Abstract
Anthropogenic activities lead to changes in characteristics of aquatic ecosystems, including alteration of turbidity and addition of invasive species. In this study, we tested how changes in turbidity and the recent invasion of an aquatic macrophyte, Egeria densa, may have changed the predation pressure by introduced largemouth bass on juvenile striped bass and delta smelt, two species that have seen a drastic decline in recent decades in the Sacramento-San Joaquin Delta. In a series of mesocosm experiments, we showed that increases in vegetation density decreased the predation success of largemouth bass. When placed in an environment with both open water and vegetated areas, and given a choice to forage on prey associated with either of these habitats, largemouth bass preyed mainly on open water species as opposed to vegetation-associated species, such as juvenile largemouth bass, bluegill or red swamp crayfish. Finally, we showed that turbidity served as cover to open water species and increased the survival of delta smelt, an endemic species at risk. We also found that such open water prey tend not to seek refuge in the vegetation cover, even in the presence of an imminent predation threat. These results provide the beginning of a mechanistic framework to explain how decreases in turbidity and increases in vegetation cover correlate with a decline of open water species in the Sacramento-San Joaquin Delta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human-induced changes of the environment are leading to a rapid alteration of global biodiversity (Vitousek 1994) and invasions by non-native species have been identified as one of the ‘Big Five’ drivers of biodiversity loss (Sala et al. 2000). The number and the speed at which non-native species are being introduced worldwide are dramatically increasing (Lockwood et al. 2007). The ecological and evolutionary consequences of invasive species include behavioural and trait shifts (Holway and Suarez 1999; Sih et al. 2010; Sih et al. 2011), niche displacement (Race 1982; Douglas et al. 1994), competitive exclusion (Porter and Savignano 1990; Carlton et al. 1999), as well as hybridization and alterations of gene flow in native species (Rhymer and Simberloff 1996). Direct predation of native species by exotic ones is among the most familiar of invader impacts (Mooney and Cleland 2001; Wilcove et al. 1998; Sih et al. 2010). Along with invasive species, habitat destruction or alteration has been identified as a major cause of biodiversity decline (Sala et al. 2000). Because habitat disturbance can facilitate invasions, habitat alteration and species invasions are often inter-related problems (Crooks 2002; Johnson et al. 2008). In some cases, invasive species may be directly responsible for habitat alterations, when they act as ecosystem engineers, altering both biotic and abiotic components of the ecosystem (Crooks 2002). Such ecosystem engineers have the capacity to drastically change habitat characteristics by rendering them less suitable to native species and more favourable to other invaders (Didham et al. 2007). Using a case study in the Sacramento-San Joaquin Delta (Delta) in California, USA, our goal is to understand how an invasive ecosystem engineer affects consumption and prey selectivity by an introduced top predator.
Over the past several decades, the Delta has seen a considerable decline in the abundance of several pelagic fish species (Feyrer et al. 2007; Sommer et al. 2007). Two species that have received significant attention include the delta smelt Hypomesus transpacificus and the striped bass Morone saxatilis, where for the latter species, the abundance of juveniles has decreased (Feyrer et al. 2007; Sommer et al. 2007). Delta smelt are small, slender-bodied open water fish endemic to the upper Sacramento-San Joaquin estuary of California (Moyle et al. 1992) and are listed as threatened under the US Endangered Species Act (USFWS 1993). Striped bass were introduced in the late 1870s and are an important sport fish in the San Francisco Estuary. The decline of these fishes has been attributed to a number of factors, including habitat alteration, changes in flow dynamics due to large-scale water diversions for municipal and agricultural water use, increased influx of contaminants, and the rise of several key invasive species that have altered food web dynamics and physical properties of the system (Sommer et al. 2007). One important invasive species is the Brazilian waterweed, Egeria densa. According to the California Department of Boating and Waterways, Egeria was introduced to the Delta in the 1960s. Today, it is highly prolific and widely distributed along shorelines and in shallow water, making up approximately 85 % of the biomass of submerged vegetation (Hestir 2010). This invasive macrophyte is considered an aquatic ecosystem engineer, as it is known to limit nutrient and light availability (Mazzeo et al. 2003), limit the re-suspension of sediments and depress phytoplankton concentrations by consuming nutrients from the water column (Yarrow et al. 2009), and enhance top-down controls by offering diurnal refuge from planktivorous fishes for zooplankton that prey on phytoplankton (Mazzeo et al. 2003)
Concurrent with the proliferation of Egeria, the Delta has seen an increase in the abundance of largemouth bass Micropterus salmoides and other non-native fishes typically residing in the littoral zone. While largemouth bass were introduced to the Delta in the early 1900s (Moyle 2002), their population expansion is relatively recent. From the 1980s to early 2000s, Brown and Michniuk (2007) reported near doubling of the percent total catch in non-native members of the family Centrarchidae, especially largemouth bass, with some regions of the Delta seeing a three- to five- fold increase. Along with these biotic changes, the Delta has also recently seen an increase in water clarity. This latter change is likely due to both a decrease in quantity of suspended sediment input due to dam construction in upstream areas (Wright et al. 2004) and also the expansion of Egeria. Even when reduced sediment input is statistically accounted for, there has been a continued clearing trend since the expansion of Egeria (Hestir 2010). Increased water clarity may in turn increase predation risk for open water species that use turbidity as a predation refuge. For example, delta smelt are associated with high-turbidity areas (Nobriga et al. 2008). Thus, a series of related changes in the Delta, including the introduction and expansion of Egeria and largemouth bass populations and increased water clarity, may work together to change predation risk for some prey species. However, the interaction of increasing density of macrophytes and changes in water clarity on predation by largemouth bass has not yet been studied.
