Abstract
Objective
To evaluate the efficacy and safety of immune checkpoint inhibitor (ICI) and chemotherapy (CT) versus CT alone in advanced non-small-cell lung cancer (NSCLC).
Methods
Databases (PubMed, Embase and Cochrane Library) were searched for relevant randomized controlled trials (RCTs). Clinical outcome measures including overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and grade 3–5 treatment-related adverse events (AEs) were analyzed by Stata 15.0 software; significance level was 0.05.
Results
Eight RCTs involving 4227 patients were included. The results showed ICI + CT significantly improved OS (hazard ratio [HR] = 0.74, 95% CI: 0.62–0.85, p < 0.001), PFS (HR = 0.66, 95% CI: 0.57 − 0.75, p < 0.001) and ORR (odds ratio [OR] = 1.89; 95% CI, 1.43–2.49, p < 0.001) compared with CT alone. Subgroup analysis indicated that significantly longer OS was also observed in subgroups including combination regimens (pembrolizumab + CT, atezolizumab + CT, ipilimumab + CT, and nivolumab + ipilimumab + CT) and PD-L1 status [negative (< 1%), positive (≥ 1%), low (1–49%) and high (≥ 50%)]. However, ICI + CT showed signifcantly higher grade 3–5 treatment-related AEs than CT (OR = 1.46, 95% CI: 1.19 − 1.79, p < 0.001).
Conclusions
ICI + CT showed better clinical efficacy than CT alone in patients with advanced NSCLC, with increased treatment-related AEs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Abbreviations: ICI, immune checkpoint inhibitor; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; RCT, randomized controlled trial; OS, overall survival; PFS, progression-free survival; ORR, objective response rate; AE, adverse event; HR, hazard ratio; OR, odds ratio; PD-1, programmed cell death protein-1; PD-L1, programmed cell death 1 ligand 1; CTLA-4, cytotoxic T lymphocyte antigen 4.
Background
Lung cancer is the leading cause of cancer-related deaths worldwide, and almost half of patients are diagnosed with advanced or metastatic disease when early symptoms appear [1, 2]. Over the past several decades, platinum-based chemotherapy was regarded as the first-line standard treatment for advanced non-small-cell lung cancer (NSCLC) patients without epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinse (ALK) mutations, with a response rate of 12.5–37%, median progression-free survival (PFS) of 4 to 8 months, and median overall survival (OS) of 8 to 13 months [3]. Due to the poor prognosis, novel and effective treatment strategies are urgently warranted for patients with advanced NSCLC.
Currently, immune checkpoint inhibitors (ICIs) including programmed cell death protein 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) and cytotoxic T ymphocyte antigen 4 (CTLA-4) inhibitors were widely used in advanced cancers. Several ICIs, such as pembrolizumab (lambrolizumab or MK-3475, a highly IgG4 monoclonal anti-PD-1 antibody), nivolumab (a fully human IgG4 anti-PD-1 antibody), atezolizumab (MPDL3280A, an IgG anti-PD-L1 antibody), and ipilimumab (a fully human monoclonal IgG1κ anti-CTLA-4 antibody) have shown promising anti-tumour activity and safety in advanced NSCLC. However, it is estimated that only 50% of patients could benefit from single-agent ICI [4], and several risk factors, such as PD-L1 expression level, EGFR and ALK genetic alteration status, are important factors affecting the curative effect. Carbone et al. [5] reported that single-agent nivolumab could not improve survival compared with chemotherapy in stage IV or recurrent NSCLC with a PD-L1 expression level of 5% or more. In the phase 3 KEYNOTE 042 trial [6], single-agent pembrolizumab resulted in prolonged PFS and OS than chemotherapy only in the PD-L1 expression level of ≥ 50% group. However, no survival benefit could be seen in the PD-L1 expression leve of ≥ 1% or ≥ 20% groups. A another study by Lisberg et al. [7] reported that pembrolizumab monotherapy showed no survival benefit in PD-L1-positive, tyrosine kinase inhibitor (TKI) naïve, and EGFR-mutant patients with advanced NSCLC.
