Abstract
The marine catfish Genidens barbus is an anadromous species from South America. The aim of the present study was to evaluate the use of lapillus otolith microchemistry (Sr:Ca; Ba:Ca and Mg:Ca ratios) as an indicator of changes in habitat use and identify the potential breeding areas and fish stocks of G. barbus populations from the Plata Basin (Paraná River Delta and De la Plata River estuary-Argentina) and Lagoa dos Patos coastal lagoon (southern Brazil). Sr:Ca, Ba:Ca and Mg:Ca ratios were measured in the core area (inner 4 rings) and external area (outer 3–4 rings) of the otoliths by ICP-OES. The Sr:Ca ratio tended to be higher in the otolith external area than in the core area, while the Ba:Ca ratio followed the opposite pattern. This suggests the displacement of fish toward higher salinity areas. The Sr:Ca, Ba:Ca, Mg:Ca ratios in the core and external areas of the otoliths from the Plata Basin differed significantly from those of the otoliths from Lagoa dos Patos. This may indicate the occurrence of two different breeding sites and at least two fish stocks in the study region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The marine catfish Genidens barbus (Lacépède 1803) is an anadromous species inhabiting estuaries and the marine continental shelf from Bahía, in Brazil, to San Blas, in Argentina (17°00’S-40°32’S) (López and Bellisio 1965). It is considered one of the most important fish resources in Uruguay, Brazil and Argentina (Reis 1986; Tavares and Luque 2004; Velasco et al. 2007; MINAGRI 2013). The marine catfish is captured by artisanal fishermen in the Lagoa dos Patos estuary (Río Grande do Sul) in Brazil, where the capture dropped from 9,000 tons (t) per year in 1972–1973 to less than 200 t per year since 1996 (IBAMA 1999; Velasco et al. 2007). Annual captures in Uruguay ranged from 90 to 150 t between 2005 and 2007 (DINARA 2013). In Argentina, the species is mainly captured in the lower section of the Plata Basin (estuarine and freshwater environments), with annual catches reaching 40–70 t (MINAGRI 2013). There are currently no fisheries regulations for this species (López et al. 2005).
The marine catfish populations from Uruguay and the Plata Basin have been poorly studied. However, some ecological aspects of the population from Lagoa dos Patos are known from previous studies (Reis 1986; Velasco and Reis 2004; Velasco et al. 2007). Adults move into that lagoon system from September to December for reproductive purposes at an age of 8–9 years to breed (Reis 1986). After females spawn in freshwater, males return to the estuarine portion of the lagoon carrying the eggs in the oropharyngeal cavity (Reis 1986; Velasco and Reis 2004). Juveniles are then released in the lower estuary waters and the adult males move to the continental shelf. Juveniles live in the estuary until the age of 3–4 years (size: 25 cm), when they change their habitat use and migrate to the ocean (Velasco et al. 2007).
Baigún et al. (2012) classified the species as vulnerable due to its complex life cycle (low fecundity, oral incubation), its restricted distribution in fresh or estuarine waters during the reproductive period, the unknown environments that inhabits during the non-reproductive period, and the critical status of the species fishery. All these factors required the development of management actions for the sustainable exploitation of the marine catfish, which relies on a better understanding of the connectivity of populations between the distribution and breeding areas in the southwestern Atlantic Ocean (Lagoa dos Patos estuary in Brazil and the Plata Basin in Argentina).
In the last decade, the analysis of the otolith microchemistry allowed the identification of migratory routes and displacements (Zlokovitz et al. 2003; Tabouret et al. 2010; Avigliano and Volpedo 2013; Avigliano et al. 2014). Fish otoliths are complex polycrystalline structures, composed of calcium carbonate (96 %) deposited as aragonite crystals in a protein matrix, and small quantities of other minerals (Campana et al. 1997). The strontium:calcium (Sr:Ca) and barium:calcium (Ba:Ca) ratios of fish otoliths are useful for the identification of migratory routes or displacements between estuarine environments because changes in these ratios are related to changes in salinity. In particular, the Sr:Ca ratio is positively correlated with water Sr:Ca ratio and salinity (Secor et al. 1995; Zlokovitz et al. 2003; Kraus and Secor 2004; Schuchert et al. 2010; Tabouret et al. 2010), while the Ba:Ca ratio is negatively correlated with water salinity (e.g. Miller 2011; Avigliano et al. 2014). In addition, the otolith magnesium:calcium (Mg:Ca) ratio has been used for the identification of fish stocks (Schuchert et al. 2010; Ferguson et al. 2011). This ratio has no relationship with water salinity but it can vary with different factors as physiological, ontogenetic, temperature and water Mg:Ca ratio (Martin and Thorrold 2005; Martin and Wuenschel 2006; Miller 2011).