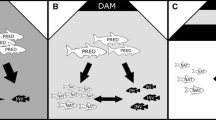
Our goal here was to examine how the expansion of Egeria may affect predator–prey interactions involving predatory, adult largemouth bass and their prey, including prey that commonly use vegetation for refuge (juvenile largemouth bass, bluegill, and crayfish) as well as delta smelt and juvenile striped bass, which were abundant in open water areas until their recent declines. Previous work suggests that high vegetation density reduces predation by adult largemouth bass (Savino and Stein 1982), which are cannibalistic on juveniles (Nobriga and Feyrer 2007). As a result, dense stands of Egeria may increase survival and recruitment of juvenile largemouth bass, even as it decreases predation success of adults. In our first experiment, we tested the prediction that juvenile largemouth bass and bluegill experience decreased predation risk from adults in the presence of high densities of Egeria. In a second experiment, we examined whether increased density of Egeria affects largemouth bass predation on an open water fish species, the striped bass. We predicted that (1) predatory largemouth bass would consume more prey located in the open water because they would be easier to locate than those hidden in the vegetation and previous research has shown that largemouth bass predation is reduced with increasing densities of vegetation (Savino and Stein 1982) and (2) this preference should be more marked as the density of vegetation increases, because vegetation-associated prey would be even more difficult to locate. Finally, our third experiment investigated the interaction between Egeria and turbidity on the survival of both vegetation-associated prey and an open water species, delta smelt. For this last experiment, we predicted that (1) as stated above, adult largemouth bass would consume more open water prey than vegetation-associated prey, and (2) the disparity between open water and vegetation-associated prey consumption should increase as the density of Egeria increases, and (3) more open water prey should be consumed under clear than turbid conditions, as increased turbidity interferes with the ability of largemouth bass to locate and capture prey (Huenemann et al. 2012).
Methods
Species collection and maintenance
Adult largemouth bass to be used as predators and juvenile largemouth bass and juvenile bluegill (Lepomis macrochirus) to be used as vegetation-associated prey items were collected by electrofishing from the Sacramento-San Joaquin Delta, CA, in July and August 2009. Adult largemouth bass were fed every second day a diet of juvenile bluegill and redear sunfish (Lepomis microlophus), while juvenile largemouth bass and bluegill were fed live bloodworms. Red swamp crayfish (Procambrus clarkii), a third vegetation-associated prey species, were collected from Putah Creek, CA (a tributary to the Yolo Bypass floodway, located within the northern Delta) in August and October 2009, using baited traps. Once in the laboratory, they were maintained on a diet of carrots. Juvenile centrachids and red swamp crayfish were chosen as vegetation-associated prey because they are commonly found in the diets of largemouth bass in the Delta (Nobriga and Feyrer 2007). These prey types are also common within the endemic range of largemouth bass (Huskey and Turingan 2001).
Striped bass (open water prey species used for Experiment 2) were obtained from the Professional Aquaculture Services in Chico, CA in August 2009 and were fed pellets (1.5 mm Silver Cup floating pellets, Nelson & Sons, Murray, UT). Delta smelt (open water prey species used for Experiment 3) were obtained from the Fish Conservation and Culture Laboratory in Byron, CA in October 2009 and were fed plankton pellets (Hikari Plankton, Kyorin, Himeji, Japan). Each species was maintained in 8000-L flow-through pools (3.6 m diameter, 0.8 m deep) equipped with an air stone. The flow-through system consisted of untreated and unfiltered well water, which was passed through a degassing column to eliminate supersaturated nitrogen gas and to replenish depleted O2 before entering the tanks. In all experiments, predators and prey were acclimated for at least two weeks. Ideally, all prey and predators would have been sourced from the wild in order to avoid a possible prey selection bias due to predator naivety of hatchery-origin fish. However, due to the low abundance of delta smelt and juvenile striped bass in the Delta resulting from their precipitous declines, it was not feasible to obtain ample numbers of juvenile striped bass or delta smelt from the wild. In addition, due to the status of delta smelt as Threatened under the Endangered Species Act, we were not authorized to collect wild delta smelt. Thus, for both Experiments 2 and 3 where hatchery-reared fish were used as open water prey species, we focus our interpretation of results on the relative differences between treatments within each experiment (low density vs. high density of vegetation in Experiments 2 and 3, and low vs. high turbidity in Experiment 3).
For Experiments 1, 2, and all vegetation-associated prey in Experiment 3, we used prey items that were within the range of appropriate sizes for predation by adult largemouth bass. Hoyle and Keast (1987) showed that largemouth bass handling time increases rapidly with prey size, and that optimal ratios for prey to predator lengths are 0.29 for bluegill (with a maximum ratio of 0.49) and 0.24 for crayfish prey, with wild largemouth bass selecting prey close to or smaller than the optimal sizes predicted by laboratory experiments. In this study, all prey species except for delta smelt (i.e., juvenile largemouth bass, bluegill, red swamp crayfish, and striped bass) were within a standard length (SL) range of 70–85 mm. The size range of adult largemouth bass was 250–400 mm SL, such that the prey: predator length ratio ranged from 0.18 to 0.34. However, for Experiment 3 in which we used delta smelt as the open water prey species, it was not possible to obtain individuals that approached the optimum size for largemouth bass consumption. As adults, delta smelt reach only 60–70 mm (Moyle 2002). In addition, they are a slender, soft-bodied fish that may be inherently easier to consume for largemouth bass than the juvenile centrarchids and crayfish that are equipped with spines or an exoskeleton. Thus, while the size and body type of delta smelt introduced unavoidable biases in Experiment 3, the range of prey sizes and types were realistic for the prey field of largemouth bass in the Delta. Furthermore, we used a higher number of delta smelt (30) than the three vegetation associated species (10 each) in order to match the number of open water and vegetation-associated prey items. As delta smelt are a slender-bodied fish, providing a higher number of delta smelt made the amount of biomass for each prey species roughly equivalent in Experiment 3. Densities of adult and juvenile largemouth bass and bluegill were comparable to densities observed during field surveys in the Delta (Conrad, unpubl. data). Crayfish density data from the Delta were not available, but 10 individuals/tank was the chosen density for crayfish in order to have comparable densities to both juvenile largemouth bass and bluegill.
