at day 53 and 0). See Supplemental Table 1 for values and statistical analysis results
Discussion
Despite recognition that many spatially separate fish stocks are connected by planktonic larval dispersal, most management policies do not account for this connectivity (Kough et al. 2013). Not incorporating connectivity into management increases risk of fishery declines (Kerr et al. 2017). This is especially true for species with long planktonic larval durations (PLD) that are managed exclusively as local stocks, such as bonefish. This study is the first to estimate bonefish larval trajectories from spawning sites in the Caribbean Sea and western Atlantic Ocean, and is especially powerful because it used high-resolution realistic ocean circulation hindcast and particle tracking simulations that accounted for temporal variability of ocean circulation for the study period (2009–2015), and estimated the level of connectivity among known management regions.
This study follows several others that have emphasized the importance of particle release location (i.e., spawning location), scaling (Cowen et al. 2006), and larval traits on patterns of dispersal predicted from biophysical models (Kough et al. 2016). A plankton larval dispersal model derived from a validated regional physical model was used. It should be noted that nothing is known about the bonefish larval stage other than the range of planktonic larval duration. It is recognized that incorporating larval behavior swimming traits is important in modeling their transport (e.g., Kudo 2001; Paris et al. 2005, 2013; Kim et al. 2007; Kough et al. 2013, 2016; McVeigh et al. 2017). However, rather than attempt to assign behavior without field or laboratory observations (i.e., no biologically realistic information is available for bonefish) we used a surface-based model as it represents a first order approximation of the drift characteristics in the surface mixed layer where these larvae occur. This reported analysis is the first-ever study on possible bonefish larval transport. It is intended to be a foundational study for bonefish, i.e., to present these general findings, upon which future research can be built. When larval behavior information becomes known for bonefish, future iterations of the model experiment will be adjusted to incorporate changing PLD and vertical migratory behaviors of the leptocephali. Importantly, this study provides new information relevant to management of bonefish, a data-poor species that supports economically important fisheries in need of information to guide management strategies. Further, the use of these refined models will be valuable to predicting distribution of juveniles and adults in the future when climate change changes the suitability of various thermal habitats over the Caribbean and southeast coast of the United States.
While the dispersal distances predicted by oceanographic models are often different than distances observed using genetic techniques (D'Aloia et al. 2015; Williamson et al. 2016; Debreu et al. 2012; Truelove et al. 2017). This study finds that the status quo of bonefish management, whereby each country manages the fishery as an independent stock, does not match the biology of the species. Many of these management regions appear connected via larval dispersal. The levels of connectivity differ based on the interaction between ocean currents and the spatial orientation of management regions, but the results show that nearly all of the existing management regions rely on a mixture of local and distant larval supply. None of the countries where bonefish occur appear to have a separate unit stock where a unit stock is defined as a self-contained, isolated population, for which fishing has no effect upon the individuals of other stocks (Holden and Raitt 1974). Future genetic research may provide further details on population connectivity. Thus, bonefish conservation must incorporate a regional component into existing management strategies.
Different marine species and their larvae have different characteristics that affect their dispersal and add complexity to the management of species assemblages (Castonguay and McCleave 1987; Holstein et al. 2014). However, most marine larvae have no or very weak swimming ability and therefore can be considered passive particles at their early stages (e.g., Kough et al. 2013; Brown et al. 2005). Moreover, for most species, including bonefish, larval behavior is completely unknown. Our models used larvae as passive particles, and showed extensive connectivity among management regions in the western Atlantic, including both the Caribbean Sea and Gulf of Mexico which is important information considering the present management regimes. Considering the wide variance in the distance that the larvae traveled, adding swimming behavior or different times for settlement, while outside the scope of this foundational work, would not likely add important information that would dispute that the present management regime needs changing to properly manage this species. Indeed, adding larval behavior to future models will likely provide more detail to a regional management strategy.