In this context, the objective of the present study was to evaluate the use of lapillus otolith microchemistry (Sr:Ca; Ba:Ca and Mg:Ca ratios) as an indicator of changes in the habitat use of G. barbus. We preferred using lapillus otoliths rather than sagittal or asteriscus otoliths because they were larger and allowed less measurement error. The otolith chemical signature was compared between the core and the external areas to confirm the change in habitat use reported by Velasco et al. (2007). The chemical signature was also compared between the core area of otoliths from different estuaries (Plata Basin in Argentina and Lagoa dos Patos in Brazil) to identify the potential breeding areas, and between the external area of otoliths from those estuaries to determine the occurrence of different fish stocks. This information is important for the sustainable exploitation of the species and the development of assessment and management models.
Materials and methods
Study area
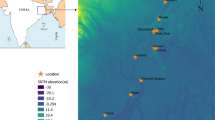
The Lagoa dos Patos is a large coastal lagoon located on the coast of Río Grande do Sul (Brazil), with a total area of 10,360 km2. The southern part of the system is occupied by an estuary, whose total area accounts for 10 % of the lagoon area. The mean salinity is around 15 g/L in the estuary (Burns et al. 2006), while the maximum depth is 6 m in the estuary (Guerrero et al. 2010) and 18 m in the navigation channel. The system is connected to the Atlantic Ocean by a narrow channel that is 4 km long and 740 m wide (Fig. 1).
The Plata Basin (3,170,000 km2) discharges into the De la Plata River estuary (30,362 km2) with an average discharge of 23,000 m3/s towards the Atlantic Ocean (Guerrero et al. 1997). This fluvio-marine environment can be divided into three regions based on water salinity: inner, middle, and outer (Fig. 1). The salinity is lower than 0.2 g/L in the inner section, while it ranges from 0.2 to 10 g/L and from 5 to 25 g/L in the middle and outer regions, respectively. The outer section is 300 km wide and the maximum depth of the De la Plata River estuary ranges between 5 and 25 m (Guerrero et al. 2010). The most important rivers in the Plata Basin are the Paraná Guazú and Uruguay Rivers (Fig. 1).
Sample collection
A total of 68 adult fish were caught with gillnets in the Lagoa dos Patos estuary in Brazil (Pelotas and San Lorenzo do Sul localities) (age range: 7–14 years; total length range: 410–810 mm), at a depth less than 5 m. In addition, 74 adult fish were collected with longlines in the Plata Basin in Argentina (Sauce and Paraná Guazú Rivers in the Paraná River Delta and the De la Plata River estuary) (age range: 7–13 years; total length range: 427–791 mm), at depths ranging from 10 to 33 m (Fig. 1). The total length (in mm) of all the animals collected was recorded and the lapillus otoliths were removed.
Age determination and sample preparation
The otoliths were washed with distilled water and photographed. The right otolith of each pair was embedded in crystal epoxy resin and sectioned transversely through the core to a thickness of 1 mm using a rotary saw equipped with a diamond blade (Dremel® 250 and 300). The number of rings in the section was counted by two independent observers under a stereomicroscope (Leica® EZ4-HD) at 40× magnification. Age determination by counting the ring number in lapillus otoliths of G. barbus was validated by Reis (1986). To avoid possible year-class effects on trace element composition of lapillus otoliths, only the otoliths with 7 or 8 rings were selected for the study (see Table 1).
