Summary
Purpose
Pyrotinib (PTN), an irreversible EGFR/HER2 dual tyrosine kinase inhibitor used for treating HER2-positive breast cancer, is primarily metabolized by cytochrome P450 (CYP)3A4 isozyme. Rifampicin (RIF) is a strong index CYP3A4 inducer. Therefore, the study aimed to elucidate the effect of RIF on PTN pharmacokinetics (PK) in Chinese healthy volunteers.
Methods
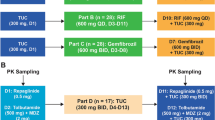
This phase I, open-label study investigated the effects of steady-state RIF administration on single-dose PK of PTN. 18 healthy participants were enrolled in this trial, who received a single oral dose of 400 mg of PTN on days 1 and 13, and were administrated with RIF 600 mg qd on days 6 through 16. RIF was administrated on an empty stomach, PTN were administrated orally in the morning 30 min after the start of the standard meal. Serial PK samples for PTN were collected on days 1 and days 13. Safety assessments were performed via clinical laboratory tests throughout the study.
Results
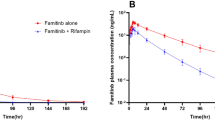
18 subjects were enrolled and 16 completed the study. RIF significantly reduced PTN exposure: Geometric least-squares mean ratios (90% CI) for PTN + RIF versus PTN alone were 0.04 (0.034,0.049), 0.04 (0.037,0.054), and 0.11 (0.09,0.124) for area under the curve from time zero to time of last quantifiable concentration (AUC0 − t), area under the curve from time zero to infinity (AUC0−∞ ), and maximum observed plasma concentration(Cmax), respectively. PTN alone and co-administered with RIF was well tolerated.
Conclusion
The exposure of PTN was significantly affected by the action of RIF. The findings suggest that concomitant strong CYP3A4 inducers should be avoided during PTN treatment. Concurrent administration of PTN and RIF was well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyrotinib maleate (PTN, Jiangsu Hengrui Pharmaceuticals Co., Ltd.) is a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor that is used to treat HER2-positive breast cancer[1], which has been approved by National Medical Products Administration (NMPA) to reach the market because of the remarkable result of the phase II study[2,3,4]. The mechanism of action of PTN is similar to that of neratinib, but it was found that PTN has better intestinal absorption due to its higher plasma exposure level[5].
PTN is slowly absorbed and widely distributed. The main advantage of PTN is that it irreversibly binds to the site of action with a stable effect. PTN is well tolerated as an oral drug with a small molecular property that enhances the ability to penetrate the blood-brain barrier[5].
Phenotyping experiments demonstrated that CYP3A4 is the most active enzyme responsible for the biotransformation of PTN[6]. Clinically, PTN is likely to be used in combination with other drugs for the treatment of breast cancer or complications[7]. If the drug used in combination is an inducer of CYP3A4 metabolic enzyme, it may reduce the exposure of PTN and affect the anti-tumor effect of PTN.
Rifampicin (RIF), a strong index inducer of CYP3A4, is recommended as a representative probe drug to evaluate the mechanism of CYP3A4-mediated interactions.[8,9,10,11].
The purpose of this study was to evaluate the effect of RIF on the PK profile and safety of oral PTN tablets in healthy Chinese subjects. Furthermore, it provides a reference and basis for dose adjustment of the concurrent administration of PTN and RIF, or other CYP3A4 inducer.
Materials and methods
Study design
This was a phase I, single-site, open-label, fixed-sequence, crossover study with three treatment periods to evaluate the effect of an oral tablet of PTN on the steady-state PK of orally administrated RIF in 18 healthy subjects. The study was conducted in accordance with the principles of the Declaration of Helsinki. A written informed consent was obtained from every subject. The study protocol was reviewed and approved by an independent Medical Ethics Committee.
All participants received a single 400 mg dose of PTN in the morning on days 1 and 13(Fig. 1). Subjects received RIF 600 mg once daily on days 6–16. PTN 400 mg once daily was co-administrated with RIF 600 mg once daily on day 13. RIF was administrated on an empty stomach, then subjects had a standard meal one hour after RIF administration. PTN were administrated orally in the morning 30 min after the start of the standard meal.
