Abstract
Background
Neoadjuvant and adjuvant immune checkpoint inhibitor treatments for non-small cell lung cancer (NSCLC) patients with resectable disease have presented promising results. This is a phase I study to evaluate the safety and efficacy of neoadjuvant toripalimab in combination with chemotherapy for NSCLC.
Methods
Treatment-naive patients with resectable NSCLC (stage II–IIIB) received two to four cycles of toripalimab (240 mg, intravenously, q3w) combined with platinum-paclitaxel chemotherapy. Surgical operation was performed approximately 4 weeks after the last cycle. The primary end point was safety. The efficacy endpoints included radiographic and pathological response rates, expression of programmed death ligand 1 (PD-L1) and molecular targets.
Results
A total of 11 patients were enrolled, consisting of 2 patients (18%) with adenocarcinoma and 9 patients (82%) with squamous cell carcinoma. All patients received two to four cycles of toripalimab plus chemotherapy and underwent radical resection. Regarding safety, 5 of 11 patients (45%) had neoadjuvant treatment-related adverse events, and 1 patient (9%) experienced grade 3 or worse treatment-related adverse events. Radiographic partial response was achieved in 10 patients, with an objective response rate of 91%. Among 11 patients, 6 (55%) achieved pathological complete response, including 1 PD-L1-negative patient.
Conclusion
Neoadjuvant toripalimab plus platinum-paclitaxel chemotherapy was tolerable and induced a pathological complete response in 55% of resectable NSCLC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the most commonly diagnosed cancers and the leading cause of cancer death worldwide. In 2020, it was estimated that there were 2.2 million cases of lung cancer and 1.8 million deaths [1]. In China, lung cancer accounted for approximately 828,000 new cases and approximately 657,000 deaths in 2016 [2]. Among all pathological types of lung cancer, non-small cell lung cancer (NSCLC) is still the predominant type. For early and locally advanced stage NSCLC, surgical resection remains the cornerstone treatment strategy, yet approximately 30-55% of patients may relapse after surgery [3]. Neoadjuvant chemotherapy in NSCLC shows a modest outcome, and an absolute improvement of 5.4% was achieved in the 5-year survival compared with that of surgery alone. However, approximately 60% of patients experience grade 3 or worse toxicity [4].
Immune checkpoint inhibitors (ICIs), especially antibodies targeting the immune inhibitory pathway of programmed death 1 (PD-1) protein, have shown remarkable efficacy in the treatment of advanced NSCLC. Initiating ICIs before resection may leverage high tumor-associated antigen levels and enhance the T-cell priming and expansion of tumor-specific T-cell clones [5, 6]. Sustained antitumor immunity could be activated and persist beyond the time of resection [7,8,9], which may result in tumor regression and the improvement of survival rates.
Clinically, neoadjuvant anti-PD-1/PD-L1 antibody monotherapy in early-stage NSCLC has shown promising efficacy according to the results of several studies. The Checkmate 159 study [10] is the first neoadjuvant immunotherapy study of NSCLC aiming to explore the safety and efficacy of neoadjuvant nivolumab in resectable stage I-IIIA NSCLC. The results showed a major pathological response (MPR) rate of 45% and a pathological complete response (pCR) rate of 10% in the enrolled patients. Only 5% (1 of 20) of patients experienced treatment-related adverse events (TRAEs) of grade 3 or worse, and no delay of surgery occurred. In addition, several trials, including MK3475-223, LCMC3 and loNESCO IFCT-1601, have explored the safety and efficacy of pembrolizumab, atezolizumab and durvalumab, respectively, for resectable NSCLC [11]. Compared with neoadjuvant monotherapy, neoadjuvant regimens consisting of ICIs plus chemotherapy might be more effective. In addition, the NADIM study [12] showed encouraging results with an MPR rate of 83% and an R0 resection rate of 100% for neoadjuvant nivolumab plus chemotherapy in NSCLC. A 24% pCR rate, 36.9% MPR rate and 54% objective response rate (ORR) were achieved in the neoadjuvant nivolumab plus platinum-based dual chemotherapy group of the CheckMate 816 study, and this treatment has been approved for the neoadjuvant therapy of resectable NSCLC by the National Comprehensive Cancer Network (NCCN) [13]. In addition, in the NEOSTAR study [14] focusing on the combination of nivolumab plus the anti-CTLA4 antibody ipilimumab for the neoadjuvant therapy of NSCLC, 33% of patients achieved an MPR or pCR.
