Abstract
Patients with hepatocellular carcinoma (HCC) outside Milan criteria (MC) may be candidates for curative therapy after successful downstaging. We aimed to identify the predictors of successful downstaging of unresectable HCC in patient by transarterial chemoembolization (TACE) outside MC. We performed a retrospective study on patients with unresectable HCC outside MC who received downstaging with TACE. Clinical and laboratory variables were recorded. We identified 101 patients with unresectable HCC who underwent initial TACE, who formed the derivation set of this study. Thirty patients who treated by TACE with the same selection criteria served as an external validation set. We performed univariate and multivariate logistic regression analyses to identify variables associated with successful downstaging. Then we did the predictive model to predict the efficiency of TACE. Of the 101 patients in the study, 26 patients (25.7%) were successfully downstaging and 75 patients (74.3%) failed downstaging. Multivariate analysis of factors to predict successful downstaging of HCC outside MC the number of tumor (P = 0.01), portal vein tumor thrombosis (PVTT)(p < 0.01), the size of tumor (P = 0.02), hepatitis B surface antigen (HBsAg) (P = 0.01), α-fetoprotein (AFP) (P = 0.02) as significant predictors of successful downstaging. Then we constructed the predictive model. The area under the ROC curve (AUROC) of the predictive equation was 0.90 (95% confidence interval, 0.83–0.95). We found in our study that the number and size of tumors, PVTT, HBsAg, and AFP are good predictors of successful downstaging of unresectable HCC in patients by TACE outside the MC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary liver cancer is the second most frequent cause of cancer death worldwide with HCC representing the most common form of primary liver cancer [1]. This highly malignant tumor portends a median survival of < 1 year without treatment [2]. A variety of treatment options are available to HCC patients depending on a number of patient and tumor characteristics [1]. Currently, curative treatment such as surgical resection, and liver transplantation serves as the best way of treatment for HCC. Unfortunately, almost 80% of patients with HCC are initially diagnosed at intermediate or advanced stage, hence being unqualified for curative treatments [3, 4].
For patients with unresectable HCC, downstaging is attempted to bring tumors within MC (a single nodule less than 5 cm in diameter, or up to 3 nodules, with the largest being less than 3 cm in diameter, with no evidence of vascular invasion or distant metastasis) by using liver-directed therapy and finally made the curative treatment available [5]. Options for conducting downstaging include systemic therapy and locoregional therapies(LRT). Moreover, the application of LRT such as TACE, radiofrequency ablation (RFA), transarterial radioembolization (TARE), stereotactic body radiation (SBRT), or a combination of therapies is more extensive [6]. TACE is the treatment approach most commonly used for unresectable HCC. Current guidelines including the barcelona clinical liver cancer(BCLC) staging system recommend TACE as the standard treatment of intermediate-stage HCC. The effectiveness of TACE as an adjuvant therapy for HCC has been documented in clinical studies [7].
TACE therapy can reduce tumor burden and further make curative treatment acceptable, which improves survival rates in unresectable HCC patients. Although TACE is currently recommended as a first-line therapy for the treatment of intermediate-stage HCC, the incidence of recurrence is high and the efficacy of TACE for unresectable HCC is discouraging. Meanwhile, the factors that predict successful downstaging by TACE have not yet been clearly established [5]. The aim of our study was to determine the factors that predict successful downstaging of HCC in patients outside MC and provide theoretical support for improving the efficacy of downstaging by TACE.