Serial blood samples for determining the plasma concentration of PTN were collected on days 1 and 13 at the following times: pre-dose, 1 h, 2 h, 3 h, 4 h, 5 h, 6 h, 8 h, 10 h, 12 h, 24 h, 48 h, 72 h, 96 h post-dose.
All blood samples were collected into evacuated heparin lithium anticoagulant tubes. Plasma was separated by centrifugation at around 2500 × g for 10 min at 25 ± 5℃, and the blood sample could be placed at room temperature no longer than one hour. The separated plasma sample should be transferred to -70℃(-60℃~-90℃)freezer, where it was stored until it was shipped for analysis.
Study subjects
Each subject was evaluated with physical examination, medical history, and laboratory testing at a screening visit conducted within 14 days prior to the first dose of drug. Eligible subjects were 18 to 45 years of age, had a minimum weight requirement of 45 kg for women and 50 kg for men, and a body mass index requirement between 19 and 24 kg/m2. Key exclusion criteria included history or presence of clinically significant medical, psychiatric disorder; concomitant chronic or acute illness; history or presence of drug addiction or excessive use of alcohol or tobacco; previous drug allergy. Participants whose QT interval longer than 470 msec corrected by Fridericia on 12-lead electrocardiogram were also excluded from the study. Participants were also excluded from the study if they had taken any drug in the past 14 days or any drugs that alter the activity of liver enzyme in the past 28 days. Subjects were required to have negative screening results for HIV-1, hepatitis B, hepatitis C, and syphilis. Concomitant medication was not permitted during the study except for dealing with adverse events or treating emergencies.
Safety evaluations
Safety evaluations included the monitoring of adverse events (AEs), physical examinations, clinical chemistry laboratory tests, vital signs, and electrocardiograms. Treatment-emergent adverse events (TEAEs) were recorded throughout the study.
Bioanalytical methods
Plasma samples were analyzed for PTN by a validated high-performance liquid chromatography tandem mass spectrometry analysis. The LLOQ (lower limit of quantification) for PTN in plasma was 1.00 ng/mL, and the upper limit of quantification was 500 ng/mL. Precision and accuracy were evaluated by replicate analyses of human plasma quality control samples prepared at five concentrations: 1.00, 3.00, 25.0, 400, and 500 ng/mL. Precision, measured as the percent coefficient of variation, the maximum of which is 5.9% across the quality control range. Accuracy, expressed as the percent difference from the mean value, ranged from − 2.3 to 5.7%. Both were within acceptance standards of 15%, except that 20% for LLOQ.
Pharmacokinetic analysis
A non-compartmental PK analysis of the PTN concentration-time data was conducted. PK analyses of plasma PTN concentration-time data were analyzed by Phoenix WinNonlin 8.1. Plasma PK parameters for PTN were calculated using actual elapsed times from dosing. The individual PK parameters that were determined included maximum observed plasma concentration (Cmax), time to Cmax (Tmax), area under the curve from time zero to the last quantifiable concentration (AUC0 − t), area under the curve from time zero to the infinite concentration (AUC0−∞), elimination half-life (t1/2), apparent volume of distribution (VZ/F), apparent clearance(CL/F).
Statistical analysis
This study was designed to estimate the magnitude of drug-interaction effect of RIF on the PK parameters of PTN. PK parameters were log-transformed and analyzed by analysis of variance to determine the point estimate and associated 90% confidence intervals for the difference between test treatment (PTN + RIF) and reference treatment (RIF alone). These values were then back-transformed to calculate the point and interval estimates for test-to-reference treatment ratios on the original scale. Geometric least-squares mean ratios and 90% confidence intervals were generated by the mixed-effect model for within-subject treatment comparisons.
Results
Demographics
Eighteen subjects, 6 females and 12 males, were enrolled and included in the safety and PK populations. Two male subjects were prematurely discontinued from the study. The two subjects withdrew due to drug induced liver damage on period 3, days 13 (PTN + RIF), with increased alanine aminotransferase (ALT ) and aspartate aminotransferase (AST) level, whose severity both were level 1.Besides, one subject vomited during the trial, which occurred within twice the median Tmax of the same group of subjects.