Due to the limitations of single-agent ICI, several randomized controlled trials [8,9,10,11,12,13,14,15] (RCTs) have evaluated the efficacy and safety of ICI and chemotherapy in the first-line treatment for advanced NSCLC. In order to evaluate the efficacy and safety of ICI + CT versus CT alone in advanced NSCLC, we performed this meta-analysis and examined the tumor response, survival of patients and treatment-related AEs in patients with advanced NSCLC.
Methods
Strategy of study screening
We identified original articles by searching databases including PUBMED, EMBASE and Cochrane Library from inception until December 2021. As for the literature search, we used any of the following key words: “immune checkpoint blockade OR immune checkpoint inhibitor OR immune therapy OR immunotherapy OR PD-1 OR PD-L1 OR pembrolizumab OR nivolumab OR atezolizumab OR tremelimumab OR avelumab OR durvalumab OR ipilimumab” AND “Non-small Cell Lung Cancer OR NSCLC” AND “chemotherapy”. To avoid missing relevant studies, we also searched manually through relevant references to identify other relevant clinical trials. Only randomized controlled studies (RCTs) that investigated the efficacy and safety of ICI + CT in the first-line treatment for advanced NSCLC were eligible for inclusion in the meta-analysis. Other inclusion criteria were articles published in English and presentation of data for any of the efficacy and safety outcomes of interest that were OS, PFS, objective response rate (ORR) and treatment related adverse events (AEs). Papers of non randomized trials, reviews, meta-analysis, letters, and case reports were excluded. The trials identified through the search were independently screened by two authors (L.F. M and J.F. H) for inclusion. Any disagreements were arbitrated by a third author (P.H. L).
Data extraction and Quality assessment
Two authors (P.H. L and S.X. H) independently extracted data concerning author details, year, study design, phase, number of patients, age, sex, and treatment regimens, and PD-L1 status according to a predefined data extraction form. Clinical outcomes including ORR, PFS, OS, grade 3–5 treatment related AEs, and treatment related deaths were recorded for further analysis. Data of OS for patients with PD-L1-negative (< 1%), PD-L1-positive (≥ 1%), PD-L1-low (1–49%) and PD-L1-high (≥ 50%) tumors was also recorded in detailed. When multiple papers of the same trial were identified, data was extracted and recorded as a single trial. If any discrepancy occured, problems were resolved by discussion and consensus. Two authors (P.H. L and H.L. W) used the Cochrane Collaboration risk of bias assessment tool to assess the risk of bias of the included RCTs [18].
Statistical analysis
Stata SE 15.0 (Stata Corporation, College Station, TX, USA) was used to conduct meta-analysis in the study. We calculated the pooled hazard ratio and 95% CI for OS and PFS and the pooled odds ratio and 95% CI for ORR and the incidence of grade 3–5 treatment related AEs. Between-study heterogeneity was analysed through I-squared (I2) tests in the meta-analysis. The heterogeneity was considered as high (either I2 > 50% or p < 0.1), then the randomized-effects model was applied; otherwise, the fixed-effects model was used. P value < 0.05 would be treated as statistically significant.
Results
Search results and study characteristics
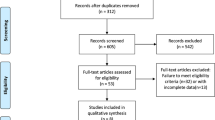
The fowchart of the selection process and detailed identifcation are shown in Fig. 1. After the duplicate removal, eight RCTs with a total of 4227 patients were included [8,9,10,11,12,13,14,15]. Among the eight global, multi-center RCTs, six [8, 9, 11, 12, 14, 15] were phase 3 studies and two [10, 13] were phase 2 studies. All patients were diagnosed with NSCLC by pathology and were adults with advanced or metastatic disease, and received ICI + CT in the first-line treatment. Across these eight trials, six trials reported OS for patients with PD-L1-negative (< 1%) tumors, and five trials reported OS for patients with PD-L1-low (1–49%) and PD-L1-high (≥ 50%) tumors, while only 4 trials reported OS for patients with PD-L1-positive (≥ 1%) tumors. All articles were published between 2012 and 2021. All the eight trials were identified in the systematic evaluation, including three pembrolizumab plus chemotherapy [8,9,10], two atezolizumab plus chemotherapy [11, 12], two ipilimumab plus chemotherapy [13, 14], and one nivolumab + ipilimumab + chemotherapy [15]. The main characteristics of the included studies are shown in Table 1.