The method applied in the present study allowed for the isolation of specific areas of the otoliths (e.g. core and external areas). The left otolith of each pair was embedded in crystal epoxy resin and two sections (940 ± 100 μm thick) were obtained per otolith and the number of rings in each section was counted under a stereomicroscope (Leica® EZ4-HD) at 40× magnification (Fig. 2). The sections were discarded if they differed in the number of rings. The outer three (in the case of 7-year-old fish) or four rings (in the case of 8-year-old fish) were eliminated from one of the two sections and the inner four rings were eliminated from the other section using a rotary saw, under a stereomicroscope. By means of this procedure two areas were obtained for the left lapillus otolith of each 7 or 8-year-old fish: a core area containing the inner four rings, and an external area containing the outer three to four rings (Fig. 2). Both areas were manually removed from the resin, washed in distilled water Mili-Q (Millipore, Sao Paulo, Brazil) and weighed. Those otolith sections in which the formation of cracks during the polishing process caused the loss of material were not included in the study. For this reason, the sample number differs between the core and external areas (Table 2).
Scheme illustrating the differential polishing of otolith transversal sections. A) Lapillus otolith embedded in crystal epoxy resin. B) Two sections cut through the core of the otolith. C) frontal view of the sections, showing the growth rings. D) Differential polishing of sections, with section one (otolith core area) containing the inner four rings (first 4 years of life) and section two (otolith external area) containing the outer four rings (5 to 8 years of life)
Otolith microchemistry
The core and external areas were digested with 10 % nitric acid during 48 h. The otolith Sr (407.771 nm), Ba (233.527 nm), Mg (285.213 nm) and Ca (317.933 nm) concentrations were determined by inductively coupled plasma-atomic emission spectrometry (ICP-OES), using a Perkin Elmer® Optima 2000 DV (Überlingen, Germany) equipped with cross-flow nebulizer and a quartz ICP torch (method EPA 200.7) (EPA 1994). A Perkin-Elmer AS-90 Plus autosampler was used for automated sample handling. The equipment was cleaned at regular intervals with MilliQ water (Millipore, Saõ Paulo, Brazil) and 10 % nitric acid matrix to prevent sample memory effects. The detection limits of the ICP-OES were 8, 10 and 10 μg/L for Ba, Sr and Mg, respectively. External calibration was carried out using the atomic spectroscopy standard QCS 21 (Quality Control Standard, Perkin Elmer® Pure, USA). All measurements were performed in triplicate (RSD < 4 %). The digestion and analytical procedures were checked by the analysis of Otolith Certified Reference Material for trace elements (FEBS-1, National Research Council, Canada). Replicate analysis of these reference materials showed good accuracy, with the following metal recovery rates: 93 % for Sr; 99 % for Ba; 105 % for Mg; 114 % for Ca.
Statistical analysis
Data were tested for normality and homogeneity of variance using the Shapiro-Wilk and Levene’s tests, respectively. The otolith Sr:Ca; Ba:Ca and Mg:Ca ratios were normally distributed (Shapiro-Wilk test, p > 0.05). However, only the Sr:Ca ratio met the assumption of homogeneity of variance (Levene’s, p > 0.05). For this reason the Mg:Ca and Ba:Ca ratios were analyzed using the non-parametric tests because they did not meet that assumption (Levene’s p < 0.05), even after being Log (x + 1) transformed (Longmore et al. 2010).
Analysis of covariance (ANCOVA) was used to assure that differences in otolith weight did not confound any stock-specific differences in elemental composition (Campana et al. 2000; Kerr and Campana 2013). ANCOVA is robust to violations of the assumption of homogeneity of variance (Olejnik and Algina 1984). The otolith Sr:Ca, Mg:Ca and Ba:Ca ratios varied significantly with otolith weight (ANCOVA, p < 0.05) and they were corrected using the common within-group slope (b) for each variable (element:Ca) on otolith weight (e.g. Galley et al. 2006; Burke et al. 2008; Longmore et al. 2010; Kerr and Campana 2013).