The mean (± standard deviation (SD)) age was 29.1 (± 6.7) years and ranged between 19 and 43 years. The mean (± SD) body mass index was 21.94 (± 1.35) kg/m2, height was 165.61 (± 8.42) cm, and weight was 60.37 (± 7.67) kg. One subject (5.6%) was minority, and other seventeen subjects (94.4%) were Han.
Safety
A total of 96 treatment emergent adverse events (TEAE) were reported in 18 (100%) of the 18 treated subjects (Table 1). Of these, Three subjects (16.7%) experienced 5 adverse events (AEs) during period 1 (PTN alone). Eighteen subjects (100%) experienced 41 AEs during period 2 (RIF alone). Eighteen subjects (100%) experienced 50 AEs during period 3 (PTN + RIF ).
Seven subjects (38.89%) experienced adverse reactions to PTN and 18 (100%) subjects experienced adverse reactions to RIF. All the severity of adverse events is level 1, No serious adverse events or non-fatal serious AEs occurred through the study. Two subjects (11.1%) experienced AEs leading to withdrawal from the trial, which may not be related to PTN, but may be related to RIF.
AEs with an incidence of ≥ 10% included chromaturia (100%), discoloration stool (100%), leukocyte count decreased (22.22%), ALT increased (22.22%), neukocyte count decreased (22.22%), increased AST level (16.67%), blood triglycerides increased (11.11%) and headache (11.11%). All adverse events are attributed to recovery/resolution.
Pharmacokinetics
In the case of using PTN alone or in combination with RIF,The PK parameters of PTN are presented in Table 2, while the comparison of the PK parameters of PTN are shown in Table 3, the mean (SD) plasma concentration time curve are shown in Fig 0.2., When PTN was combined with RIF, the AUC0 − t, AUC0−∞, and Cmax of PTN were decreased by 96%, 96%, and 89%, respectively. Furthermore, PTN clearance was significantly increased, and its half-life was shortened from 15.725 h to 6.261 h.
Discussion
PTN is primarily metabolized to produce O-despicoline (M1), carbonylation M1 (M2), dehydro-pyrrolidine (M5-1), carbonyl-N-methyl Pyrrolidine (M7) and hydroxymethylpyrrolidine (M8-1) and double oxidation and dehydrogenation metabolites (M9) by CYP3A4[6]. Therefore, when PTN was in combination with CYP3A4 inducer, it induced the metabolism of PTN and decreased its exposure in the body. Considering that recommended dose of PTN in phase II clinical trial of PTN was 400 mg, and the minimum effective dose is 240 mg, consequently, this study chose 400 mg as the single dose of PTN.
According to the guidelines for drug-drug interactions, this study chose Rifampicin as a strong inducer of CYP3A4 to investigate drug-drug interactions.
It has been reported that full induction is reached 1 week after starting RIF, so the drug-drug interaction was evaluated after 11 days of RIF administration to ensure full inhibition. The dose of RIF is set as 600 mg qd according to regulatory guidance generally recommends in the evaluation of a drug as a perpetrator of drug interaction[8,9,10,11].
-
The study shown that repeated dosing of RIF for 11 days resulted in a decrease of 96%, 96%, and 89% in AUC0 − t, AUC0−∞, and Cmax of PTN, respectively. When PTN co-administering with the itraconazole, a potent CYP3A4 inhibitor, the exposure of PTN was increased[12].According to the guidelines for drug-drug interactions, it is recommended to conduct trials of PTN which co-administrated with CYP3A4 moderate inducer in order to strongly guide risk management.
Compared with lapatinib, PTN has a more comprehensive target, and its inhibitory effect on the target is irreversible, thereby inhibiting tumor growth more effectively; compared with neratinib, PTN has a higher bioavailability[3,4,5]. Therefore, PTN occupies a higher position in the molecular targeted therapy of breast cancer. The current phase I and phase II clinical studies have confirmed its good pharmacokinetics and safety. For the moment, phase III clinical studies are being actively carried out to extend indications of PTN as well as combination regimens.
-
This study has some obvious limitations. Firstly, this study was conducted in a small number of healthy volunteers, and the findings may not be applicable to the wider population or specific populations. Secondly, CYP3A gene polymorphisms were not considered in this study, which may have an impact on the metabolic disposal of PTN. Therefore, the effects of CYP3A gene polymorphisms on the drug-drug interactions of PTN needs to be further studied.