Efficacy
Data of OS, PFS, and ORR was reported in all the eight included trials. Randomized-effects model was used in these outcome measurements because of the significant heterogeneity (I2 > 50%). The forest plot of these outcomes are showed in Fig. 2.
The meta-analysis indicated that ICI + CT significantly improved OS (HR = 0.74, 95% CI: 0.62–0.85, p < 0.001) (Fig. 2 A), PFS (HR = 0.66, 95% CI: 0.57 − 0.75, p < 0.001) (Fig. 2B), and (OR = 1.89; 95% CI, 1.43–2.49, p < 0.001) (Fig. 2 C) in comparison to CT alone. Subgroup analysis showed that improved OS for ICI + CT was observed in either pembrolizumab plus chemotherapy, atezolizumab plus chemotherapy, ipilimumab plus chemotherapy or nivolumab plus ipilimumab plus chemotherapy group (HR = 0.57, 95% CI: 0.45 − 0.69, p < 0.001; HR = 0.82, 95% CI: 0.70 − 0.95, p < 0.001; HR = 0.90, 95% CI: 0.77 − 1.04, p < 0.001, and HR = 0.70, 95% CI: 0.52 − 0.88, p < 0.001, respectively) (Fig. 3).
In the PD-L1 subgroups, ICI + CT was associated with significantly longer OS than CT in either PD-L1-negative, PD-L1-positive, PD-L1-low and PD-L1-high group (HR = 0.59, 95% CI: 0.49 − 0.68, p < 0.001; HR = 0.58, 95% CI: 0.48 − 0.68, p < 0.001; HR = 0.63, 95% CI: 0.52 − 0.75, p < 0.001, and HR = 0.55, 95% CI: 0.42 − 0.68, p < 0.001, respectively). (Fig. 4).
Safety
The incidence of grade 3–5 treatment-related AEs were reported in all the eight publications. Randomized-effects model was used because of the high heterogeneity (I2 > 50%). As shown in Fig. 5 A, ICI + CT significantly increased the incidence of grade 3–5 treatment-related AEs (OR = 1.46, 95% CI: 1.19–1.79, p < 0.001) compared with CT alone.
Among the eight trials, treatment-related deaths were reported in seven trials. Fixed-effects model was used because of the low heterogeneity (I2 < 50%). As shown in Fig. 5B, there was no statistical difference in the incidence of treatment-related deaths between the ICI + CT and CT groups (OR = 1.94, 95% CI: 0.97–3.88, p = 0.061).
Quality of the included studies
The risks of bias of the included studies in this meta-analysis are summarized in Fig. 6. The methodological quality was assessed as high in all the eight RCTs.
Discussion
Immune checkpoint inhibitors have played an important role in the treatment of advanced NSCLC nowadays [19, 20]. In recent years, many clinical trials showed that ICI combination therapies offered a better survival benefit than monotherapies in advanced NSCLC [17, 21–22]. Mo et al. [23] reported that ICI combination therapies including ICI + CT, double-agent ICIs (nivolumab plus ipilimumab) and ICIs plus targeted therapy plus chemotherapy could significantly improve OS and PFS over monotherapies in patients with advanced NSCLC. However, despite the advent of novel ICI combination therapies, the optimal first-line setting for advanced NSCLC has not been established, and the combination of ICI and CT has become one of the most promising approaches in the treatment of advanced NSCLC. Due to the insufficient evidence regarding the efficacy and safety of ICI + CT versus CT alone in advanced NSCLC and the controversial role of PD-L1 as a prognostic predictor, a meta-analysis is warranted to provide more evidence for clinical use of this treatment strategy.