As a monoclonal antibody targeting PD-1, toripalimab has been approved in locally advanced or metastatic melanoma, recurrent or metastatic nasopharyngeal carcinoma, and urothelial carcinoma in China. Neoadjuvant toripalimab in locally advanced colorectal cancer has shown tolerable safety and promising efficacy [15]. In a phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III NSCLC, toripalimab plus platinum-based doublet chemotherapy yielded a high MPR rate, manageable toxicity, and feasible resection rate [16]. The current study aims to assess the safety and efficacy of neoadjuvant toripalimab plus platinum-paclitaxel chemotherapy in resectable stage II-IIIB NSCLC to provide more evidence for neoadjuvant immunotherapy.
Methods
Study design
This study was a single-center, single-arm, phase I study. The inclusion criteria were as follows: (a) Eligible patients were 18 to 75 years of age, and (b) had histologically and cytologically confirmed stage II-IIIB NSCLC, which was considered to be surgically resectable. Besides, (c) all the patients had an Eastern Cooperative Oncology Group performance status score of 0 to 1 (on a 5-point scale, a higher score indicates more severe disabilities), (d) adequate pulmonary function and other organ functions [17]. The key exclusion criteria were as follows: (a) ongoing systemic immunosuppressive therapy; (b) active autoimmune diseases and previous allogeneic organ transplantation or hemopoietic stem cell transplantation; (c) hypersensitivity to any monoclonal antibodies; (d) history of interstitial lung disease or active and uncontrolled infection; (e) grade III to grade IV congestive heart failure; (f) uncontrolled hypertension or uncontrolled hypercalcemia; (g) artery thrombosis, embolism, or ischemia within 6 months before study treatment; (h) coagulation disorders requiring warfarin treatment; and (i) any other known malignant tumor.
The enrolled patients received two to four cycles of toripalimab (240 mg, every 3 weeks, intravenous) plus standardized platinum-paclitaxel chemotherapy. For patients with N0 disease, surgical resection was conducted after two cycles treatment without disease progression (assessed per RECIST version 1.1). For patients with N1-2 disease, one or two further cycles would be given before surgical resection based on judgement by multidisciplinary diagnosis and treatment. Surgical operation was planned to be performed approximately 4 weeks after the last cycle of combined therapy. The study was performed on the basis of the Declaration of Helsinki and Good Clinical Practice. The Ethics Committee of the Affiliated Hospital of Qingdao University reviewed and approved the protocol and amendments of our study (QYFYKYLL471311920). All patients provided written informed consent. Contrast-enhanced computed tomography (CT) was performed to evaluate baseline tumor staging. Contrast-enhanced CT was performed 1 month after surgery and then every 3 months until disease progression, death, or 2 years after surgery.
Pathological assessments
Surgical samples of primary tumors from the lung and lymph nodes were staged according to the criteria of the American Joint Committee on Cancer (eighth edition). Routine hematoxylin and eosin staining was used to identify the percentage of residual viable tumors and to assess primary tumors [18]. If no viable tumor cells were found in the surgical sample of a patient, pCR was considered to have been achieved [19]. The expression of programmed death ligand 1 (PD-L1) and molecular targets was evaluated. Tumor biopsies were obtained before treatment and during surgery. PD-L1 expression was detected using immunohistochemical staining with primary antibodies against PD-L1 (22C3) in 10 of 11 patients. Moreover, genetic testing was performed using next-generation sequencing in 9 of 11 patients. The details of the PD-L1 expression assay and the results of genetic testing of the enrolled patients are listed in Table 1.