Conclusions
This study evaluated the extent of drug-drug interactions between RIF, a putative strong CYP3A4 inducer, and PTN, a known substrate of CYP3A4. As expected, co-administration of RIF and PTN in healthy subjects resulted in a significantly reduction of PTN in AUC0 − t, AUC0−∞, and Cmax compared with PTN alone. The findings suggest that in the clinical application of PTN, the dose should be avoided in combination with CYP3A4 inducers.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Huang T, Luo X, Wu B et al (2020) Pyrotinib enhances the radiosensitivity of HER2overexpressing gastric and breast cancer cells[J]. Oncol Rep 44(6):2634–2644
Blair HA, Pyrotinib (2018) First Global Approval[J]. Drugs 78(16):1751–1755
Su B, Huang T, Jin Y et al (2021) Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways[J]. Gastric Cancer 24(2):352–367
Fei Q (2019) Pyrotinib or Lapatinib Combined With Capecitabine in HER2-Positive Metastatic Breast Cancer With Prior Taxanes, Anthracyclines, and/or Trastuzumab: A Randomized, Phase II Study.[J]. J Clin oncology: official J Am Soc Clin Oncol 37(29):2610–2619
Xuhong JC, Qi XW, Zhang Y et al (2019) Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer[J]. Am J Cancer Res 9(10):2103–2119
Meng J, Liu XY, Ma S et al (2019) Metabolism and disposition of pyrotinib in healthy male volunteers: covalent binding with human plasma protein[J]. Acta Pharmacol Sin 40(7):980–988
Lin Y, Lin M, Zhang J et al (2020) Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis[J]. Cancer Res Treat 52(4):1059–1066
Li J, Rockich K, Yuska B et al (2020) An Open-Label Study to Assess the Effect of Itraconazole and Rifampin on Parsaclisib Pharmacokinetics When Administered Orally in Healthy Participants[J]. J Clin Pharmacol 60(11):1519–1526
Elin M, Svensson S, Murray MO, Karlsson et al (2015) Rifampicin and rifapentine significantly reduce concentrations of bedaquiline, a new anti-TB drug[J]. J Antimicrob Chemother 70:1106–1114
Niladri Chattopadhyay T, Kanacher M, Casjens et al (2018) CYP3A4-mediated effects of rifampicin on the pharmacokinetics of vilaprisan and its UGT1A1-mediated effects on bilirubin glucuronidation in humans.[J]. Br J Clin Pharmacol 84:2857–2866
Zhou X, Pant S, Nemunaitis J et al (2017) Effects of rifampin, itraconazole and esomeprazole on the pharmacokinetics of alisertib, an investigational aurora a kinase inhibitor in patients with advanced malignancies[J]. Investigational New Drugs
Yue L, Qian Z, Chao L et al Multiple administrations of itraconazole increase plasma exposure to pyrotinib in Chinese healthy adults[J]. Drug Design, Development and Therapy,2021:15 2485–2493
Acknowledgements
We would like to thank all the subjects who participated in this study, study coordinator, the clinical investigators and support staff.
Funding
This study was supported by scientific research project of Jiangsu Medical Products Administration (202106).
Author information
Authors and Affiliations
Contributions
Designed Research: Wei Qian, Hui-ping wang. Performed Research : Ming-min Cai, Ting Dou, Lu Tang, Qiu-yue Sun. Analyzed Data: Ming-min Cai, Zi-hong Zhai. Wrote Manuscript: Ming-min Cai, Wei Qian. Language Modification:Ming-min Cai, Wei Qian.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in the study involving human participants were conducted in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent of publication was obtained from all authors.
Conflict of interest
The author reports no conflicts of interest in this work.
Disclosure of potential conflicts of interest
The author reports no conflicts of interest in this work.
Research involving Human Participants and/or Animals
The phase I clinical trial was approved by Ethics Committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, Mm., Dou, T., Tang, L. et al. Effects of rifampicin on antineoplastic drug pyrotinib maleate pharmacokinetics in healthy subjects. Invest New Drugs 40, 756–761 (2022). https://doi.org/10.1007/s10637-022-01241-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01241-7