The level of connectivity between management zones depends on the interaction between ocean currents and spatial orientation of management zones. For example, the currents in the Bahamas and the north coast of Cuba, which are separate management regions, suggest high levels of retention. However, the proximity of these management regions also results in moderate to high levels of inter-region connectivity for bonefish. Managers must be cautious, however, to resist the temptation to apply results across species. For example, many of the 26 sites in our study are near spawning aggregation sites for over 30 species of fish, including snappers and groupers, which are important commercial and recreational fishing resources (e.g., Claro and Lindeman 2003; Kobara and Heyman 2010; Kobara et al. 2013). However, the level of connectivity between the north coast of Cuba and the Bahamas management zones differs from findings for snapper larvae originating from spawning sites on Cuba’s north coast (Kough et al. 2016). Kough et al. (2016) models found high rates of larval retention on the north coast of Cuba, and minimal levels of dispersal. In this study we found high levels of retention as well as moderate to high levels of inter-region connectivity. These differences are likely due to spawning locations, moon phase and PLD. Snappers spawn on reef edges (Kough et al. 2016), whereas bonefish spawn in the upper 50 m of the water column in offshore waters with total depth exceeding 1000 m (Danylchuk et al. 2011). Using drifters to estimate particle trajectories, Hidalgo et al. (2002) found that starting position influenced the pathways of the drifters. Moreover, the PLD for snapper larvae used by Kough et al. (2016) was 25–40 d, whereas in this study we used 53 d for bonefish (PLD range 41–71 d). The combination of offshore spawning locations and longer PLD in the mostly strong currents related to the Gulf Stream and Antilles Current contributed to estimates of higher connectivity for bonefish compared with snappers.
Management zones that are separated by long stretches of open ocean may also be connected due to their proximity to the Western Boundary Current. This appears to be the case for some spawning sites on the Yucatan Peninsula and southwest Cuba, where there is retention of some larvae, but which also provide larvae to the Florida Keys. For example, the model estimates that a large portion of larvae spawned at site 16, in the northeastern Yucatan Peninsula, and sites 17 and 18, near the Mexico-Belize border, are dispersed to the open ocean and Florida Keys. And though local retention is dominant for south Cuba sites, the furthest western site (site 12) appears to exhibit connectivity to the northwest Caribbean and Florida Keys.
The Florida Keys management zone is unique among the regions examined in this study in that it is the recipient of unidirectional transport of larvae from multiple upstream sources. The Florida Keys geographic location is not adjacent to other management regions. Thus, there is no multi-directional larval exchange, such as between the Bahamas and northern Cuba management regions. The Florida Keys is also the last downstream location (northernmost latitude) adjacent to the WBC of climatic conditions and sufficient habitat suitable to support a large bonefish population. A large portion of the larvae emanating from Florida Keys predicted spawning sites are entrained in the WBC and lost, either to the open ocean or to latitudes north of bonefish geographic range. This is unlike the sites at Los Roques, which also lose most larvae to downstream transport, but these larvae remain within the bonefish geographic range so likely contribute to other management regions. Thus, the Florida Keys bonefish population likely relies upon larvae from upstream sources and local retention of larvae from the western-most spawning sites in the Florida Keys, which are retained by local currents rather than dispersed by the WBC.
The scenario of high levels of larval supply to the Florida Keys from external sources is not unprecedented. The management of the commercial and recreational fisheries for Caribbean spiny lobster (Panulirus argus) in the Florida Keys is similarly based on region-wide connectivity via a long PLD phase (Kough et al. 2013). Indeed, lobster management presents a special challenge in the Caribbean because of high levels of connectivity in a mosaic of independently managed fisheries that should really be managed under a regional framework (Kough et al. 2016). It appears that bonefish fishery management may face a similar challenge.
The Yucatan Peninsula management regions of Belize and Mexico also present a unique challenge. On the one hand, the model estimates that three of the four sites have equal or higher rates of larval dispersal than retention: site 16 appears to lose nearly all of its larvae to dispersal, sites 17 and 18 lose a large portion of larvae to dispersal but also have notable rates of retention. Site 19, in southern Belize, shows the highest rates of retention, but this site is on an offshore atoll with a limited bonefish population, so might not have sufficient reproductive capacity to act as a source for the region. And unlike the Florida Keys, despite being adjacent to the WBC, there are no identifiable, consistent, external sources of larvae from locations of known high bonefish abundance.