Egeria stems were collected from the Sacramento-San Joaquin Delta in June and July 2009 and maintained in flow-through pools. Bunches of Egeria were prepared by tying either 11 stems (~12 g) or 50 stems (~51 g) of Egeria to a brick to create low and high-density bricks, respectively. These densities were chosen to reflect relevant densities of Egeria observed in the Sacramento-San Joaquin Delta (Conrad et al. unpubl. data). All Egeria bricks were checked for weight and densities before each experiment.
Experiment 1: Does Egeria density affect predation by largemouth bass in the absence of open water habitats?
This experiment took place in August 2009 in three 2000-L outdoor flow-through pools (1.8 m diameter, 0.7 m deep; 6 L/min; water: 20 ± 2 °C; turbidity: 0–0.05 NTU), located on the UC Davis campus. Pools contained either no vegetation, low density vegetation (109 stems/m2), or high density vegetation (462 stems/m2) in the form of 25 low- or high-density Egeria bricks positioned evenly throughout the pool floor. One adult largemouth bass was added in each pool 24 h prior to the start of the trial. After the acclimation period, 10 juvenile largemouth bass and 10 juvenile bluegill were added to the pool at 1000 h. Predator and prey were left to interact for 22 h, after which predator, prey and vegetation were removed. The number of prey from each species was counted; the pool was emptied, rinsed, and refilled with clean water and vegetation was added as needed for the next trial.
We ran nine replicates (n = 9/treatment) for each of the three treatments (no, low, or high density of Egeria) using a randomized complete block design, whereby each predatory largemouth bass (the blocking factor) was used once in each vegetation treatment and the same pools were used in repeated trials. To control for hunger level, adult largemouth bass were starved for 24 h prior to being used in the experiment.
Experiment 2: Does Egeria density affect predation by largemouth bass when open water habitats are available?
Here, we investigated whether predation rate and prey choice by adult largemouth bass on four types of prey (juvenile striped bass in the open water, and juvenile largemouth bass, juvenile bluegill, and crayfish in the vegetation) were affected by Egeria density. We set up mesocosms so that prey and predators could have access to three types of microhabitat: vegetation (high or low density), edge of vegetation, or open water. We recorded habitat preference of all species, prey species consumed by predators, and prey mortality (e.g., found dead in the tank, with the cause of mortality unknown).
The experiment took place in August-September 2009 in four 8000-L outdoor flow-through pools (3.6 m diameter, 0.8 m deep, flow: 6 L/min, turbidity: 0–0.05 NTU). Half of the pool was kept clear, while the other half contained either a low (108 stems/m2) or a high (490 stems/m2) density of Egeria. Adult largemouth bass were arbitrarily paired at the beginning of the experiment and housed in 2800-L flow-through pools (2.1 m diameter, 0.8 m deep). We used a randomized block design, whereby each eight pairs of adult largemouth bass (the blocking factor) was used once in each of the two vegetation treatments (i.e., high or low density) in repeated trials, to achieve n = 8/treatment. To control for hunger level of the predator, the pairs were starved for 2 days prior to being used. Two weeks prior to starting the experiment, predatory largemouth bass were fed a mix of juvenile largemouth bass, juvenile bluegill, juvenile striped bass, and crayfish to ensure that predators had recent experience with all four types of prey.
At 1200 h, 10 juvenile largemouth bass, 10 juvenile bluegill, 10 juvenile striped bass, and 10 crayfish were added to the pool. After a 30-min acclimation period, two adult largemouth bass were added to the pool. Prey and predators were left to interact for 44 h (more time was allowed for the adult largemouth bass to acclimatize and to allow all subjects stabilize their habitat preferences in the larger pools used for Experiments 2 and 3). After this period, prey and predators were captured using seine and dip nets. The number of prey remaining from each species was counted; the pool was emptied, rinsed, and filled for the next trial. Surviving prey were returned to their initial holding tanks after the prey for the next trial were removed. We did not keep track of individual prey fish, so some prey may have been used in more than one replicate. We do not believe that reusing fish biased our results because behavioral observations (see below) on fish at the beginning of the experiment (when they were naïve to the treatments) yielded similar outcomes as our observations at the end of the experiment, when some fish were presumably reused.
Behavioral observations were conducted every 2 h between 0600 h and 0400 h (the next day) during the final 22 h of the trial. Observations consisted of scoring the position of each species in the pool (0: in the vegetation; 1: within 0.2 m of the edge of the vegetation; 2: in the open) to obtain information on habitat use for each species. All individuals of a given species were usually found within the same habitat type. In a few cases, juvenile largemouth bass were found both on the edge and in the open, so they were given a score of 1.5.
Experiment 3: Do Egeria density and turbidity interact to affect largemouth bass predation on open-water and vegetation-associated prey?
