Summary
Purpose Immune-related adverse events (IrAEs) are auto-immune reactions associated with immune checkpoint inhibitor-based therapy (ICI). To date, little is known about immunotherapy-induced pneumonitis (IIP). In this study, we investigated the clinical and CT features of IIP in non-small cell lung cancer (NSCLC) patients treated with ICI. Methods CT images and clinical data of 98 NSCLC patients in our hospital were retrospectively analyzed after ICI therapy, and the incidence, onset time, CT findings, grade, treatment and prognosis of IIP were recorded. Results Nineteen patients developed IIP, which occurred 42∼210 days after ICI therapy, and the median time was 97 days. The CT findings for IIP showed multifocal ground-glass opacity (GGO) in 5 cases, patchy shadows in 6 cases, mixed distribution of patchy and strip-like shadows in 4 cases, and patchy shadows with honeycomb lung in 4 cases. The mean age and proportions of smokers, CD3+ and CD4+ of T lymphocyte subset in patients with IIP were significantly higher than those in patients without IIP (all p < 0.05). Among 19 patients with IIP, there were 10 patients with grade 1 ~ 2 and 9 patients with grade 3 ~ 4; 13 patients received hormone therapy, 12 of them were improved or stable, and 1 patient got worse after hormone therapy. No deaths from IIP were found. Conclusion IIP is a relatively rare but serious adverse event, and it is sensitive to hormone therapy. Its CT manifestations are diverse, and timely detection and treatment are the keys to reduce IIP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the malignant tumor with the highest morbidity and mortality in the world, which is a serious threat to human health [1,2,3]. In recent years, several immune checkpoint inhibitors (ICI) including programmed death protein-1 (PD-1) or programmed death protein ligand-1 (PD-L1) inhibitors have been Food and Drug Administration (FDA)–approved for certain types of advanced cancer, and have brought significant long-term survival benefits to patients with advanced lung cancer [4]. The therapy principle of PD-1/PD-L1 inhibitors is to activate suppressed anti-tumor immune cells such as T cells, but overactivated immune cells will attack human normal cells, so the adverse reaction spectrum of immunotherapy is completely different from that of chemotherapy or targeted therapy. Although the overall incidence of adverse reactions in immunotherapy is low, there will still be serious immune related adverse events (irAEs) in patients receiving immunotherapy, such as immunotherapy-induced pneumonitis (IIP), immune-related myositis or myocarditis and so on [5, 6].
IIP is defined as the presence of infiltrates on thoracic imaging and clinical symptoms of cough, shortness of breath, or wheezing, and the absence of microbiological evidence of infection in patients who were currently receiving immunotherapy [7]. At present, there are few reports about IIP in the world, but with the usage of PD-1/PD-L1 inhibitors, it is particularly important to evaluate and monitor the incidence, clinical features, treatment and prognosis of IIP as soon as possible [8]. A study also suggested that the overall mortality associated with PD-1/PD-L1 inhibitor treatment was 0.45%, of which deaths caused by IIP were the most common (28.0%) [9]. It can be seen that although the overall incidence of IIP is not common, serious IIP have serious life-threatening consequences if it is not handled properly. So, clinicians need to pay more attention to the rare but serious adverse event [10].
Therefore, the goal of our study was to summarize and analyze the CT manifestations, clinical features, onset time, grade, treatment and prognosis of IIP in advanced NSCLC patients after ICI monotherapy, so as to provide some reference information for early detection and treatment of IIP.
Materials and methods
Patient population
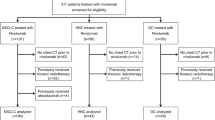
This study was approved by the ethics committee of our hospital, and written informed consent was obtained from all participants. CT and clinical data of patients with advanced NSCLC who received PD-1/PD-L1 inhibitor monotherapy in our hospital from January 2017 to June 2020 (including patients enrolled in clinical studies and non-clinical studies) were retrospectively analyzed. Inclusion criteria: (1) Pathologically confirmed non-small cell lung cancer, according to the eighth edition of the AJCC staging as III~IV; (2) Received ICI treatment; (3) Age > 18 years old; (4) Performed CT scan of lung before and after immunotherapy. Exclusion criteria: (1) Lack of complete clinical and lung CT data; (2) Prior radiotherapy or chemotherapy for lung cancer; (3) Prior tuberculosis and fungi infectious diseases in the lung before immunotherapy. Finally, 98 NSCLC patients were selected for the further analysis, including 19 patients with IIP and 79 patients without IIP. Their CT manifestations, incidence, onset time, grade, treatment results and prognosis of IIP were recorded.