Regions such as the Florida Keys and Yucatan Peninsula may be more susceptible to the temporal variability of ocean current dynamics in delivering larvae, so they may experience more variation in population size. Populations that receive variable, if infrequent, peaks in larval supply can be maintained via the storage effect (Warner and Chesson 1985). In this scenario, years with low larval influx would be reflected in year-classes with low abundance, the overall bonefish population would be maintained by individuals from years of high larval supply, and the population would be dominated by these high-recruitment year classes. We assume that the greater number of larvae reaching an area will result in greater recruitment over long time periods. Prolonged gaps in larval supply, however, may cause population declines. Anecdotal information from recreational fishermen and fishing guides, and more recent analysis of catch data (Kroloff et al. this issue; Rehage et al. this issue; Santos et al. 2017, this issue), suggest a long-term decline in bonefish abundance (1990s to present). During informal interviews over the last several years, many fishing guides in the Florida Keys reported that they had not seen juvenile and subadult bonefish in many years. In some cases, they had not seen juvenile bonefish in >15 yr. (A. Adams unpubl. data), suggesting that a lack of larval recruitment was the cause. Indeed, Klarenberg et al. (this issue) found that both recruitment failures and adult mortality were likely causes of the decline. More recently in the Florida Keys, bonefish abundance appears to be increasing rapidly, dominated by size classes (300–400 mm FL, one to two years old; size/age from Crabtree et al. 1996) which suggest recent recruitment events. This may reflect the rescue effect (Gotelli 1991), whereby the Florida Keys bonefish population receive larvae from external sources that prevents local extinction and helps to maintain population size. The challenge in devising a strong management strategy for the Florida Keys is determining the extent that the bonefish population is dependent upon local and distant sources, and incorporating local and regional factors accordingly.
Temporal variability in ocean currents can affect larval transport, so it is important to include multiple year estimates of connectivity via larval dispersal. Indeed, Kough et al. (2016), relying on 10 years of data, found that more years of model simulation reduced the variance of the predictions for larval destinations. Realizing that 10 years of data is difficult to obtain for many species and locations, they surmised that five years is a sufficient compromise between estimating trends and overweighting rare dispersal events. Inclusion of temporal variability is especially important for bonefish and other species with prolonged spawning seasons and will likely be considered in our future research. Indeed, Wallace and Tringali (2016) found two distinct genetic clusters throughout the regional bonefish population in the Caribbean Sea and western north Atlantic Ocean, and posited that these clusters might represent temporally separate spawning groups. For example, there may be different groups that spawn in winter and spring, such as for Atlantic cod (Kovach et al. 2010). Given seasonal differences in dispersal distance observed in this study, this possibility is worthy of additional examination.
Given the high level of connectivity among management regions due to larval dispersal, threats to bonefish spawning locations should be of special concern. In Cuba, for example a reported 20 tons of bonefish per year are harvested from a pre-spawning aggregation site (Jorge Angulo, University of Florida, pers. comm.), nets are set to intercept bonefish migrating to a pre-spawning aggregation site in Quintana Roo, Mexico (Addiel Perez, ECOSUR, pers. comm.), and spawning migrations are targeted for harvest in some locations in the Bahamas (A. Adams pers. obs.). In addition, the characteristics of bonefish spawning locations (proximity of deep water to a protected shoreline) makes them vulnerable to development (Adams et al. this issue). Combining our knowledge of the effects of spawning aggregation declines on other species (e.g., Sadovy De Mitcheson et al. 2008), and dispersal of bonefish larvae among management regions, suggests that loss of spawning output will have both intra- and inter-regional consequences for bonefish.
The influence of climate change on geographic range of bonefish is also an important consideration. The larvae that are transported to northern of latitudes that are presently unsuitable for bonefish inhabitation, for example, may eventually be within the temperature range of bonefish physiological tolerances, and thus may provide suitable habitats when considering climate change (Liu et al. 2015; Muhling et al. 2015).