In this experiment, we investigated the effects of both Egeria density and turbidity levels on open water and vegetation prey. The experiment took place in October and November 2009 and followed a 2 × 2 design, using four pools, whereby species were exposed to a high or low density of Egeria (as in Experiment 2) and were maintained in either clear or turbid water (n = 12/treatment). As in Experiment 2, Egeria covered only half the pool, allowing species to choose their preferred microhabitat. Turbidity was created by adding 150 mL of nano-algae (Instant Algae, Nannochloropsis, Reed Mariculture, Campbell, CA), which yielded a Secchi reading of ~34 cm (with high vegetation: mean ± SD = 33.5 ± 2.7 cm, with low vegetation: 34.3 ± 3.9 cm, n = 64). Nannochloropsis is used at the delta smelt rearing facility to provide the fish with optimal feeding conditions in their holding tanks: the increase in turbidity results in an increased response to zooplankton feed added separately (J. Lindberg, personal communication). Readings using a turbidimeter indicated a turbidity level of 2.7 ± 0.5 NTU. We could clearly see the bottom of the pool (60 cm below water surface) in the low turbidity treatments, hence, we were unable to provide a meaningful Secchi reading (turbidimeter reading: 0.1 ± 0.1 NTU). Two air stones were positioned in the pool to ensure that the nano-algae would not settle, and flow was turned off during the trials to maintain turbidity levels. Secchi readings confirmed that the turbidity was held constant throughout the trials.
Due to the predator naivety of the delta smelt, we introduced one adult largemouth bass in the smelt holding pool when the smelt first arrived to the experimental facility, and let the bass interact with the delta smelt for 2 h. While we did not quantify predation by the bass during these acclimation trials, the smelt shoaled more tightly and clearly avoided the area of the pool containing the largemouth bass.
Pool size, starvation of adult largemouth bass prior to the trials, and blocking of predators for the study design was as described above for Experiment 2. We conducted a total of 48 trials.
At 1200 h, 10 juvenile largemouth bass, 10 juvenile bluegill, 30 delta smelt (average 56 ± 6 mm FL), and 10 crayfish were added to the pool. After a 20-min acclimation period, two adult largemouth bass were added to the pool. Prey and predators were left to interact for 44 h, after which, prey, predator, and vegetation were removed from the pool. The number of prey from each species was counted; the pool was emptied, rinsed, and filled for the next trial. Density of vegetation and turbidity were switched between pools to control for pool effects between trials.
After each trial, we found unconsumed or partially consumed smelt carcasses at the surface and at the bottom of the pools. To test whether this mortality was related to handling stress or to predation, we ran three additional predator-free trials in each of the four treatments, for which we introduced 30 delta smelt, 10 bluegill, and 10 crayfish in each of the pools and counted the number of surviving prey after 44 h. We did not introduce juvenile largemouth bass as we observed them pursuing smelt during our observations, and dead delta smelt were retrieved from the juvenile largemouth bass holding pool, presumably regurgitated by the juvenile bass. Thus, these trials provided an estimate of non-predation related mortality that we could use to correct the observed predation in the trials containing predators. After their last trials, juvenile and adult largemouth bass were sacrificed using a lethal dose of MS222. Stomach content analyses revealed that both juvenile and adult largemouth bass had consumed delta smelt.
The predator-free trials indicated that delta smelt death occurred in the absence of potential predators (mean mortality: 1.0 out of 30 in clear water, low vegetation, 1.6 in clear water, high vegetation, 0.3 in both turbid conditions). To account for this non-predation mortality, we used a corrected number of smelts in our analysis (total number of smelts dying due to predation = number of carcasses + number of missing smelts − mean death unrelated to predation in the treatment).
Information on habitat use of each species was obtained through observations conducted at dawn (~0715), 1000, 1300, 1600 and dusk (~1900 h) on the second day of the trial. As in Experiment 2, observations consisted of scoring the position of each species in the pool using the same scoring system. It was not possible to collect information on habitat preference in turbid pools; hence, only data from clear pools were collected.
Statistical analyses
To investigate whether vegetation density and/or turbidity affected total prey consumption by predators (regardless of the specific prey species consumed), we calculated the total number of prey eaten in each trial and applied a square-root transformation to normalize the data. Transformed data were tested for normality with a Shapiro-Wilk test. We then performed a one-way (two-way for Experiment 3) ANOVA followed by Tukey post-hoc tests (Experiment 1). We also calculated effect size for significant differences following the procedure in Cohen (1992). Specific predator individuals or pairs were included as a random factor in all analyses.
In addition to the initial ANOVA to determine the effect of experimental treatments on total prey consumption, we were also interested in whether vegetation density and/or turbidity level influenced the composition of prey species consumed by predators. To explore this question, we calculated the number of each prey species consumed in each trial, and applied a square-root transformation to normalize the data. We assumed that the numbers of prey eaten for each species were not independent of each other, so we analyzed them together using a MANOVA. We tested the MANOVA assumption of multivariate normality following the procedure in Burdenski (2000), in which the Mahalanobis distance (D2) is calculated for entire suite of prey species consumed, and the resulting values of D2 are graphically compared with the chi-square distribution. We performed a one-way (two-way for Experiment 3) MANOVA with the transformed proportion of each prey species consumed as a response variable (two for Experiment 1, four for Experiments 2 and 3). If the MANOVA indicated a significant treatment effect, it was followed by individual ANOVAs on each prey species to determine if multiple or a specific prey species were affected. Predators were introduced as a random factor in all the analyses.
Even after the square root transformation, not all the data for each experiment met the assumption of a normal distribution for the ANOVA on total prey consumption (non-normal data for Experiment 1) or the MANOVA on individual species consumption (non-normal data for Experiments 1 and 2). However, research to date indicates that both the ANOVA and MANOVA are robust against violations of the normality assumption (Finch 2005; Schmider et al. 2010). Indeed, Finch (2005) reports that when the normality assumption is violated, the parametric MANOVA slightly outperformed its non-parametric equivalent in terms of reducing the Type I error rate and maintaining statistical power.
To bolster the MANOVA analysis of a treatment effect on species composition of the consumed prey for Experiments 2, we also computed the relative selectivity for each prey type following Chesson (1983) to investigate whether prey choice by predators was affected by vegetation density. The relative selectivity index (α i ) is calculated as:
where n i0 is the number of prey of type i at the beginning of the experiment, r i is the number of prey type i that were consumed by the predator, and m is the total number of different prey types.