Methods
Patients
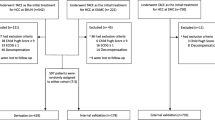
This retrospective experiment has been approved by the Clinical Trial Ethics Committee of the Affiliated Hospital of Southwest Medical University, China (Acceptance Number:KY2021156). Informed consent is an oral or written consent obtained from all participating adult experimenters and the parents or legal guardians of minors or incapacitated adults. We identified 101 patients with unresectable HCC who underwent initial TACE from October 2018 to June 2020 in the Affiliated Hospital of Southwest Medical University, China and performed a retrospective cohort analysis. We included patients with HCC lesions outside MC who were Child–Pugh class A-B, BCLC B-C, had good performance status, had no evidence of tumor invasion into distant metastases. In the previous study, HCC patients with PVTT, palliative TACE treatment can be an accessible effective measure to improve the OS and PFS for both type I and type II PVTT patients [8]. Therefore, The efficacy of TACE in patients diagnosed as advanced HCC accompanied with different types of PVTT is unclear. The inclusion of patients in our study was not restricted by PVTT and the number of HCC lesions. Exclusion criteria were 1) patients with distant metastases,; 2) unable to undergo TACE due to poor liver function (for example: advanced Child–Pugh class C, presence of refractory ascites, severe hepatic encephalopathy); 3) ECOG performance status greater than 1; 4) assessed without appropriate imaging diagnosis before initial TACE; 5) initial TACE for ruptured HCC; 6) complete main portal vein obstruction without collateral circulation; The diagnosis of HCC was made in accordance with American Association for the Study of Liver Diseases (AASLD) guidelines (contrast imaging showing an arterial hyperenhancement pattern with venous phase wash out; or histological diagnosis after liver biopsy). These patients formed the derivation set of this study.
From July 2020 to October 2020, another cohort of 30 patient treated in the Affiliated Hospital of Southwest Medical University, China by TACE with the same selection criteria was analyzed as an independent external validation set. Downstaging was considered to have failed if there was 1 of following: (1) progression of tumor as noted by an increased in the number or size of HCC lesions, (2) HCC lesions invading the hepatic or portal vessels, (3) distant metastases.
Collection of data
We recorded demographic data including age, sex, etiology of the liver disease, laboratory variables including HBsAg, white blood cell count, red blood cell count (RBC), erythrocyte number, hemoglobin, platelet count, prothrombin time(PT), activated partial thromboplastin time(APTT), thrombin time(TT), fibrinogen, Alanine aminotransferase(ALT), aspartate transaminase(AST), albumin(ALB), total bilirubin(TBIL),direct bilirubin(DBIL), indirect bilirubin(IBIL), lactic dehydrogenase(LHD), total bile acid(TBA), glutamyltransferase(GGT), alkaline phosphatase(ALP), prealbumin(PA), total cholesterol(TC), carbamide, uric acid, creatinine and tumor markers including AFP, CA199, CA50, CA242 and CA724.
All laboratory values and tumor markers were determined 1 day before TACE. In addition, we recorded the tumor characteristics, such as the number of HCC lesions, the distribution of HCC lesions, and the longest diameter of HCC lesions. The efficacy of TACE to the entire therapeutic regimen was initially evaluated every 8 weeks after initial TACE. After 6 months, the efficacy was assessed every 12 weeks, until meet the MC or mRECIST definition of progressive disease.
Statistical analysis
In this study, the Kolmogorov–Smirnov method was used to test the normality of each continuous quantitative data in the sample. Quantitative data of normal distribution is expressed by mean ± SD; quantitative data of non-normal distribution data is expressed by interquartile range. And categorical data are shown as frequency and proportion. The quantitative data of normal distribution adopts independent sample t test; the quantitative data of non-normal distribution adopts the nonparametric Wilcoxon rank sum test, and the categorical data adopts Chi-square test, Fisher’s exact probability method or rank sum test.
Univariate logistic regression analysis was performed to identify variables that could predict successful downstaging. A P value of 0.05 or less was considered significant. The variables found to be significant in univariate analysis, as well as variables thought to be clinically significant, were all included in the multivariate analysis. Logistic regression analysis was used for model development. Predictive accuracy and sensitivity of the model were performed using receiver operating characteristic(ROC) curves. All analyses were performed with SPSS version 25 software(IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
In the derivation cohort (n = 101), the mean age was 57.43 ± 10.44, and 75.2% of patients were men. A total of 88.1% and 11.9% of patients were diagnosed at BCLC stage A and B. Convention TACE (C-TACE) was performed in 57 patients, and drug-eluting beads TACE (DEB-TACE) was used in 44 patients. In terms of tumor factors, the number of tumor is less than 3(62.3%), and 69 (68.4%) patients of tumor distribution was observed in multiple lobe.
The clinical factors of the derivation (n = 101) and external (n = 30) validation sets prior TACE are summarized in Table 1. There were no significant differences in baseline characteristics between the derivation and validation set.
Table 1. Baseline characteristics in patients between the derivation and validation set.