Summary
This study is the first to estimate bonefish larval dispersal from spawning sites for this economically important species in the Caribbean. The analyses utilized a high-resolution realistic ocean circulation hindcast and online particle tracking simulations that accounted for 1) temporal variability of ocean circulation in 2009–2015 (instead of using the mean circulation as many earlier studies did) and 2) the spawning patterns of bonefish with relation to moon phases, seasons, and years. This study will guide future use of more advanced larval transport and hydrodynamics models that can be used to refine results. Specifically, certain larval behaviors can be incorporated into the circulation model with finer resolution for regions with high larval concentrations. This study will also guide research aimed at prioritizing habitat protections for bonefish throughout the Caribbean. Given that bonefish are increasingly seen as a useful conservation tool because their economic and cultural value make them useful as an umbrella species (Adams and Murchie 2015), the implications of this study apply more broadly to other marine species.
References
Adams AJ (2016) Guidelines for evaluating the suitability of catch and release fisheries: lessons learned from Caribbean flats fisheries. Fish Res. https://doi.org/10.1016/j.fishres.2016.09.027
Adams AJ, Murchie KJ (2015) Recreational fisheries as conservation tools for mangrove habitats. In: Murchie KJ, Daneshgar PP (eds) Mangroves as fish habitat, vol 83. American Fisheries Society, Symposium, Bethesda, pp 43–56
Adams AJ, Shenker J, Jud Z, Lewis JP, Danylchuk AJ (this issue) Identifying pre-spawning aggregation sites for bonefish (Albula vulpes) in the Bahamas to inform habitat protection and species conservation. Environ Biol Fish
Boucek RE, Lewis P, Stewart BD, Jud ZR, Adams AJ (this issue) Measuring site fidelity and homesite-to-pre-spawning site connectivity of Bonefish (Albula vulpes): using mark-recapture to inform habitat conservation. Environ Biol Fish
Brown CA, Jackson GA, Holt SA, Holt GJ (2005) Spatial and temporal patterns in modeled particle transport to estuarine habitat with comparisons to larval fish settlement patterns. Estuar Coast Shelf Sci 64:33–46. https://doi.org/10.1016/j.ecss.2005.02.004
Caldwell PC, Merrifield MA, Thompson PR (2015) Sea level measured by tide gauges from global oceans — the Joint Archive for Sea Level holdings (NCEI Accession 0019568), Version 5.5, NOAA National Centers for Environmental Information, Dataset, https://doi.org/10.7289/V5V40S7W
Castonguay M, McCleave JD (1987) Vertical distributions, diel and ontogenetic vertical migrations and net avoidance of leptocephali of Anguilla and other common species in the Sargasso Sea. J Plankton Res 9:195–214. https://doi.org/10.1093/plankt/9.1.195
Chapman DC (1985) Numerical Treatment of Cross-Shelf Open Boundaries in a Barotropic Coastal Ocean Model. J Phys Oceanogr 15:1060–1075. https://doi.org/10.1175/1520-0485
Claro R, Lindeman KC (2003) Spawning aggregation sites of Snapper and Grouper species (Lutjanidae and Serranidae) on the Insular Shelf of Cuba. Gulf and Caribbean Research 14:91–106
Colton DE, Alevizon WS (1983a) Feeding ecology of Bonefish in Bahamian waters. Trans Am Fish Soc 112:178–184
Colton DE, Alevizon WS (1983b) Movement patterns of the Bonefish (Albula vulpes) in Bahamian waters. US National Marine Fisheries Service Fishery Bulletin 81:148–154
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of Connectivity in Marine Populations. Science 311:522–527. https://doi.org/10.1126/science.1122039