In relation to the objectives of this study, we conducted the following analysis or comparisons: 1) The element:Ca ratios were compared between the core and external areas of the otoliths using paired t-Test (Sr:Ca ratio) or the non-parametric equivalent Wilcoxon test (Ba:Ca and Mg:Ca ratios) in order to evaluate the change in habitat use. 2) The element:Ca ratios in the otolith core area were compared between the study estuaries (Argentina vs Brazil) to determine the occurrence of different breeding sites with t-Test (Sr:Ca ratio) or the non-parametric equivalent Mann–Whitney U test (Ba:Ca and Mg:Ca ratios). 3) The element:Ca ratios in the otolith external area were compared between Argentina and Brazil to determine the occurrence of different fish stocks with t-Tests (Sr:Ca ratio) and Mann–Whitney U test (Ba:Ca and Mg:Ca ratios). 4) Scatter plots were used to represent graphically the relationships between the Sr:Ca, Ba:Ca and Mg:Ca ratios (see Ferguson et al. 2011; Avigliano et al. 2014). 5) The Hotelling T-square test (Jørgensen and Rajeswaran 2005) was used to evaluate the otolith multi-elemental fingerprints and detect differences in the multi-elemental composition of the core and external areas of otoliths from different sampling sites. This test is appropriate for small samples and is robust to violations of the assumptions of normality and homogeneity of variance (Jørgensen and Rajeswaran 2005). 6) A Canonical Discriminant Analysis (CDA) of the elemental concentration of the otolith core and external areas was performed to test the accuracy of using that variable (element:Ca) for the identification of fish origin site (e.g. Longmore et al. 2010; Kerr and Campana 2013). To determine the discriminatory importance of each element:Ca ratios (i.e. the value of each variable that contributed most to the separation of the groups) across all discriminant functions, the mean discriminant coefficient was calculated using the following equation (Backhaus et al. 2006): Mean discriminant coefficient bj = Σ |bjk|*EAKk (k = 1, k = ….) where bjk is the standardized discriminant function coefficient for the variable j with respect to the discriminant function k, and EAk is the proportion of the eigenvalue of the discriminant function k in relation to the sum of all eigenvalues. All statistical tests were performed using the InfoStat® software.
Results
Element ratios
The Sr:Ca ratio (Table 2) was similar between otolith areas both (core vs external) in Argentina and Brazil. Moreover, the Sr:Ca ratio (Table 2) in the core and external areas was significantly higher in Argentina compared to Brazil.
The Ba:Ca and Mg:Ca ratios (Table 2) were significantly lower in the external than in the core area of otoliths from both study sites. The Ba:Ca ratio (Table 2) in the core and external areas was significantly higher in Brazil compared to Argentina. However, the opposite pattern was observed for the Mg:Ca ratio (Table 2).
Furthermore, a separation of values corresponding to Brazil from those corresponding to Argentina was observed for all the relationships analyzed (Sr:Ca vs Mg:Ca, Sr:Ca vs Ba:Ca, Ba:Ca vs Mg:Ca) and for both otolith areas (see Fig. 3). There were four fish collected in Lagoa do Patos that had isotopic signatures that differed from the other fish caught at this location (Fig. 3).
Multivariate analysis
Results from the Hotelling’s T-square test revealed significant differences in the multi-element signatures of the otolith core (p < 0.0001) and external (p < 0.001) areas between Argentina and Brazil. The Canonical Discriminant Analysis showed a separation between the groups from Argentina and those associated with Brazil, based on the elemental concentration of both otolith areas (Table 3). The CDA proved to have greater accuracy in classifying the fish caught in Argentina than those collected in Brazil (core area: 71.4 %; external area: 63.6 %) (Table 3). Based on the mean discriminant coefficients, the otolith Ba:Ca ratio was identified as the most important variable, followed by the otolith Mg:Ca and Sr:Ca ratios, both for the core (bj = 0.75, bj = − 0.03, bj= − 0.06, respectively) and external (bj = 0.57, bj = − 0.13, =bj= − 0.86, respectively) areas of the otoliths.
Discussion
Several studies have demonstrated that the otolith Sr:Ca and Ba:Ca ratios are closely related to the salinity of water bodies where fishes live (e.g. Kraus and Secor 2004; Miller 2011; Avigliano and Volpedo 2013). Both the Plata Basin and Lagoa Dos Patos are characterized by a pronounced salinity gradient, and otolith Sr:Ca ratio is positively correlated (Albuquerque et al. 2012; Avigliano and Volpedo 2013). In the present study, the Sr:Ca ratio in the core and external areas of otoliths from fish collected in Argentina was significantly higher than that from fish collected in Brazil, while the opposite pattern was observed for otolith Ba:Ca. This is in agreement with the wider salinity range of the De la Plata River estuary in Argentina (2–30 g/L, according to Guerrero et al. 2010) in comparison to Lagoa dos Patos in Brazil (2–20 g/L, according to Burns et al. 2006).