Results of downstaging
Of the 101 patients in the study, 26 patients (25.7%) were successfully downstaging and 75 patients (74.3%) failed downstaging. The distinction between the clinical and laboratory variables among patients who were successfully downstaging and those who failed downstaging by univariate and multivariate logistic analysis is given in Table 2.
Table 2. The distinction between the clinical and laboratory variables among derivation set by univariate and multivariate logistic analysis.
Predictors of successful downstaging
Multivariate analysis of factors to predict successful downstaging of HCC outside MC the number of tumor (P = 0.01), PVTT(P < 0.01), the size of tumor (P = 0.02), HBsAg (P = 0.01), AFP (P = 0.02) as significant predictors of successful downstaging. Factors such as age, gender, the type of TACE, and laboratory variables such as total bilirubin and albumin, were not significant upon multivariate analysis. Then we did the predictive model to predict the efficiency of TACE. Bring AFP, PVTT, the number of tumor, the size of tumor and HBsAg to construct the predictive equation:
The predictive power of the predictive equation was evaluated by ROC curve analysis. The area under the ROC curve (AUROC) of the predictive equation was 0.90 (95% confidence interval, 0.83–0.95) (Fig. 1).
Validation of the predictive model
For the purpose of externally validating this predictive equation, we collected data among a second set of patients (n = 30) undergoing TACE in the Affiliated Hospital of Southwest Medical University, China. The area under the ROC curve (AUROC) of the predictive equation was 0.86 (95% confidence interval, 0.72–0.95) (Fig. 2).
Discussion
TACE is the treatment approach most commonly used for unresectable HCC. The effectiveness of TACE as an adjuvant therapy for HCC has been documented in clinical studies [5]. Downstaging therapy with TACE, as a selected local–regional strategy, reduces tumor burden and further make radical treatment acceptable, which improves survival rates in unresectable HCC patients. A retrospective study have shown that 1-year, 2-year and 3-year accumulating PFS were 68.8%, 40.6% and 31.3%, respectively after down-staging therapy by TACE; 1-year, 2-year and 3-year accumulating OS were 84.4%, 71.9% and 53.1%, respectively after down-staging therapy by TACE. Kaplan–Meier curves showed that successful down-staging was correlated with longer PFS and OS [9]. In related studies, of the 179 patients after initial TACE, 44 (25%) achieved tumor of downstaging to within Milan criteria and showed significantly longer survival than non-downstaged ones (P = 0.02) [10]. However, to our best knowledge, there is still no study about the factors affecting the efficacy of TACE on hepatocarcinoma downstaging.
It is still controversial as to which kind of patients can benefit from the TACE, related to the heterogeneity of the patients covered in the various studies and the diversity of the clinical elements influencing prognosis importance. In our retrospective study, we have attempted to try to find the relevant factors affecting the efficacy of TACE on hepatocarcinoma downstaging.
In the study, AFP, PVTT, HBsAg, the number of tumor and tumor diameter were noted to be the independent predictors of successful downstaging through multivariate analysis. Recently, several studies showed that AFP can improve the predictive accuracy of post-LT survival in patients with HCC. Regarding downstaging of HCC in patients outside Milan, Yao et al. showed that an AFP > 1000 ng/ml was predictive factor of failed downstaging in a total of 122 HCC patients enrolled in the downstaging protocol treated by LRT. Only 1 of 8 patients with an AFP greater than 1000 ng/ml were successfully downstaged in their study [11]. Similarly, in our study only 5 of 44 patients with AFP level higher than 1000 ng/ml were successfully downstagd. Whereas 21 out of 57 patients with the AFP level lower than 1000 ng/ml, were successfully downstaged. In a previous study showed that AFP can not only promote the proliferation of hepatocellular carcinoma cells and the formation of tumor blood vessels, but also enhance the antiapoptosis effect of cancer cells [12]. Thus, AFP plays an important role in the development and progression of HCC. It is could be an explanation of our finding in this study.
Meanwhile, we found that PVTT was also an important predictive factor to evaluate the efficacy of downstaging by TACE. Patients with PVTT usually have an aggressive disease course, decreased liver function reserve, limited treatment options, hinger recurrence rates after treatment, and, therefore, worse overall survival. Among untreated HCC patients with PVTT, the median overall survival has been reported as low as 2 to 4 months. Many aspects of PVTT have impacted the theoretical and practical safety and efficacy of treatment, for example, disordered blood flow and associated impairment of liver function, heat-sink effects of blood flow in the area of the PVTT, and tumor location in the blood vessel [13]. These data imply that PVTT may present adverse effect on the efficacy of downstaging.