Crabtree RE, Harnden CW, Snodgrass D, Stevens C (1996) Age, growth, and mortality of bonefish, Albula vulpes, from the waters of the Florida Keys. Fish Bull 94:442–451
D’Aloia CC, Bogdanowicz SM, Francis RK, Majoris JE, Harrison RG, Buston PM (2015) Patterns, causes, and consequences of marine larval dispersal. Proc Natl Acad Sci U S A 112:13940–13945
Danylchuk AJ, Cooke SJ, Goldberg TL, Suski CD, Murchie KJ, Danylchuk SE, Shultz A, Haak CR, Brooks E, Oronti A, Koppelman JB, Philipp DP (2011) Aggregations and offshore movements as indicators of spawning activity of Bonefish (Albula vulpes) in the Bahamas. Mar Biol 158:1981–1999
Debreu L, Marchesiello P, Penven P, Cambon G (2012) Two-way nesting in split-explicit ocean models: Algorithms, implementation and validation. Ocean Model 49:1–21. https://doi.org/10.1016/j.ocemod.2012.03.003
Fairall CW, Bradley EF, Rogers DP, Edson JB, Young GS (1996) Bulk parameterization of air-sea fluxes for Tropical Ocean-Global Atmosphere Coupled-Ocean Atmosphere Response Experiment. J Geophys Res 101:3747–3764. https://doi.org/10.1029/95JC03205
Fedler A (2010) The economic impact of flats fishing in the Bahamas. Report to the Everglades Foundation, Palmetto Bay, Florida. https://www.bonefishtarpontrust.org/downloads/research-reports/stories/bahamas-flats-economic-impact-report.pdf
Fedler A (2013) Economic impact of the Florida Keys flats fishery. Report to Bonefish & Tarpon Trust, Key Largo Florida. https://www.bonefishtarpontrust.org/downloads/research-reports/stories/BTT%20-%20Keys%20Economic%20Report.pdf
Fedler A (2014) Economic impact of flats fishing in Belize. Report to Bonefish & Tarpon Trust, Key Largo, Florida. https://www.bonefishtarpontrust.org/downloads/research-reports/stories/2013-belize-economic-study.pdf
Flather RA (1976) A tidal model of the north-west European continental shelf. Memoires Societe Royale des Sciences de Liege 10:141–164
Gotelli NJ (1991) Metapopulation models: the rescue effect, the propagule rain, and the core-satellite hypothesis. Am Nat 138(3):768–776
Haak CR, Power M, Cowles G, Danylchuk A (this issue) Hydrodynamic and isotopic niche differentiation between juveniles of two sympatric cryptic bonefishes, Albula vulpes and Albula goreensis in The Bahamas. Environ Biol Bish
Haidvogel DB, Arango H, Budgell WP, Cornuelle BD, Curchitser E, Di Lorenzo E, Fennel K, Geyer WR, Hermann AJ, Lanerolle L, Levin J, McWilliams JC, Miller AJ, Moore AM, Powell TM, Shchepetkin AF, Sherwood CR, Signell RP, Warner JC, Wilkin J (2008) Ocean forecasting in terrain-following coordinates: Formulation and skill assessment of the Regional Ocean Modeling System. Journal of Computational Physics, Predicting Weather, Climate and Extreme Events 227:3595–3624. https://doi.org/10.1016/j.jcp.2007.06.016
Haley V (2009) Acoustic telemetry studies of Bonefish (Albula vulpes) movement around Andros Island, Bahamas: implications for species management. Master’s thesis. Florida International University, Miami
Hidalgo SE, Shima JS, Hellberg ME, Thorrold SR, Jones GP, Robertson DR, Morgan SG, Selkoe KA, Ruiz GM, Warner RR (2002) Evidence of self-recruitment in demersal marine populations. Bull Mar Sci 70:251–271
Holstein DM, Paris CB, Mumby PJ (2014) Consistency and inconsistency in multispecies population network dynamics of coral reef ecosystems. Mar Ecol Prog Ser 499:1–18. https://doi.org/10.3354/meps10647
Holden MJ, Raitt DFS (eds) (1974) Manual of Fisheries Science Part 2 – Methods of Resource Investigation and their Application. FAO, Rome, p 255