Our meta-analysis shows that ICI + CT significantly improved OS (HR = 0.74, 95% CI: 0.62–0.85, p < 0.001), PFS (HR = 0.66, 95% CI: 0.57 − 0.75, p < 0.001), and (OR = 1.89; 95% CI, 1.43–2.49, p < 0.001) compared with CT alone in advanced NSCLC, and significantly longer OS was observed in either pembrolizumab + chemotherapy, atezolizumab + chemotherapy, ipilimumab + chemotherapy, and nivolumab + ipilimumab + chemotherapy subgroup (all p < 0.001), indicating that ICI + CT is more effective than CT alone in the first-line treatment of advanced NSCLC. Similar results were found in other malignant tumors. In the phase 3 KEYNOTE-355 trial [24], pembrolizumab plus chemotherapy showed improved PFS versus chemotherapy among patients with metastatic triple-negative breast cancer with combined positive score (CPS) of 10 or more. In the CheckMate 649 trial [25], nivolumab in combination with chemotherapy was associated with significantly longer OS and PFS versus chemotherapy alone in previously untreated patients with advanced gastric, gastro-oesophageal junction, or oesophageal adenocarcinoma. In the IMpower133 trial [26], atezolizumab + chemotherapy resulted in significantly longer OS and PFS than chemotherapy alone in the first-line treatment of extensive-stage small-cell lung cancer (SCLC). These results suggest combined inhibition of immune checkpoint PD-1/PD-L1/CTLA-4 signaling pathway and chemotherapy resulting in enhanced anti-tumor activity. Preclinical studies suggests that PD-1/PD-L1/CTLA-4 checkpoint inhibitors increase T cells’ responses and reduce the acquired immune system tolerance which is overexpressed by cancer and tumor icroenvironment [27], and chemotherapeutic agents may increase immune-potentiating effects under certain condition, thereby enhancing the anti-tumor immune effects in tumors [28]. In addition, biomarkers for predicting an enhanced benefit for ICI combination therapies remain elusive, and whether PD-L1 can be used as a biomarker to predict outcome is controversial [29, 30]. In this study, compared with CT alone, ICI + CT showed significantly longer OS in either PD-L1-negative, PD-L1-positive, PD-L1-low and PD-L1-high group (all P < 0.001). The results were consistent with those from Landre et al. [31], who reported that PD-1/PD-L1 inhibitor plus chemotherapy showed improved OS, PFS and ORR versus CT alone for negative or < 1% PDL1expressing in the firstline treatment for metastatic NSCLC. These indicate that the addition of ICI to chemotherapeutic agents could benefit patients regardless PD-L1 expression levels, and PD-L1 can not be used as a biomarker to predict outcome for patients treated with ICI + CT in advanced NSCLC.
Regarding toxicities, the safety and tolerability profile of single-agent ICI was well established in cancers [32,33,34]. However, ICI combination therapies were reported to show increased treatment-related AEs over monotherapies in many studies. In the phase 3 CheckMate 649 trial [25], nivolumab plus chemotherapy significantly increased the incidence of grade 3–5 treatment-related AEs (59% vs. 44%) versus chemotherapy alone in patients with advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma. A recent meta-analysis [23] demonstrates that ICI combination therapies, including ICI + CT, were associated with significantly increased grade 3 or higher AEs (OR = 1.30, 95% CI: 1.03–1.57, p = 0.007) compared with monotherapies. In our study, the meta-analysis showed that the incidence of grade 3–5 treatment-related AEs in the ICI + CT group were significantly higher than that in the CT group (OR = 1.46, 95% CI: 1.19 − 1.79, p < 0.001). Treatment-related deaths showed similar between the two groups (OR = 1.94, 95% CI: 0.97–3.88, p = 0.061). Although the general safety profile of ICI + CT was found to be worse than that of CT, the incidence of treatment-related deaths is overall rare (0.7–1.9%) (Table 1), and the toxicities were manageable with appropriate monitoring.
Despite encouraging results, our study has several limitations. First, patients in each trial received different combination regimens (pembrolizumab plus chemotherapy, atezolizumab plus chemotherapy, ipilimumab plus chemotherapy, and nivolumab plus ipilimumab plus chemotherapy), and the anti-tumor mechanisms of ICIs (including PD-1, PD-L1 and CTLA-4 inhibitors) are different, which add heterogeneity to our analysis. Second, the number of included studies is small and only one trial was included in the nivolumab plus ipilimumab plus chemotherapy group, which may lead to a limitation in the evaluation of results in this study. Finally, the follow-up time among each trial is different, and the data of OS from some included trials were not mature enough because of the limited follow-up time.