The Sr:Ca ratio tended to be higher in the otolith external area than in the core area, while the Ba:Ca ratio followed the opposite pattern. These trends are consistent with the change in habitat use throughout ontogeny, from a low salinity environment (estuary) to a high salinity one (continental shelf). This agrees with the displacement pattern previously reported by Velasco and Reis (2004) for G. barbus from Brazil, based on fish length distribution and growth parameters. They found that juvenile catfish (up to 4 years old) inhabit lower salinity (estuarine) waters, while pre-adults and adults (over 4 years of age) are distributed in coastal.
The change in habitat use has been reported by other authors for euryhaline species from Lagoa dos Patos and Plata Basin as Lycengraulis grossidens (Mai et al. 2014) and Micropogonias furnieri (Albuquerque et al. 2012). Like catfish, some populations of these species migrate from the estuaries to ocean during ontogeny.
Mg incorporation into carbonates is affected by kinetic and metabolic effects. There is evidence of temperature effects on the incorporation of Mg in biotic aragonite as well as ontogenetic, growth, and precipitation rate effects (e.g. Martin and Thorrold 2005; Martin and Wuenschel 2006; Miller 2011).
In this study, the otolith Mg:Ca ratio was higher in the otolith core area than in the external area (Table 2). This could be due to different factors, for example a change in the rate of incorporation of Mg during ontogeny, or it could be related to habitat use change (e.g. temperature, water Mg:Ca).
The water temperature varied from 12 °C in winter to 27.5 °C in summer in Laguna dos Patos (Brazil) (Muxagata et al. 2012), when this varied from 8 °C to 24 °C in Plata Basin (Argentina) (Guerrero et al. 1997). In this paper, otolith Mg:Ca ratio was significantly higher in fishes from Argentina than in fishes from Brazil. These results are consistent with those reported by Gaetani and Cohen (2006), where a negative effects of temperature on Mg incorporation in abiotic aragonite have been reported. However, for juvenile anadromous Chinook salmon (Oncorhynchus tshawytscha), otolith Mg:Ca was positively correlated with temperature and mean daily otolith and somatic growth rate (Miller 2011). Furthermore, Mg incorporation was not related to the effect of temperature on otolith Mg:Ca in euryhaline species (spot Leiostomus xanthurus, gray snapper Lutjanus griseus and chinook salmon Oncorhynchus tshawytscha) (Martin and Thorrold 2005; Martin and Wuenschel 2006; Miller 2011). In conclusion, the factors affecting the incorporation of Mg in the otolith are highly variable among species and should be tested experimentally. However, the differences found in the Mg:Ca ratios between the otolith core and external areas may also reflect a change of habitat use consisting in the displacement from an estuarine to a marine environment.
The different Sr:Ca, Ba:Ca, Mg:Ca ratios and chemical signature of the core and external areas of otoliths from Argentina compared to those from Brazil may indicate the occurrence of two different breeding sites and two populations of the species in the southwestern Atlantic Ocean. According to our results, the two populations remain separated during the breeding and adult periods. This could be an indication that most fishes tend to select as spawning site, the estuary where they were born. This hypothesis should be tested with other methodologies, such as tagging or genetics analysis.
The homing behavior was previously reported for other large Silurids from Plate Basin as Pseudoplatystoma corruscans (Pereira et al. 2009). Furthermore, homing and anadromy are two behavior life-history trails that are well attested in various group of fish, especially the family Salmonidae (e.g. Salmo, Salvelinus, Oncorhynchus), but also in other groups as Clupeidae (American shad Alosa spadissima) (Marschall et al. 1998; Quinn et al. 1999; McDowall 2001). Marschall et al. (1998) and Quinn et al. (1999) reported that the Atlantic salmon (Salmo salar) and sockeye salmon (Oncorhynchus nerka) show a strong tendency to return to their natal site. The anadromous behavior provides fish the opportunity for more rapid growth, and higher fecundity through the exploitation of rich food resources, while homing fosters adaptation of stocks to favorable local spawning conditions (McDowall 2001).