Kerr LA, Hintzen NT, Cadrin SX, Clausen LW, Dickey-Collas M, Goethel DR, Hatfield EMC, Kritzer JP, Nash RDM (2017) Lessons learned from practical approaches to reconcile mismatches between biological population structure and stock units of marine fish. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsw188
Kim H, Kimura S, Shinoda A, Kitagawa T, Sasai Y, Sasaki H (2007) Effect of El Niño on migration and larval transport of the Japanese eel (Anguilla japonica). ICES J Mar Sci 64:1387–1395. https://doi.org/10.1093/icesjms/fsm091
Klarenberg et al (this issue) Use of a dynamic population model to estimate mortality and recruitment trends for Bonefish in the Florida Keys. Environ Biol Fish
Kobara S, Heyman WD (2010) Sea bottom geomorphology of multi-species spawning aggregation sites in Belize. Mar Ecol Prog Ser 405:243–254. https://doi.org/10.3354/meps08512
Kobara S, Heyman WD, Pittman SJ, Nemeth RS (2013) Biogeography of transient reef-fish spawning aggregations in the Caribbean: a synthesis for future research and management. Oceanog Mar Biol: Annual Review 51:281–326
Kough AS, Claro R, Lindeman KC, Paris CB (2016) Decadal analysis of larval connectivity from Cuban snapper (Lutjanidae) spawning aggregations based on biophysical modeling. Mar Ecol Prog Ser 550:175–190. https://doi.org/10.3354/meps11714
Kough AS, Paris CB, Iv MJB (2013) Larval Connectivity and the International Management of Fisheries. PLoS One 8:e64970. https://doi.org/10.1371/journal.pone.0064970
Kovach AI, Breton TS, Berlinsky DL, Maceda L, Wirgin I (2010) Fine-scale spatial and temporal genetic structure of Atlantic cod off the Atlantic coast of the USA. Mar Ecol Prog Ser 410:177–195
Kroloff E, Heinen JT, Rehage J, Braddock K, Santos RO (this issue) A Key Informant Analysis of Local Ecological Knowledge and Perceptions of Bonefish Decline in South Florida. Environ Biol Fish
Kudo K (2001) Larval vertical-migration strategy of Japanese EEL. In: MTS/IEEE Oceans 2001. An Ocean Odyssey. Conference Proceedings (IEEE Cat. No.01CH37295). Presented at the MTS/IEEE Oceans 2001. An Ocean Odyssey. Conference Proceedings (IEEE Cat. No.01CH37295), pp. 870–875 vol. 2. https://doi.org/10.1109/OCEANS.2001.968231
Lett C, Verley P, Mullon C, Parada C, Brochier T, Penven P, Blanke B (2008) A Lagrangian tool for modeling ichthyoplankton dynamics. Environ Model Softw 23:1210–1214. https://doi.org/10.1016/j.envsoft.2008.02.005
Li Y, He R, Manning JP (2014) Coastal connectivity in the Gulf of Maine in spring and summer of 2004–2009. Deep Sea Research Part II: Topical Studies in Oceanography, Harmful Algae in the Gulf of Maine: Oceanography, Population Dynamics, and Toxin Transfer in the Food Web 103:199–209. https://doi.org/10.1016/j.dsr2.2013.01.037
Liu Y, Lee SK, Enfield DB, Muhling BA, Lamkin JT, Muller-Karger FE, Roffer MA (2015) Impact of global warming on the Intra-Americas Sea: part-1. A dynamic downscaling of the CMIP5 model projections. J Mar Syst 148:56–69
Marchesiello P, McWilliams JC, Shchepetkin A (2001) Open boundary conditions for long-term integration of regional oceanic models. Ocean Model 3:1–20. https://doi.org/10.1016/S1463-5003(00)00013-5
McClain CR, Hardy SM (2010) The dynamics of biogeographic ranges in the deep sea. Proceedings of the Royal Society of London B: Biological Sciences rspb20101057. https://doi.org/10.1098/rspb.2010.1057
McVeigh DM, Eggleston DB, Todd AC, Young CM, He R (2017) The influence of larval migration and dispersal depth on potential larval trajectories of a deep-sea bivalve. Deep Sea Res Part 1: Oceanographic Research Papers 127:57–64
Mojica R, Shenker JM, Harnden CW, Wanger DE (1995) Recruitment of Bonefish, Albula vulpes, around Lee Stocking Island, Bahamas. Fish Bull 93:666–674