This selectivity index ranges from 0 (total avoidance of prey type) to 1 (only prey type selected), and can be interpreted as the preference for one prey type relative to the average preference for alternative prey types. These values were then used in a one-way ANOVA to analyze the effect of habitat characteristics on predator selectivity. This analysis approach has been used previously to investigate the effect of increasing turbidity on prey selectivity by largemouth bass (Shoup and Wahl 2009). We did not conduct the selectivity analysis for Experiment 3 because of our observation that juvenile largemouth bass consumed delta smelt in addition to the adults. Since the selectivity analysis requires an assumption of equivalent encounter rates with predators, and delta smelt faced a larger number of predators than other prey types, it was not appropriate for Experiment 3.
Finally, to assess whether species microhabitat use differed between vegetation treatments (Experiments 2 and 3 only), we computed the average position score for each species in each trial, and applied an arcsine transformation to normalize the data. The position of each species may be related, so we performed a MANOVA, using the four species’ positions scores as our response variables. For Experiment 3, the effect of turbidity on species microhabitat use could not be assessed, since no observations could be carried out in the turbid pools.
Results
Experiment 1: Does Egeria density affect predation by largemouth bass in the absence of open water habitats?
The square root transformation did not successfully normalize the data (low density, P = 0.019; high density, P < 0.0001); however, the ANOVA results are still robust in the case that the data are not normal (Schmider et al. 2010). The one-way ANOVA revealed a significant effect of Egeria density on the total number of prey consumed during the trials (F2,16 = 5.6, P = 0.015), with no effect of the individual predator as a blocking factor (F8,16 = 1.0, P = 0.46). Post-hoc tests indicated a decrease in overall prey consumption as density of Egeria increases (no vegetation vs. high density: P = 0.012, Cohen’s d measure of effect size = −1.52 Fig. 1).
Does vegetation density affect the consumption of specific vegetation-associated species by predators? The number of prey consumed decreased with increased Egeria density (MANOVA, Pillai’s Trace: F4,32 = 2.7, P = 0.050, Fig. 1), with no effect of the individual predator (F16,32 = 1.5, P = 0.18). Univariate analyses found that an increase in vegetation density led to a decrease in the consumption of bluegill (ANOVA, F2,16 = 4.2, P = 0.035), but not of juvenile largemouth bass (F2,16 = 1.9, P = 0.19, Fig. 1). When the two vegetation treatments were combined [no vegetation vs. vegetation (low + high)], we found that the presence of vegetation marginally reduced the consumption of juvenile largemouth bass (F1,17 = 3.5, one-tailed P = 0.04), but we also found an effect of predator block for juvenile largemouth (F8,17 = 3.0, P = 0.027) that was not present for bluegill (F8,17 = 0.71, P = 0.68). Due to a very low consumption of prey in the high density treatment (the predator did not consume any prey in 7 of 9 trials), we could not compare prey preference between vegetation treatments.
Experiment 2: Does Egeria density affect predation by largemouth bass when open water habitats are available?
The transformed data on total prey consumption was normally distributed (low density, P = 0.117; high density, P = 0.230). There was no effect of vegetation density on the total number of prey consumed (ANOVA, F1,7 = 0.5, P = 0.82, Fig. 2) and no effect of predator block (F7,7 = 2.5, P = 0.13). Similarly, multivariate analyses revealed no effect of vegetation density on the consumption of any prey type (MANOVA, Pillai’s Trace: F4,4 = 1.4, P = 0.37, Fig. 2) or predator block (F28,28 = 1.38, P = 0.20), and no effect of vegetation density on fish microhabitat use (Pillai’s Trace: F4,11 = 0.2, P = 0.93, Fig. 3). As there was no effect of vegetation density or predator block, we did not run ANOVAs on individual prey species. Visual inspection of the habitat preference scores indicated that juvenile largemouth bass, juvenile bluegill, and crayfish were located in the Egeria, while striped bass preferred open water. Adult largemouth bass were observed mainly in the Egeria.
Because the vegetation-associated prey species (juvenile largemouth, juvenile bluegill, and crayfish) were consumed at such a low frequency that meaningful selectivity indices could not be calculated on the individual species, we combined them to compute the selectivity of predators for vegetation-associated species versus striped bass located in the open water. However, there was no effect of either vegetation density (ANOVA, F1,5 = 1.0, P = 0.36) or predator blocks (F7,5 = 0.79, P = 0.63) on predator selectivity. Regardless of vegetation density, predators preferentially consumed species associated with open water, i.e., striped bass (Fig. 2).
Experiment 3: Do Egeria density and turbidity interact to affect largemouth bass predation on open-water and vegetation-associated prey?
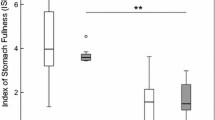
The transformed data on total consumption were normally distributed in all treatments (clear-low vegetation, P = 0.321; clear-high, P = 0.657; turbid-low, P = 0.567; turbid-high, P = 0.471). The total number of prey consumed decreased with increasing turbidity (two-way ANOVA, F1,11 = 11.0, P = 0.007, Cohen’s d measure of effect size = −0.66 Fig. 4), but there was no effect of vegetation density (F1,11 = 1.68, P = 0.22), and no interaction (F1,11 = 1.63, P = 0.23) on the total number of prey consumed by the predators. We also found a predator block effect (F11,11 = 3.8, P = 0.016), an interaction between block and turbidity (F11,11 = 5.78, P = 0.004), but no interaction between block and vegetation density (F11,11 = 1.5, P = 0.26). Species-specific analyses and the MANOVA on whether treatments influenced the number of individuals consumed of each prey species were not run for this experiment because delta smelt were the main species consumed by largemouth bass, while juvenile largemouth bass, juvenile bluegill, and crayfish made up a negligible proportion of the prey eaten (Fig. 4).