Conclusions
Compared with CT alone, ICI + CT greatly enhances OS, PFS, and ORR rates in the first-line treatment for advanced NSCLC, with increased grade 3–5 treatment-related AEs. Survival benefit was observed for ICI + CT among all patients regardless PD-L1 expression levels. PD-L1 can not be used as a biomarker for predicting outcome for patients treated with ICI + CT. Due to the limitations in our study, further investigations are required.
Availability of data and materials
Not applicable.
References
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, Paz-Ares L (2017) Lung cancer: current therapies and new targeted treatments. Lancet. Jan 21;389(10066):299–311
Duma N, Santana-Davila R, Molina JR (2019) Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc. Aug;94(8):1623–1640
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology Group (2002) ;. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. Jan 10;346(2):92 – 8. doi: https://doi.org/10.1056/NEJMoa011954. PMID: 11784875
Darvin P, Toor SM, Sasidharan Nair V, Elkord E Immune checkpoint inhibitors: recent progress and potential biomarkers.Exp Mol Med. 2018 Dec13;50(12):1–11
Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR Jr, Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA (2017 Jun) CheckMate 026 Investigators. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 22(25):2415–2426
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G (2019 May) KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 4(10183):1819–1830
Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, Coluzzi P, Ledezma B, Mendenhall M, Hunt J, Wolf B, Jones B, Madrigal J, Horton J, Spiegel M, Carroll J, Gukasyan J, Williams T, Sauer L, Wells C, Hardy A, Linares P, Lim C, Ma L, Adame C, Garon EB (2018 Aug) A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC. J Thorac Oncol 13(8):1138–1145
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC (2018) ; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. May 31;378(22):2078–2092
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM (2018 Nov) KEYNOTE-407 Investigators. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 22(21):2040–2051
Awad MM, Gadgeel SM, Borghaei H, Patnaik A, Yang JC, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Altan M, Jalal SI, Panwalkar A, Gubens M, Sequist LV, Saraf S, Zhao B, Piperdi B, Langer CJ (2021 Jan) Long-Term Overall Survival From KEYNOTE-021 Cohort G: Pemetrexed and Carboplatin With or Without Pembrolizumab as First-Line Therapy for Advanced Nonsquamous NSCLC. J Thorac Oncol 16(1):162–168
West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, Sadiq A, Sandler A, Lin W, Ochi Lohmann T, Archer V, Wang L, Kowanetz M, Cappuzzo F (2019 Jul) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20(7):924–937
Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, Longeras PD, Goldschmidt J Jr, Novello S, Orlandi F, Sanborn RE, Szalai Z, Ursol G, Mendus D, Wang L, Wen X, McCleland M, Hoang T, Phan S, Socinski MA (2021 Apr) Atezolizumab Plus Chemotherapy for First-Line Treatment of Nonsquamous NSCLC: Results From the Randomized Phase 3 IMpower132 Trial. J Thorac Oncol 16(4):653–664
Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, Reck M (2012) Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. Jun 10;30(17):2046-54
Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, Han B, Ganea DE, Von Pawel J, Vladimirov V, Fadeeva N, Lee KH, Kurata T, Zhang L, Tamura T, Postmus PE, Jassem J, O’Byrne K, Kopit J, Li M, Tschaika M, Reck M (2017 Oct) Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J Clin Oncol 20(30):3449–3457
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, Alexandru A, Sakai H, Lingua A, Salman P, Souquet PJ, De Marchi P, Martin C, Pérol M, Scherpereel A, Lu S, John T, Carbone DP, Meadows-Shropshire S, Agrawal S, Oukessou A, Yan J, Reck M (2021 Feb) First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 22(2):198–211
Zhou Y, Chen C, Zhang X, Fu S, Xue C, Ma Y, Fang W, Yang Y, Hou X, Huang Y, Zhao H, Hong S, Zhang L (2018 Dec) Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis. J Immunother Cancer 22(1):155
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M (2018 Jun) IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 14378(24):2288–2301