Moon JH, Pang IC, Yang JY, Yoon WD (2010) Behavior of the giant jellyfish Nemopilema nomurai in the East China Sea and East/Japan Sea during the summer of 2005: A numerical model approach using a particle-tracking experiment. J Mar Syst 80:101–114. https://doi.org/10.1016/j.jmarsys.2009.10.015
Muhling BA, Liu Y, Lee SK, Lamkin JT, Roffer MA, Muller-Karger FE, Walter IIIJF (2015) Potential impact of climate change on the Intra-Americas Sea: Part-2. Implications for Atlantic bluefin tuna and skipjack tuna adult and larval habitats. J Mar Syst 148:1–13
North EW, Schlag Z, Hood RR, Li M, Zhong L, Gross T, Kennedy VS (2008) Vertical swimming behavior influences the dispersal of simulated oyster larvae in a coupled particle-tracking and hydrodynamic model of Chesapeake Bay. Mar Ecol Prog Ser 359:99–115. https://doi.org/10.3354/meps07317
Orlanski I (1976) A simple boundary condition for unbounded hyperbolic flows. J Comput Phys 21:251–269. https://doi.org/10.1016/0021-9991(76)90023-1
Paris CB, Cowen RK, Claro R, Lindeman RC (2005) Larval transport pathways from Cuban snapper (Lutjanidae) spawning aggregations based on biophysical modeling. Mar Ecol Prog Ser 296:93–106
Paris CB, Helgers J, van Sebille E, Srinivasan A (2013) Connectivity Modeling System: A probabilistic modeling tool for the multi-scale tracking of biotic and abiotic variability in the ocean. Environ Model Softw 42:47–54. https://doi.org/10.1016/j.envsoft.2012.12.006
Putman NF, He R (2013) Tracking the long-distance dispersal of marine organisms: sensitivity to ocean model resolution. J R Soc Interface 10:20120979. https://doi.org/10.1098/rsif.2012.0979
Putman NF, Mansfield KL, He R, Shaver DJ, Verley P (2013) Predicting the distribution of oceanic-stage Kemp’s ridley sea turtles. Biol Lett 9:20130345. https://doi.org/10.1098/rsbl.2013.0345
Qian H, Li Y, He R, Eggleston DB (2014) Connectivity in the Intra-American Seas and implications for potential larval transport. Coral Reefs 34:403–417. https://doi.org/10.1007/s00338-014-1244-0
Rehage JS, Santos RO, Kroloff EKN, Heinen JE, Lai Q, Black B, Boucek RE, Adams AJ (this issue) How has the quality of bonefishing changed? Quantifying temporal patterns in the South Florida Flats Fishery using local ecological knowledge. Environ Biol Fishes
Roberts CM (1997) Connectivity and Management of Caribbean Coral Reefs. Science 278:1454–1457. https://doi.org/10.1126/science.278.5342.1454
Sadovy De Mitcheson YS, Cornish A, Domeier M, Colin PL, Russell M, Lindeman KC (2008) A global baseline for spawning aggregations of reef fishes. Conserv Biol 22(5):1233–1244
Santos R, Rehage JS, Adams AJ, Black BD, Osborne J, Kroloff EKN (2017) Quantitative assessment of a data-limited recreational bonefish fishery using a timeseries of fishing guides reports. PLoS One 12(9):e0184776. https://doi.org/10.1371/journal.pone.0184776
Santos, RO, Rehage JS, Kroloff EKN, Heinen JE, Adams AJ (this issue) Combining data sources to elucidate spatial patterns in 1 recreational catch and effort: fisheries-dependent data and local ecological knowledge applied to the South Florida bonefish fishery. Environ Biol Fishes
Shchepetkin AF, McWilliams JC (2005) The regional oceanic modeling system (ROMS): a split-explicit, free-surface, topography-following-coordinate oceanic model. Ocean Model 9:347–404. https://doi.org/10.1016/j.ocemod.2004.08.002
Sikirić MD, Janeković I, Kuzmić M (2009) A new approach to bathymetry smoothing in sigma-coordinate ocean models. Ocean Model 29:128–136. https://doi.org/10.1016/j.ocemod.2009.03.009