Outcome evaluation
The primary end point was safety, and all the patients were monitored for TRAEs according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0 [20, 21]. The efficacy endpoints included radiographic and pathological response rates, including the ORR and pCR rate. Radiographic response evaluation was conducted by the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 [22].
Results
Patients and treatment
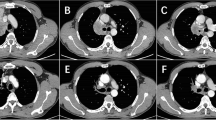
From October 15th, 2019, through July 28th, 2021, a total of 11 patients were enrolled. All of these patients were eligible according to the inclusion criteria of the study. Among the 11 enrolled patients, 9 patients (81.8%) had squamous cell carcinoma and were staged II to IIIA (according to the eighth edition of the TNM Classification for Lung Cancer-IASLC) [23]. A total of 18.2% (2/11) of patients had potentially resectable adenocarcinoma of stage IIIB, including one patient with stage T3N2M0 and another patient with stage T4N2M0. Lymph node involvement was found in 72.7% (8 of 11) of patients, and 90.9% (10 of 11) of patients were current or former smokers. The basic information of the enrolled patients is listed in Table 1; Fig. 1.
Clinical activity and pathological response
After two to four cycles of preoperative toripalimab plus platinum-paclitaxel chemotherapy, 10 patients (91.9%) had a radiographic PR with an ORR of 91%, and 1 patient (9.1%) had stable disease (SD). All enrolled patients underwent R0 resection with lobectomy and lymphadenectomy. There were no treatment-related delays of surgical operation. The median interval between the last dose of toripalimab plus platinum-paclitaxel chemotherapy and surgery was 35 days (range from 27 days to 46 days). Among the 11 patients with evaluable surgical tumor tissue samples, 6 patients (55%) achieved pCR, including 5 patients with squamous cell carcinoma and 1 patient with adenocarcinoma. One patient had pathological SD after neoadjuvant therapy. Among the 6 patients who achieved pCR, there was one patient with negative expression of PD-L1 protein. The outcomes of patients who received neoadjuvant therapy are displayed in Fig. 1.
After surgical operation, 10 patients received adjuvant treatment, among whom 4 patients received sustained toripalimab treatment up to 1 year. Significantly, the postoperative pathology of one patient showed complex small cell carcinoma, and therefore, this patient was given four cycles of adjuvant chemotherapy with an etoposide-platinum regimen. By the last follow-up on May 20th, 2022, none of the enrolled patients experienced recurrence. The median follow-up time was 20.40 months, and the recurrence-free survival (RFS) of this cohort of patients was not reached.
Safety and Feasibility
Neoadjuvant toripalimab plus platinum-paclitaxel chemotherapy was not associated with any previously unreported toxic side effects in this study. During the period of neoadjuvant treatment, TRAEs of any grade occurred in 45% of patients (5 of 11 patients), and 18% of patients (2 of 11 patients) experienced grade 3 or worse TRAEs. Among the TRAEs, neutropenia and alanine aminotransferase increased were the two most common TRAEs, both with an incidence of 27% (2 of 11 patients), while grade 3 or higher neutropenia occurred in 27% of patients (2 of 11 patients). The detailed information for TRAEs is summarized in Table 2.
PD-L1 expression analysis and genetic testing
PD-L1 expression was evaluated in 10 patients. pCR occurred in both PD-L1-positive and PD-L1-negative tumors. Additionally, routine genetic testing for NSCLC (including EGFR, ALK, MET, HER2, ROS1, BRAF, RET, NTRK1, PIK3CA, and MEK1) was conducted using surgical resection samples in 7 of 11 patients. None of these patients harbored common driver genetic alterations except for one patient with a KRAS mutation.