In our study, HBsAg is a powerful predictive factor of efficacy of TACE. Long-term prognosis is unsatisfactory for patients with HBV-related disease because of frequent recurrence and poor residual hepatic function [14]. In the previous, Jing-Feng Liu et al. showed the evidence that low pre- or post-operative levels of HBsAg may be associated with better long-term survival in patients with HBV-related HCC. Patients with low pre-operative serum levels of HBsAg showed significantly higher OS than those with high serum levels at 1 year (90.5% vs 85.3%), 3 years (78.0% vs 70.6%), and 5 years (69.4% vs 52.6%;P < 0.01) [15]. This poor prognosis is probably due in part to chronic HBV infection, which promotes not only carcinogenesis of recurrent HCC but also excessive inflammation and fibrosis in the liver, further reducing residual hepatic function [15]. Therefore, high HBsAg levels in serum may negatively affect the efficacy of downstaging.
In the previous study, Christian Toso et al. showed that to establish a reliable selection policy by LRT, size/number or total tumor volume(TTV) of HCC have to be taken into account [16]. In related studies, combining a variety of LRT, TTV was noted to be an excellent independent predictor of successfully downstaging. Arvind R et al. indicated that for every 1 cm3 increase in TTV, the odds of successful downstaging decreased by 2%. At a TTV cutoff of 200 cm3, 76% of patients below this threshold were successfully downstaged, whereas only 4.5% of patients outside this threshold were successfully downstaged [5]. Different studies showed larger tumors were assumed to have a higher incidence of satellite nodules and vascular invasion. So the consequent relation between larger TTV and the aggressive clinicopathological character of HCC led to the valuable studies of the prognostic value of TTV [17]. Therefore, we perform a multivariate regression analysis on the number of tumor and tumor diameter, which proved the number of tumor and tumor diameter can be used as the predictors of downstaging efficacy by TACE.
We have created statistically predictive model based on a predictive logistic regression model tailored to the individual patient and give accurate efficacy information of TACE in these patients. The model is simple and easy-to-use, intergrating 5 predictors that constitute the essentials of preoperative clinical evaluation. The predictive performance of the model was further certified by external validation set. The area under the ROC curve (AUROC) of the predictive equation was 0.90 (95% confidence interval, 0.83–0.95). The area under the ROC curve (AUROC) of the predictive equation by external validation set was 0.86 (95% confidence interval, 0.72–0.95).
Furthermore, The outcome of downstaging was not affected by age and gender in our study. The type of TACE did not influence the outcome of downstaging. Unexpectedly, molecular targeting drug did not influence the outcome of downstaging in our study. A randomized phase III study conducted in Japan evaluated the effectiveness of sorafenib therapy when initiated after TACE. Four hundred and fifty-eight patients were randomized to either sorafenib or placebo, with a median time to randomization of 9.3 weeks). The study failed to show that the addition of sorafenib after TACE prolonged PFS and OS [18]. A recent article suggested that the arterial blood supply of the tumor may be associated with the efficacy of sorafenib. HCC tumors with a good arterial blood supply benefited more than those with a poor arterial supply [19]. The results in our study may be related to this factor. In addition, this suggests that the optimal timing and efficacy of molecular targeting drug in relation to TACE has yet to be determined, which needs further studies [20].
The present study had several limitations. The limitation of the present study are the small number of cases, the retrospective observational design of the study, and difficulty showing the small statistical significance. In this retrospective study, it is difficult to control confounding factors, leading to possible deviations in the result. Based on the promising results, assessment of a larger number of cases, well-designed randomized controlled trials, and comparison with other locoregional therapies are essential to further propose the importance of the TACE.
Conclusion
By combining 5 risk factors of pre-TACE, a novel, validated predictive model was constructed for predicting the efficacy of TACE on downstaging. The results showed that the model had good predictive performance. It is warranted that the model should be tested in prospective clinical trials.
Data availability
Haomin Lin should be contacted if someone wants to request the data.