Truelove NK, Kough AS, Behringer DC, Paris CB, Box SJ, Preziosi RJ, Butler MJ (2017) Biophysical connectivity explains population genetic structure in a highly dispersive marine species. Coral Reefs 36(1):233–244
Wallace EM, Tringali MD (2016) Fishery composition and evidence of population structure and hybridization in the Atlantic bonefish species complex (Albula spp.). Mar Biol 163:142. https://doi.org/10.1007/s00227-016-2915-x
Warner RR, Chesson PL (1985) Coexistence mediated by recruitment fluctuations: a field guide to the storage effect. Am Nat 125(6):769–787
Warner JC, Sherwood CR, Arango HG, Signell RP (2005) Performance of four turbulence closure models implemented using a generic length scale method. Ocean Model 8:81–113. https://doi.org/10.1016/j.ocemod.2003.12.003
Williamson DH, Harrison HB, Almany GR, Berumen ML, Bode M, Bonin MC, Choukroun S, Doherty PJ, Frisch AJ, Saenz-Agudelo P, Jones GP (2016) Large-scale, multidirectional larval connectivity among coral reef fish populations in the Great Barrier Reef Marine Park. Mol Ecol 25:6039–6054
Xue H, Incze L, Xu D, Wolff N, Pettigrew N (2008) Connectivity of lobster populations in the coastal Gulf of Maine: Part I: Circulation and larval transport potential. Ecol Model 210:193–211. https://doi.org/10.1016/j.ecolmodel.2007.07.024
Yin Y, Lin X, Li Y, Zeng X (2014) Seasonal variability of Kuroshio intrusion northeast of Taiwan Island as revealed by self-organizing map. Chin J Oceanol Limnol 32:1435–1442. https://doi.org/10.1007/s00343-015-4017-x
Young CM, He R, Emlet RB, Li Y, Qian H, Arellano SM, Van Gaest A, Bennett KC, Wolf M, Smart TI, Rice ME (2012) Dispersal of deep-sea larvae from the intra-American seas: simulations of trajectories using ocean models. Integr Comp Biol 52:483–496. https://doi.org/10.1093/icb/ics090
Zeng X, He R (2016) Gulf Stream variability and a triggering mechanism of its large meander in the South Atlantic Bight. J Geophys Res Oceans 121:8021–8038. https://doi.org/10.1002/2016JC012077
Zeng X, Li Y, He R, Yin Y (2015a) Clustering of Loop Current patterns based on the satellite-observed sea surface height and self-organizing map. Remote Sensing Letters 6:11–19. https://doi.org/10.1080/2150704X.2014.998347
Zeng X, He R, Xue Z, Wang H, Wang Y, Yao Z, Guan W, Warrillow J (2015b) River-derived sediment suspension and transport in the Bohai, Yellow, and East China Seas: A preliminary modeling study. Continental Shelf Research, Coastal Seas in a Changing World: Anthropogenic Impact and Environmental Responses 111:112–125. https://doi.org/10.1016/j.csr.2015.08.015
Zeng X, Li Y, He R (2015c) Predictability of the Loop Current Variation and Eddy Shedding Process in the Gulf of Mexico Using an Artificial Neural Network Approach. J Atmos Ocean Technol 32:1098–1111. https://doi.org/10.1175/JTECH-D-14-00176.1
Zhang WG, Wilkin JL, Levin JC, Arango HG (2009) An Adjoint Sensitivity Study of Buoyancy- and Wind-Driven Circulation on the New Jersey Inner Shelf. J Phys Oceanogr 39:1652–1668. https://doi.org/10.1175/2009JPO4050.1
Acknowledgements
Research support provided by Bonefish and Tarpon Trust; NSF grants: OCE1029841, OCE1559178; NOAA grants: NA11NOS0120033, NA14NMF4540061, NA16NOS0120028; NASA grants: NNX10AU06G, NNX12AP84G, NNX13AD80G, NNX14AO73G; The Gulf of Mexico Research Initiative Grant 2015-V-487 are much appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, X., Adams, A., Roffer, M. et al. Potential connectivity among spatially distinct management zones for Bonefish (Albula vulpes) via larval dispersal. Environ Biol Fish 102, 233–252 (2019). https://doi.org/10.1007/s10641-018-0826-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-018-0826-z