As described above, we observed that delta smelt faced a higher risk of predation compared to the other prey species because delta smelt were consumed by both adult largemouth bass and juvenile largemouth bass (which did not consume the other prey species available). However, between experimental treatments, the number of potential predators of delta smelt was the same, and the ANOVA indicates a clear effect of turbidity on the number of delta smelt surviving to the conclusion of the trials.
Does vegetation density affect species microhabitat use? As crayfish always stayed in the vegetation (mean score = 0, SD = 0), we removed the crayfish habitat use data from the analysis. Vegetation density did not affect microhabitat use of the other species (MANOVA, Pillai’s Trace: F3,20 = 0.50, P = 0.69, Fig. 5). Visual inspection of the mean scores indicated that juvenile largemouth, juvenile bluegill, and crayfish were mainly located in the Egeria, while delta smelt preferred the open water. Adult largemouth bass were observed mainly in the Egeria. In the predation-free trials (all species present except largemouth bass), habitat associations of delta smelt, bluegill, and crayfish, were all similar to their associations in the presence of largemouth bass.
Discussion
The results of our study provide insights into how predation by introduced largemouth bass on vegetation-associated prey, as well as open water species, is influenced by the habitat-altering characteristic of an invasive macrophyte. Our first experiment indicated that when confined in Egeria, predatory largemouth bass suffer reduced foraging rates, as seen by the decrease in total prey consumption in vegetated pools. A density of 480 stems/m2 led to a complete suppression in feeding by the largemouth bass in 78 % of the trials, which is in accordance with previous studies investigating the effect of habitat complexity on centrarchid foraging success (Savino and Stein 1982). Thus, Egeria provides a refuge for juvenile largemouth bass and other centrarchids, in line with results from Durocher et al. (1984), which showed that largemouth bass recruitment is directly proportional to the density of submerged vegetation. The relationship between habitat preference and survival of planktivorous fish in response to piscivorous predators has been observed and reported in many studies (Sih 1987; Turner and Mittelbach 1990). Although these concepts are not novel, our results highlight the dual role of the invasive macrophyte on invasive largemouth bass: Egeria provides a refuge from predators to the early life stages of centrarchids thereby increasing their recruitment, while decreasing the foraging efficiency of the adults.
The results of Experiment 2 and 3 indicated, however, that when open water prey are available, and predators and prey have a choice between vegetation and open water habitats, macrophyte density did not affect predator foraging success or prey survival. Instead, we found that adult (as well as juveniles in Experiment 3) largemouth bass foraged almost exclusively on species associated with open water (striped bass and delta smelt), explaining why macrophyte density did not affect their foraging success. While it is important to note that striped bass and delta smelt were the only captively-reared prey species in these experiments, the results nonetheless corroborate our hypothesis that largemouth bass preferentially forage in open water habitat, rather than vegetated habitat. Indeed, stomach content analyses performed on wild-caught largemouth bass sampled from vegetated habitats in the Delta, and elsewhere, have shown that juvenile largemouth bass, bluegill, and crayfish are among the most common prey species (Nobriga and Feyrer 2007; Weinersmith, unpubl. data). Thus, the predators used in our experiments most likely had a well-developed search image for these vegetation-associated species, yet they selected prey in the open water when they were available.
The results of Experiment 3 also show that turbidity provides a predation refuge for open water species, such as delta smelt, since smelt mortality due to predation was higher under clear conditions than under turbid conditions. There is a growing literature showing turbidity-mediated alterations in predator–prey interactions (Gregory 1993; Bonner and Wilde 2002; Lehtiniemi et al. 2005; VanLandeghem et al. 2011). Turbidity degrades transmission of visual information, and consequently has strong negative effects on capture success of some predators (Mazur and Beauchamp 2003; Zamor and Grossman 2007; Huenemann et al. 2012). For instance, Shoup and Wahl (2009) showed that prey selectivity by largemouth bass was altered under turbid conditions. This experiment also had the somewhat problematic result that juvenile largemouth bass were observed to consume adult delta smelt. While this unanticipated result made the selectivity analysis untenable, this is an important observation which suggests that given suitable conditions, juvenile largemouth bass may be predators of delta smelt just as adult largemouth bass may be. This observation suggests that where adult or even juvenile delta smelt may overlap in habitat and range with juvenile largemouth bass, they may be subject to a significant predation risk.
Native species in the Delta and striped bass prefer more turbid environments (Feyrer and Healey 2003), while many of the invasive species prefer relatively clear environments. This distribution difference may reflect alternate antipredator strategies. Although Egeria provided a refuge from predators to other species, neither striped bass nor delta smelt were found to use it, even in the face of likely predation. This observation suggests that the historic turbidity of the Delta may have provided enough of a refuge from predators, leading open water species not to seek additional cover. This also suggests that a decrease in Delta turbidity levels, driven to some degree by the spread of Egeria and other submerged aquatic vegetation (Hestir 2010), may leave native species helpless in the face of piscivores by decreasing the availability of optimal habitat and by making these species more susceptible to predation. Taken together, the results of our three experiments suggest that the two invasive species (Egeria densa and largemouth bass) have the potential to interact synergistically to aggravate the decline of native species. Largemouth bass were introduced to the Delta prior to 1900 (Moyle 2002) but in the absence of refuge for early-stage juveniles, the presence of largemouth bass per se was not likely a drastic source of mortality for native species. Egeria densa’s role as a habitat-modifier is well established (Mazzeo et al. 2003), and the rapid proliferation of Egeria in the past 30 years (Brown and Michniuk 2007) has provided an extensive predator refuge for early-stage centrarchids while decreasing open water habitat. This suggests a positive interaction between Egeria and largemouth bass through increased largemouth bass recruitment.