References
Titano J, Voutsinas N, Kim E (2019) The Role of Radioembolization in Bridging and Downstaging Hepatocellular Carcinoma to Curative Therapy. Semin Nucl Med 49(3):189–196
Kim Y et al (2017) Downstaging therapy followed by liver transplantation for hepatocellular carcinoma beyond Milan criteria. Surgery 162(6):1250–1258
Villanueva A (2019) Hepatocellular Carcinoma. N Engl J Med 380(15):1450–1462
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet 391(10127):1301–1314
Murali AR et al (2016) Predictors of Successful Downstaging of Hepatocellular Carcinoma Outside Milan Criteria. Transplantation 100(11):2391–2397
Parikh ND, Waljee AK, Singal AG (2015) Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl 21(9):1142–1152
Jiang J et al (2017) Nomogram for individualized prediction of recurrence after postoperative adjuvant TACE for hepatitis B virus-related hepatocellular carcinoma. Medicine (Baltimore) 96(32):e7390
Tang Q et al (2021) Efficacy and Safety of Transarterial Chemoembolization in Elderly Patients of Advanced Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Retrospective Study. Front Oncol 11:646410
Cai L et al (2021) Drug-eluting bead transarterial chemoembolization is an effective downstaging option for subsequent radical treatments in patients with hepatocellular carcinoma: A cohort study. Clin Res Hepatol Gastroenterol 45(4):101535
Yasui Y et al (2018) Up-to-seven criteria as a useful predictor for tumor downstaging to within Milan criteria and Child-Pugh grade deterioration after initial conventional transarterial chemoembolization. Hepatol Res 48(6):442–450
Yao FY et al (2015) Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 61(6):1968–1977
Wang X, Wang Q (2018) Alpha-Fetoprotein and Hepatocellular Carcinoma Immunity. Can J Gastroenterol Hepatol 2018:9049252
Liu PH, Huo TI, Miksad RA (2018) Hepatocellular Carcinoma with Portal Vein Tumor Involvement: Best Management Strategies. Semin Liver Dis 38(3):242–251
Poon RT et al (2000) Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol 18(5):1094–1101
Qiu JF et al (2017) Pre- and post-operative HBsAg levels may predict recurrence and survival after curative resection in patients with HBV-associated hepatocellular carcinoma. J Surg Oncol 116(2):140–148
Toso C et al (2009) Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology 49(3):832–838
Zakaria HM et al (2020) Total tumor volume as a prognostic value for survival following liver resection in patients with hepatocellular carcinoma. Retrospective cohort study Ann Med Surg (Lond) 54:47–53
Kudo M et al (2011) Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 47(14):2117–2127
Zhu Q et al (2015) Arterial blood supply of hepatocellular carcinoma is associated with efficacy of sorafenib therapy. Ann Transl Med 3(19):285
Dinh VY et al (2016) Pilot Study of Intrahepatic Artery Chemotherapy in Combination with Sorafenib in Hepatocellular Carcinoma. Anticancer Res 36(7):3555–3563
Funding
This work was supported by the National Natural Science Foundation of China (81803019).
Author information
Authors and Affiliations
Contributions
SS and YL designed the study, HL, FP and BL and prepared first and final draft of the article; YG, CF and XY recorded the clinical and laboratory variables and prepared Table-1 and Table-2; HL and BL performed univariate and multivariate logistic regression analyses to identify the variables associated with successful downstaging and prepared Figure-1 and Figure-2. SS is corresponding author, provided critical feedback and helped to modify manuscript. All authors reviewed the manuscript. The authors deny any conflicts of interest.
Corresponding authors
Ethics declarations
Ethics approval
This retrospective experiment has been approved by the Clinical Trial Ethics Committee of The Affiliated Hospital of Southwest Medical University, China (Acceptance Number:KY2021156).
Informed consent
Informed consent is an oral or written consent obtained from all participating adult experimenters and the parents or legal guardians of minors or incapacitated adults.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the clinical and laboratory variables.
Competing interests
The authors deny any conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, H., Luo, B., Peng, F. et al. The efficacy of transarterial chemoembolization in downstaging unresectable hepatocellular carcinoma to curative therapy: a predicted regression model. Invest New Drugs 40, 1146–1152 (2022). https://doi.org/10.1007/s10637-022-01261-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01261-3