Furthermore, four marine catfish collected in Brazil showed the same chemical signature of the otolith core and external areas as those collected in Argentina. These specimens were also assigned to the Argentina group based on the CDA results (see Fig. 3a, b, f and Table 3). It is possible that they were born and stayed in Argentina until they became adults and then moved to Brazil, where they were collected. This may explain present results. However, there is no previous evidence suggesting the displacement of juveniles or adults between the two study sites, since this is the first study that directly examines the migratory behavior of G. barbus. In this regard, we hope that the information reported in the present study will serve as a basis for future researches (e.g. tagging studies) focused on the species displacements. The diversion of homing behavior would not be a novelty, especially in salmonids (Cury 1994; McDowall 2001). According to McDowall (2001), a low percentage of straying in species that he may have long-term evolutionary advantages. This way the fish could move into new environments, and perhaps more beneficial in terms of food and reproduction, especially if the natal site has changed. Furthermore, a low migration rate between the spawning sites also would increase the genetic variability of populations (Cury 1994). Some studies on Atlantic salmon estimated that to maintain a genetic homogeneity within subpopulations of 2500–10,000 individuals, the migration rate between the populations has to be less than only one individual per year (see Cury 1994).
The method applied (polishing of the otoliths in thin layers and the analysis of the Sr:Ca, Mg:Ca and Ba:Ca ratios in the two delimited areas) proved to be a good indicator of fish habitat and a useful tool to identify potential fish stocks and study the displacements of G. barbus. Other authors have developed similar methodologies, for example, Arslan and Secor (2008) has isolated the otolith core of blackfin tuna using different milling methods. However, this is the first time that otolith rings are mechanically separated. In principle, it would be possible to isolate the core, rings, or a combination of these, which are needed to study. We recommend the use of this methodology to other species, especially for anadromous fish. However, there are some limitations, for instance, the methodology could be very laborious for species with small otoliths.
In conclusion, the results showed that the habitat use differed among the two age classes that were assessed. Moreover, the element fingerprints differed between fish caught in Argentina and those caught in Brazil. The data suggests that the fishes caught in Argentina and Brazil are from separate breeding sites and represent two separate stocks. Present results should be taken into account for sustainable and conservation management policies. As a contribution, we recommend stocks-specific regulations for the marine catfish. On the other hand, we also recommend defining areas for temporary closure (during reproductive migration) and species-specific regulations to ensure the spawning and breeding. Limit or control fishing in areas where fishes migrate between stocks could help to maintain genetic exchange. Furthermore, an increase in oversight and control practices should be present among the measures to be taken by the policymakers and stakeholders due to the lack of agents in the fishing area. Also a strong educational campaign about Best Management Fishing Practices for this resource will generate a population awareness of the actual situation and future options. Finally, the creation of legal fish landing ports in both estuaries will contribute to generate reliable fishing statistics and ensure the proper management of the two stocks identified in this study.
References
Albuquerque CQ, Miekeley N, Muelbert JH, Walther BD, Jaureguizar AJ (2012) Estuarine dependency in a marine fish evaluated with otolith chemistry. Mar boil 159(10):2229–2239
Arslan Z, Secor DH (2008) High resolution micromill sampling for analysis of fish otoliths by ICP-MS: effects of sampling and specimen preparation on trace element fingerprints. Mar Environ Res 66(3):364–371