Discussion
Neoadjuvant therapy transforms locally advanced NSCLC into resectable disease and improves the prognosis of patients. The PD-1 inhibitor-based neoadjuvant regimen achieved satisfactory efficacy and tolerable safety in neoadjuvant treatment for NSCLC according to the latest studies [12,13,14]. Toripalimab, a monoclonal antibody targeting PD-1, is also considered a choice for neoadjuvant immunotherapy of NSCLC and demonstrated robust efficacy based on a series of small-sample-size trials [16], as shown in Table 3. Nearly all enrolled patients (96.7-100%) underwent R0 resection after toripalimab-based neoadjuvant therapy combined with standardized chemotherapy. Regarding the safety of neoadjuvant therapy, although TRAEs commonly occurred in the enrolled patients with incidences of 22.2-87.5%, few patients (0-18.2%) had grade 3 or worse TRAEs and no treatment-related deaths occurred in these studies. In addition, toripalimab-based neoadjuvant therapy also achieved perfect performance in changing the outcomes of the enrolled patients. The MPR rate of these studies ranged from 40.90 to 66.70%, and the pCR rate ranged from 18.2 to 50%. In this study, the neoadjuvant administration of toripalimab plus platinum-paclitaxel chemotherapy in patients with stage II-IIIB resectable NSCLC was associated with tolerable adverse events (any TRAEs: 45.5%; grade 3 or worse TRAEs: 18.2%), and pCR was achieved in 55% of patients. Most inspiringly, we observed six patients who obtained pCR for primary tumors, including 5 squamous cell NSCLCs and 1 adenocarcinoma, which included one PD-L1 expression-negative patient, suggesting that PD-1 inhibitor-based neoadjuvant therapy could benefit NSCLC patients regardless of the immunogenic profiles, including the expression level of PD-L1.
In addition, toripalimab-based neoadjuvant therapy for resectable NSCLC patients might induce a better RFS after surgery and adjuvant therapy with or without sustained immunotherapy. Based on the results of the final follow-up, no recurrences were found in the enrolled patients in the TOGATHER study and our study after median follow-up times of 6.00 months and 20.40 months, respectively. Nevertheless, due to the short follow-up time, whether this high pCR rate and long RFS time with neoadjuvant therapy with toripalimab and chemotherapy will lead to an improvement in the overall survival of resectable NSCLC patients still warrants long-term follow-up and exploration.
Additionally, according to the genetic testing results in our study, we found that among the 6 patients who achieved pCR, 1 had TP53 mutations with a PD-L1 expression of 10% and a pathological type of squamous cell carcinoma. Although evidence has shown that in lung adenocarcinoma, TP53 and KRAS mutations may predict the efficacy of anti-PD-1/PD-L1 immunotherapy [24], there is still no general agreement in squamous NSCLC, and more in-depth studies are needed to define the role of these molecular targets in NSCLC. Indeed, there are some limitations of our study: the small number of patients enrolled in our study and the short postoperative follow-up period; the postoperative adjuvant treatment groups were not randomized, and not all the patients received toripalimab treatment, which causes a bias in evaluating the DFS rate; and not all the patients had available detection results of PD-L1 expression and genetic testing, which limited the accuracy of the predictive analysis. Admittedly, the small sample size without rigorous estimation in this study is hard to draw robust conclusion. Further research is needed to identify patients who may benefit most from toripalimab plus chemotherapy regimens, and adequately powered trials are awaited to establish clinically meaningful benefits.
Conclusion
In conclusion, our findings suggested that neoadjuvant toripalimab plus platinum-paclitaxel chemotherapy for resectable NSCLC patients was tolerable, and the high pCR rate (55%) is inspiring.
Availability of supporting data
All data generated during this study are included in this published article.