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group; Cochrane Statistical Methods Group (2011 Oct) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 18:343:d5928
Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N, Hruban C, Guthrie VB, Rodgers K, Naidoo J, Kang H, Sharfman W, Georgiades C, Verde F, Illei P, Li QK, Gabrielson E, Brock MV, Zahnow CA, Baylin SB, Scharpf RB, Brahmer JR, Karchin R, Pardoll DM, Velculescu VE (2017 Mar) Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov 7(3):264–276
Tay RY, Heigener D, Reck M, Califano R (2019 Nov) Immune checkpoint blockade in small cell lung cancer. Lung Cancer 137:31–37
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, Sakai H, Albert I, Vergnenegre A, Peters S, Syrigos K, Barlesi F, Reck M, Borghaei H, Brahmer JR, O’Byrne KJ, Geese WJ, Bhagavatheeswaran P, Rabindran SK, Kasinathan RS, Nathan FE, Ramalingam SS (2019 Nov) Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 21(21):2020–2031
Wei Y, Du Q, Jiang X, Li L, Li T, Li M, Fan X, Li Y, Kariminia S, Li Q (2019 Jun) Efficacy and safety of combination immunotherapy for malignant solid tumors: A systematic review and meta-analysis. Crit Rev Oncol Hematol 138:178–189
Mo DC, Huang JF, Luo PH, Huang SX, Wang HL (2021 Jul) The efficacy and safety of combination therapy with immune checkpoint inhibitors in non-small cell lung cancer: A meta-analysis. Int Immunopharmacol 96:107594
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, Iwata H, Masuda N, Otero MT, Gokmen E, Loi S, Guo Z, Zhao J, Aktan G, Karantza V, Schmid P (2020 Dec) KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 5(10265):1817–1828
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. Jun 4:S0140-6736(21)00797-2
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV (2018) ; IMpower133 Study Group. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. Dec 6;379(23):2220–2229. doi: https://doi.org/10.1056/NEJMoa1809064. Epub 2018 Sep 25. PMID: 30280641
Rotte A Combination of CTLA-4 and PD-1 blockers for treatment of cancer.J Exp Clin Cancer Res. 2019 Jun13;38(1):255
Apetoh L, Ladoire S, Coukos G, Ghiringhelli F (2015 Sep) Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol 26(9):1813–1823
Walk EE, Yohe SL, Beckman A, Schade A, Zutter MM, Pfeifer J, Berry AB, College of American Pathologists Personalized Health Care Committee (2020 Jun) The Cancer Immunotherapy Biomarker Testing Landscape. Arch Pathol Lab Med 144(6):706–724
Patel SP, Kurzrock R (2015 Apr) PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther 14(4):847–856
Landre T, Des Guetz G, Chouahnia K, Taleb C, Vergnenègre A, Chouaïd C (2020 Feb) First-line PD-1/PD-L1 inhibitor plus chemotherapy vs chemotherapy alone for negative or < 1% PD-L1-expressing metastatic non-small-cell lung cancers. J Cancer Res Clin Oncol 146(2):441–448
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. Jun 28;366(26):2443–54
Nishijima TF, Shachar SS, Nyrop KA, Muss HB (2017 Apr) Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist 22(4):470–479
Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD (2015 Dec) Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26(12):2375–2391
Acknowledgements
The authors would like to thank Binyang County People’s Hospital.
Funding
None.
Author information
Authors and Affiliations
Contributions
L.F. M coordinated the data collection and conceived the original idea. J.F. H provided statistical analysis. L.F. M and P.H. L wrote the manuscript, all other Authors facilitated data collection and critically reviewed the manuscript for important intellectual contents.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors. No need for ethical approval and informed consent.
Consent for publication
All authors consent to publish this paper in this present form.
Conflict of interest
The authors declare no conflicts of interest.
Disclosure of potential conflicts of interest
None.
Research involving Human Participants and/or Animals
None.
Informed consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, LF., Huang, JF., Luo, PH. et al. The efficacy and safety of immune checkpoint inhibitor plus chemotherapy in patients with advanced non-small-cell lung cancer: a meta-analysis. Invest New Drugs 40, 810–817 (2022). https://doi.org/10.1007/s10637-022-01232-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01232-8