It is important to note that due to the life history strategies and recent drastic decline of these open water fishes, the current spatial and temporal overlap between these open water species and largemouth bass may be limited. However, recent evidence indicates that juvenile striped bass have undergone a general shift in distribution from offshore areas to inshore, shallower habitat, possibly due to a relatively higher food supply in the latter areas (Sommer et al. 2011). In addition, recent work on wild delta smelt has shown that they will move inshore or offshore with the tide, depending on whether they are trying to move upstream, downstream, or maintain their position (W. Bennett, pers. comm.). When they move into shoreline habitats, they may come in close proximity with Egeria and largemouth bass; however, as results from this study indicate, delta smelt are more likely to seek unvegetated areas. The frequency and duration of spatial overlap between adult largemouth bass, juvenile striped bass, and/or delta smelt will determine the extent to which largemouth bass are important predators of these species. To place the results of these mesocosm studies in context, we are also completing an analysis of an extensive field survey to determine the extent to which largemouth bass overlap with open water fish species throughout the Sacramento-San Joaquin Delta. Additionally, we are examining the diets of largemouth bass throughout the Delta from habitats that differ in Egeria density and turbidity to determine when and how often open water species are found in their diets.
Conservation and management scientists have acknowledged that multiple factors determine the success of invaders and their effects on native ecosystems (Facon et al. 2006). However, even while employing a generally more informative multi-criterion approach, interactive effects are often overlooked (Moffett and Sarkar 2006). While over 11,000 studies have focused on the effects of habitat modification, and 3,500 on the role of invaders on biodiversity loss, only 1.2 % of those publications has ever studied them together, and less than 0.03 % have mentioned any interactive effects between the two drivers (Didham et al. 2005). The present study, although not capturing all the complexities of the Delta, provides some insights on how these two factors may interact. Studying the effects of multiple drivers simultaneously may reveal highly complex interactions that must be understood to make better management decisions.
References
Bonner TH, Wilde GR (2002) Effects of turbidity on prey consumption by prairie stream fishes. Trans Am Fish Soc 131:1203–1208. doi:10.1577/1548-8659(2002)131<1203:eotopc>2.0.co;2
Brown LR, Michniuk D (2007) Littoral fish assemblages of the alien-dominated sacramento—San Joaquin Delta, California, 1980–1983 and 2001–2003. Estuaries Coasts 30:186–200
Burdenski T (2000) Evaluating univariate, bivariate, and multivariate normality using graphical and statistical procedures. Multiple Linear Regression Viewpoints 26:15–28
Carlton JT, Geller JB, Reaka-Kudla ML, Norse EA (1999) Historical extinctions in the sea. Annu Rev Ecol Syst 30:515–538. doi:10.1146/annurev.ecolsys.30.1.515
Chesson J (1983) The estimation and analysis of preference and its relationship to foraging models. Ecol 64:1297–1304
Cohen J (1992) A power primer. Psychol Bull 112:155–159
Crooks JA (2002) Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97:153–166
Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM (2007) Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol Evol 22:489–496. doi:10.1016/j.tree.2007.07.001
Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ (2005) Are invasive species the drivers of ecological change? Trends Ecol Evol 20:470–474. doi:10.1016/j.tree.2005.07.006
Douglas ME, Marsh PC, Minckley WL (1994) Indigenous fishes of western North-America and the hypothesis of competitive displacement - Meda fulgida (Cyprinidae) as a case study. Copeia 9–19
Durocher PP, Provine WC, Kraai JE (1984) Relationship between abundance of largemouth bass and submerged vegetation in Texas reservoirs. N Am J Fish Manag 4:84–88
Facon B, Genton BJ, Shykoff J, Jarne P, Estoup A, David P (2006) A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol Evol 21:130–135. doi:10.1016/j.tree.2005.10.012
Feyrer F, Healey MP (2003) Fish community structure and environmental correlates in the highly altered southern Sacramento-San Joaquin Delta. Environ Biol Fish 66:123–132. doi:10.1023/a:1023670404997
Feyrer F, Nobriga ML, Sommer TR (2007) Multidecadal trends for three declining fish species: habitat patterns and mechanisms in the San Francisco Estuary, California, USA. Can J Fish Aquat Sci 64:723–734
Finch H (2005) Comparison of the performance of nonparametric and parametric MANOVA test statistics when assumptions are violated. Methodol Eur J Res Methods Behav Soc Sci 1:27–38
Gregory RS (1993) Effect of turbidity on the predator avoidance behaviour of juvenile chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 50:214–246
Hestir EL (2010) Trends in estuarine water quality and submerged aquatic vegetation invasion. Dissertation, University of California, Davis
Holway DA, Suarez AV (1999) Animal behavior: an essential component of invasion biology. Trends Ecol Evol 14:328–330
Hoyle JA, Keast A (1987) The effect of prey morphology and size on handling time in a piscivore, the largemouth bass (Micropterus salmoides). Can J Zool 65:1972–1977
Huenemann TW, Dibble ED, Fleming JP (2012) Influence of turbidity on the foraging of largemouth bass. Trans Am Fish Soc 141:107–111
Huskey SH, Turingan RG (2001) Functional and morphological bases of intraspecific variation in the feeding ecomorphology of largemouth bass, Micropterus salmoides. Am Zool 41:1479–1479