Avigliano E, Riaños Martinez C, Volpedo AV (2014) Combined use of otolith microchemistry and morphometry as indicators of the habitat of the silverside (Odontesthes bonariensis) in a freshwater-estuarine environment. Fish Res 149:55–60
Avigliano E, Volpedo AV (2013) Use of otolith strontium:calcium ratio as indicator of seasonal displacements of the silverside (Odontesthes bonariensis) in a freshwater-marine environment. Mar Freshw Res 64(8):746–751
Backhaus K, Erichson B, Plinke W, Weiber R (2006) Multivariate analysemethoden. Eine anwendungsorientierte Einführung, Springer, Berlin
Baigún CRM, Colautti D, López HL, Van Damme PA, Reis RE (2012) Application of extinction risk and conservation criteria for assessing fish species in the lower La Plata River basin, South America. Aquat Conserv Mar Freshw Ecosyst 22(2):181–197
Burke N, Brophy D, King P (2008) Otolith shape analysis: its application for discriminating between stocks of Irish Sea and Celtic Sea herring (Clupea harengus) in the Irish Sea. ICES J Mar Sci 65:1670–1675
Burns MD, Garcia AM, Vieira JP, Bemvenuti MA, Marques DM, Condini DM (2006) Evidence of habitat fragmentation affecting fish movement between the Patos and Mirim coastal lagoons in southern Brazil. Neotrop Ichthyol 4(1):69–72
Campana SE, Chouinard GA, Hanson JM, Frechet A, Brattey J (2000) Otolith elemental fingerprints as biological tracers of fish stocks. Fish Res 46(1):343–357
Campana SE, Thorrold SR, Jones CM et al (1997) Comparison of accuracy, precision and sensitivity in elemental assays of fish otoliths using the electron microprobe, PIXE and laser ablation ICPMS. Can J Fish Aquat Sci 54:2068–2079
Cury P (1994) Obstinate nature: an ecology of individuals. Thoughts on reproductive behavior and biodiversity. Fish Aquat Sci 51(7):1664–1673
DINARA (2013) Dirección Nacional de Recursos Acuáticos, Uruguay. http://www.dinara.gub.uy/web_dinara/. Accessed 20 Dec 2013
EPA (1994) Methods for the determination of metals in environmental samples, supplement 1, Ohio
Ferguson GF, Warda TM, Bronwyn M, Gillanders M (2011) Otolith shape and elemental composition: complementary tools for stock discrimination of mulloway (Argyrosomus japonicus) in southern Australia. Fish Res 110:75–83
Gaetani GA, Cohen AL (2006) Element partitioning during precipitation of aragonite from seawater: a framework for understanding paleoproxies. Geochim Cosmochim Acta 70:4617–4634
Galley E, Wright P, Gibb F (2006) Combined methods of otolith shape analysis improve identification of spawning areas of Atlantic cod. ICES J Mar Sci 63:1710–1717
Guerrero RA, Acha EM, Lasta CA (1997) Physical oceanography of the Río de la Plata Estuary, Argentina. Cont Shelf Res 17(7):727–742
Guerrero RA, Piola AR, Molinari G, Osiroff AP (2010) Climatología de temperatura y salinidad en el Río de la Plata y su Frente Marítimo, Argentina-Uruguay. 1° edn. Mar del Plata, Argentina
IBAMA (1999) Desembarque de pescados no Rio Grande do Sul. Annual Report, Centro de Pesquisa do Rio Grande, RS, Brasil
Jørgensen B, Rajeswaran J (2005) A Generalization of Hotelling’s T 2. Commun Stat Theor 34(11):2179–2195
Kerr LA, Campana SE (2013) Chemical Composition of Fish Hard Parts as a Natural Marker of Fish Stocks. In: Cadrin SX, Kerr LA, Mariani S (Eds.). Stock identification methods: applications in fishery science. Academic Press. pp 205–234
Kraus RT, Secor ER (2004) Incorporation of strontium into otoliths of an estuarine fish. J Exp Mar Biol Ecol 302:85–106
Longmore C, Fogarty K, Neat F, Brophy D, Trueman C, Milton A, Mariani S (2010) A comparison of otolith microchemistry and otolith shape analysis for the study of spatial variation in a deep-sea teleost, Coryphaenoides rupestris. Environ Biol Fishes 89(3–4):591–605
López HL, Miquelarena AM, Gómez JP (2005) Biodiversidad y Distribución de la Ictiofauna Mesopotámica. Miscelánea 14:311–354
López RB, Bellisio NB (1965) Contribución al conocimiento del Tachysurus barbus (Lacepede), bagre del mar argentino (Pisces. Ariidae). In: Anais do Segundo Congreso Latino-Americano de Zoología, São Paulo, Brasil, pp 145–153
Mai AC, Condini MV, Albuquerque CQ, Loebmann D, Saint’Pierre TD, Miekeley N, Vieira JP (2014) High plasticity in habitat use of Lycengraulis grossidens (Clupeiformes, Engraulididae). Estuar Coast Shelf Sci 141:7–25