Abbreviations
- NSCLC:
-
non-small cell lung cancer
- ICIs:
-
immune checkpoint inhibitors
- PD-1:
-
programmed death 1
- MPR:
-
major pathological response
- pCR:
-
pathological complete response
- TRAEs:
-
treatment-related adverse events
- ORR:
-
objective response rate
- NCCN:
-
National Comprehensive Cancer Network
- CT:
-
computed tomography
- PD-L1:
-
programmed death ligand 1
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- SD:
-
stable disease
References
Sung H et al (2020) : GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71, 209–249 (2021)
Zheng RS, Zeng ZS, Wang HM, Sun SM, Chen KX, Li R, Wei L, He WQ (2022) J. Cancer incidence and mortality in China, 2016. JNCC 2(1), 1–9
Dziedzic DA, Rudzinski P, Langfort R, Orlowski T (2016) Polish Lung Cancer Study, G. Risk factors for local and distant recurrence after Surgical treatment in patients with non-small-cell Lung Cancer. Clin Lung Cancer 17:e157–e167
Group NM (2014) -a.C. preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet (London England) 383:1561–1571
Topalian SL, Taube JM, Pardoll DM(2020) Neoadjuvant checkpoint blockade for cancer immunotherapy.Science367
Caushi JX et al (2021) Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature 596:126–132
Liu J et al (2016) Improved efficacy of Neoadjuvant compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov 6:1382–1399
Friedman J et al (2020) Neoadjuvant PD-1 Immune Checkpoint Blockade reverses functional immunodominance among Tumor Antigen-Specific T cells. Clin cancer research: official J Am Association Cancer Res 26:679–689
Suzuki S et al (1990) Association of tumour burden with the efficacy of programmed cell death-1/programmed cell death ligand-1 inhibitors for treatment-naive advanced non-small-cell lung cancer. European journal of cancer (Oxford, England: 161, 44–54 (2022)
Forde PM et al (2018) Neoadjuvant PD-1 blockade in Resectable Lung Cancer. N Engl J Med 378:1976–1986
Shu CA et al (2020) Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21:786–795
Provencio M et al (2020) Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21:1413–1422
Ettinger DS et al (2021) NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Cancer Network: JNCCN 19:254–266
Cascone T et al (2021) Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 27:504–514
Hu H et al (2022) Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol 7:38–48
Zhao ZR, Yang CP, Chen S, Yu H, Lin YB, Lin YB, Qi H, Jin JT, Lian SS, Wang YZ, You JQ, Zhai WY, Long H (2021) Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III non-small-cell lung cancer. Oncoimmunology 10(1):1996000
Oken MM et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Pataer A et al (2012) Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 7:825–832
Huynh C et al (2021) Pathological complete response as a surrogate endpoint after neoadjuvant therapy for lung cancer. Lancet Oncol 22:1056–1058
Revised National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 for adverse event reporting
Ni J et al (2021) Clinical recommendations for perioperative immunotherapy-induced adverse events in patients with non-small cell lung cancer. Thorac cancer 12:1469–1488
Eisenhauer EA et al(1990) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer (Oxford, England: 45, 228–247 (2009)
Goldstraw P et al (2016) The IASLC Lung Cancer Staging Project: proposals for revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM classification for Lung Cancer. J Thorac Oncol 11:39–51
Dong ZY et al (2017) Potential predictive value of TP53 and KRAS Mutation Status for response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin cancer research: official J Am Association Cancer Res 23:3012–3024
Acknowledgements
We thank the patients, their families, and the participating study teams for making this study possible.
Funding
This work was supported by a grant from the Special Funding for Qilu Sanitation and Health Leading Talents Cultivation Project (to Helei Hou).
Author information
Authors and Affiliations
Contributions
Conception/design: Helei Hou and Xiaochun Zhang;
Collection and/or assembly of data: Yongjie Wang, Dantong Sun, Jingjuan Zhu and Man Jiang;
Data analysis and interpretation: Yongjie Wang, Dantong Sun, Xuchen Zhang, Na Zhou, Chuantao Zhang and Tianjun Li;
Manuscript writing: Helei Hou and Dantong Sun;
Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Ethical Approval and Consent to participate
The protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University.
Consent for publication
Written informed consent was obtained from individual or guardian participants.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, H., Wang, Y., Sun, D. et al. Neoadjuvant toripalimab plus platinum-paclitaxel chemotherapy in stage II-III non-small cell lung cancer: a single-center, single-arm, phase I study in China. Invest New Drugs 41, 86–92 (2023). https://doi.org/10.1007/s10637-022-01324-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01324-5