Johnson PTJ, Olden JD, vander Zanden MJ (2008) Dam invaders: impoundments facilitate biological invasions into freshwaters. Front Ecol Environ 6:359–365. doi:10.1890/070156
Lehtiniemi M, Engstrom-Ost J, Viitasalo M (2005) Turbidity decreases anti-predator behaviour in pike larvae, Esox lucius. Environ Biol Fish 73:1–8. doi:10.1007/s10641-004-5568-4
Lockwood JL, Hoopes MF, Marchetti MP (2007) Invasion ecology. Blackwell, London
Mazur MM, Beauchamp DA (2003) A comparison of visual prey detection among species of piscivorous salmonids: effects of light and low turbidities. Environ Biol Fish 67:397–405. doi:10.1023/a:1025807711512
Mazzeo N et al (2003) Effects of Egeria densa Planch. beds on a shallow lake without piscivorous fish. Hydrobiologia 506:591–602. doi:10.1023/b:hydr.0000008571.40893.77
Moffett A, Sarkar S (2006) Incorporating multiple criteria into the design of conservation area networks: a minireview with recommendations. Divers Distrib 12:125–137. doi:10.1111/j.1366-9516.2005.00202.x
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci United States Am 98:5446–5451. doi:10.1073/pnas.091093398
Moyle PB (2002) Inland fishes of California. University of California Press, California
Moyle PB, Herbold B, Stevens DE, Miller LW (1992) Life history and status of delta smelt in the Sacramento-San Joaquin estuary, California. Trans Am Fish Soc 121:67–77. doi:10.1577/1548-8659(1992)121<0067:lhasod>2.3.co;2
Nobriga ML, Feyrer F (2007) Shallow-water piscivore-prey dynamics in California’s Sacramento-San Joaquin Delta. San Franscisco Estuary and Watershed Sci 5
Nobriga ML, Sommer TR, Feyrer F, Fleming K (2008) Long-term trends in summertime habitat suitability for delta smelt (Hypomesus transpacificus). San Francisco Estuary and Watershed Sci 6
Porter SD, Savignano DA (1990) Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecol 71:2095–2106. doi:10.2307/1938623
Race MS (1982) Competitive displacement and predation between introduced and native mud snails. Oecologia 54:337–347. doi:10.1007/bf00380002
Rhymer JM, Simberloff D (1996) Extinction by hybridization and introgression. Annu Rev Ecol Syst 27:83–109. doi:10.1146/annurev.ecolsys.27.1.83
Sala OE et al (2000) Biodiversity—Global biodiversity scenarios for the year 2100. Sci 287:1770–1774. doi:10.1126/science.287.5459.1770
Savino JF, Stein RA (1982) Predator–prey interaction between largemouth bass and bluegills as influenced by simulated, submersed vegetation. Trans Am Fish Soc 111:255–266. doi:10.1577/1548-8659(1982)111<255:piblba>2.0.co;2
Schmider E, Ziegler M, Danay E, Beyer L, Bühner M (2010) Is it really robust? Reinvestigating the robustness of ANOVA against violations of the normal distribution assumption. Methodol Eur J Res Methods Behav Soc Sci 6:147–151
Shoup DE, Wahl DH (2009) The effects of turbidity on prey selection by piscivorous largemouth bass. Trans Am Fish Soc 138:1018–1027
Sih A (1987) Predators and prey lifestyles: an evolutionary overview. In: Kerfoot WC, Sih A (eds) Predation: direct and indirect impacts on aquatic communities. New England University Press, Hanover, pp 203–224
Sih A et al (2010) Predator–prey naivete, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621. doi:10.1111/j.1600-0706.2009.18039.x
Sih A, Ferrari MCO, Harris D (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4:367–387
Sommer T et al (2007) The collapse of pelagic fishes in the Upper San Francisco Estuary. Fish 32:270–277. doi:10.1577/1548-8446(2007)32[270:tcopfi]2.0.co;2
Sommer T, Mejia F, Hieb K, Baxter R, Loboschefsky EJ, Loge FJ (2011) Long-term shifts in the lateral distribution of age-0 striped bass Morone saxatilis in the San Francisco estuary. Trans Am Fish Soc 140:1451–1459
Turner AM, Mittelbach GG (1990) Predator avoidance and community structure—interactions among piscivores, planktivores, and plankton. Ecol 71:2241–2254. doi:10.2307/1938636
United States Fish and Wildlife Service (1993) Determination of threatened status for the delta smelt. Federal register 58:12854–12864
VanLandeghem MM, Carey MP, Wahl DH (2011) Turbidity-induced changes in emergent effects of multiple predators with different foraging strategies. Ecol Freshw Fish 20:279–286
Vitousek PM (1994) Beyond global warming—Ecology and global change. Ecol 75:1861–1876
Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E (1998) Quantifying threats to imperiled species in the United States. Biosci 48:607–615. doi:10.2307/1313420
Wright SA, Schoellhamer DH, David H (2004) Trends in the sediment yield of the Sacramento River, California, 1957–2001. San Franscisco Estuary and Watershed Sci 2
Yarrow M, Marin VH, Finlayson M, Tironi A, Delgado LE, Fisher F (2009) The ecology of Egeria densa Planchón (Liliopsida: Alismatales): a wetland ecosystem engineer? Rev Chil Hist Nat 82:299–313
Zamor RM, Grossman GD (2007) Turbidity affects foraging success of drift-feeding rosyside dace. Trans Am Fish Soc 136:167–176. doi:10.1577/t050316.1
Acknowledgements
We thank Andrew Bibian, Talene Baghdassarian, Ann Chang, Erik Hallen, and Paul Lutes for their help with animal care and technical assistance with the trials. We thank Joan Lindberg and Luke Ellison at the Fish Conservation and Culture Laboratory of the University of California Davis for supplying the delta smelt for the study and for their guidance in caring for delta smelt. This study was conducted under the University of California Davis Animal care protocol number: 15422. Financial support was provided by the Interagency Ecological Program and the US Bureau of Reclamation (R10AC20090) to LC, AS, KW and MY, and by NSERC to MF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrari, M.C.O., Ranåker, L., Weinersmith, K.L. et al. Effects of turbidity and an invasive waterweed on predation by introduced largemouth bass. Environ Biol Fish 97, 79–90 (2014). https://doi.org/10.1007/s10641-013-0125-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-013-0125-7