Marschall EA, Quinn TP, Roff DA, Hutchings JA, Metcalfe NB, Bakke TA, Saunders RL, Poff NL (1998) A framework for understanding Atlantic salmon (Salmo salar) life history. Can J Fish Aquat Sci 55(S1):48–58
Martin GB, Thorrold SR (2005) Temperature and salinity effects on magnesium, manganese, and barium incorporation in otoliths of larval and early juvenile spot Leiostomus xanthurus. Mar Ecol Prog Ser 293:223–232
Martin GB, Wuenschel MJ (2006) Effect of temperature and salinity on otolithelement incorporation in juvenile gray snapper Lutjanus griseus. Mar Ecol Prog Ser 324:229–239
McDowall RM (2001) Anadromy and homing: two life-history traits with adaptive synergies in salmonid fishes? Fish Fish 1:78–85
Miller JA (2011) Effects of water temperature and barium concentration on otolith composition along a salinity gradient: implications for migratory reconstructions. J Exp Mar Biol Ecol 40:42–52
MINAGRI (2013) Ministerio de Agricultura y Pesca de la Nación Argentina, Argentina. http://www.minagri.gob.ar/site/pesca/pesca_maritima/02-desembarques/anio.php?anio=2012 (date of Access:12/12/2013)
Muxagata E, Waldemar JA, Barbosa CN (2012) Acartia tonsa production in the Patos Lagoon estuary, Brazil. ICES J Mar Sci 69(3):475–482
Olejnik SF, Algina J (1984) Parametric ANCOVA and the rank transform ANCOVA when the data are conditionally non-normal and heteroscedastic. J Educ Behav Stat 9(2):129–149
Pereira LHG, Foresti F, Oliveira C (2009) Genetic structure of the migratory catfish Pseudoplatystoma corruscans (Siluriformes: Pimelodidae) suggests homing behaviour. Ecol Freshw Fish 18(2):215–225
Quinn TP, Volk EC, Hendry AP (1999) Natural otolith microstructure patterns reveal precise homing to natal incubation sites by sockeye salmon (Oncorhynchus nerka). Can J Zoo 77(5):766–775
Reis EG (1986) Age and growth of the marine catfish, Netuma barba (Siluriformes, Ariidae), in the estuary of the Patos Lagoon (Brasil). Fish Bull 84(3):679–686
Schuchert PC, Arkhipkin AI, Koenig AE (2010) Traveling around Cape Horn: Otolith chemistry reveals a mixed stock of Patagonian hoki with separate Atlantic and Pacific spawning grounds. Fish Res 102:80–86
Secor DH, Henderson-Arzapalob A, Piccoli PM (1995) Can otolith microchemistry chart patterns of migration and habitat utilization in anadromous fishes? J Exp Mar Biol Ecol 192:15–33
Tabouret HG, Bareille F, Clverie C, Pecheyran P, Prouzet P, Donard OF (2010) Simultaneous use of strontium:calcium and barium:calcium ratios in otoliths as markers of habitat: application to the European eel (Anguilla anguilla) in Adour Basin, South West France. Mar Environ Res 70:35–45
Tavares LER, Luque JL (2004) Community ecology of the metazoan parasites of white sea catfish, Netuma barba (Osteichthyes: Ariidae), from the coastal zone of the state of Rio de Janeiro, Brazil. Braz J Biol 64(1):169–176
Velasco G, Reis EG (2004) Changes in growth seasonality throughout Netuma barba (Lacépède, 1803) (Siluriformes, Ariidae) Ontogeny. Braz J Biol 64(4):913–914
Velasco G, Reis EG, Vieira JP (2007) Calculating growth parameters of Genidens barbus (Siluriformes, Ariidae) using length composition and age data. J Appl Ichthyol 23:64–69
Zlokovitz ER, Secor DH, Piccoli PM (2003) Patterns of migration in Hudson River striped bass as determined by otolith microchemistry. Fish Res 63:245–259
Acknowledgments
Authors are indebted to CONICET (PIP 112-20120100543CO), ANPCyT (PIP 2010–1372), Universidad de Buenos Aires (UBACYT 20620110100007) and Instituto de Oceanografia from Fundação Universidade Federal do Rio Grande (FURG) for financial support. We thank the Editor and anonymous reviewers for their constructive comments, which helped us to improve the manuscript. The authors declare that the present study was approved by CONICET and Ministry of Agriculture, Livestock and Fisheries of Argentina, and the Animal Use Ethics Committee (CEUA) from FURG, Brazil. Therefore, there are no ethical issues or conflicts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avigliano, E., Velasco, G. & Volpedo, A.V. Use of lapillus otolith microchemistry as an indicator of the habitat of Genidens barbus from different estuarine environments in the southwestern Atlantic Ocean. Environ Biol Fish 98, 1623–1632 (2015). https://doi.org/10.1007/s10641-015-0387-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-015-0387-3