CT protocol
Siemens 64-slice spiral CT scanner was used to perform lung examination. The scan range was from the tip of the lung to the bottom of the lung. Scanning parameters were as follows: tube voltage 120 kV, tube current 120∼200 mA, scan thickness 5.0 mm, pitch 1.087∼1.375, reconstruction thickness 1.25 mm, lung window reconstruction with high-resolution bone algorithm, window level − 550∼-700HU, window width 1000∼1500HU.
ICI therapy of NSCLC patients
In 98 NSCLC patients, the types of immunotherapy injection and the respective number of patients were as follows: Nivolumab (Opdivo, America, BMS company, 100 mg/10 ml per bottle) in 36 cases, Pembrolizumab (Keytruda, America, Merk company, 100 mg/4 ml per bottle) in 16 cases, Sintilimab (China, 100 mg/10 ml per bottle) in 30 cases, Toripalimab (China, 240 mg/6 ml per bottle) in 16 cases.
Diagnosis and grade of IIP
The diagnosis of IIP is determined through the patient’s clinical manifestations, blood test results, CT findings, and whether glucocorticoid therapy is effective. IIP is defined as the presence of infiltrates on thoracic imaging and clinical symptoms of cough, shortness of breath, or wheezing, and the absence of microbiological evidence of infection in patients who were currently receiving immunotherapy [11]. The severity or grading of IIP was divided into grades 1 to 4 according to the common terminology criteria for adverse events (CTCAE) [12, 13]. They were seen in Table 1.
Statistical analyses
SPSS23.0 software was used to statistically analyze the data. An independent samples t-test was used to assess the differences in numeric variable (age).While Chi-Squared tests were used to compare the difference in categorical variables (gender, smoking status, clinical stage, PD-L1 expression, gene status, and pathological types, etc.) in 98 NSCLC patients between IIP and non-IIP groups. A p value < 0.05 was set, indicating a statistically significant difference.
Results
Clinical characteristics of patients with NSCLC
A total of 98 patients with stage III and IV NSCLC were treated with PD-1/PD-L1 inhibitors, with a mean age of 58.87 ± 11.38 years (range: 37∼78 years), 57 males and 41 females. Among them, 19 patients (19.4%) developed IIP. The mean age and proportions of smokers, CD3+ and CD4+ of T lymphocyte subset in patients with IIP were significantly higher than those in patients without IIP (all p < 0.05). However, there were no significantly statistical differences in gender, Eastern Cooperative Oncology Group (ECOG) score, clinical stage, histological type, gene status, PD-L1 expression, ICI usage, and basic diseases between IIP and non-IIP groups (all p > 0.05). They were seen in Table 2.
Onset time and symptoms of patients with IIP
The onset time of 19 patients with IIP was 42∼210 days after immunotherapy, and the median onset time was 97 days. There were 6 cases (31.6%) with grade 1 IIP, 4 cases (21.1%) with grade 2 IIP, 7 cases (36.8%) with grade 3 IIP and 2 cases (10.5%) with grade 4 IIP. Among them, 13 patients (68.4%) had clinical manifestations, including chest pain (n = 4), shortness of breath (n = 8), dyspnoea (n = 4), cough and sputum (n = 10), respiratory failure (n = 1) and fever (n = 3). Some cases had multiple clinical manifestations.
CT findings of patients with IIP
Of the 19 patients with IIP, there were various imaging manifestations on their lung-window CT images, including multifocal ground glass opacities (GGO) in 5 cases (26.3%), pure patchy shadow in 6 cases (31.5%), patchy mixed strip-like shadow in 4 (21.1%), patchy shadow combined with honeycomb lung in 4 (21.1%). Infiltrating lesions in lung tissue presented asymmetric distribution in 17 cases (89.5%). The lesions were located in the periphery of the lung in 8 cases (42.1%), and the lower or middle lung lobe in 17 cases (89.5%). Multiple lung lobes were involved in 12 cases (63.2%), while a single lung lobe was involved in 7 cases (36.8%).
Treatment and prognosis of patients with IIP
Of the 19 patients with IIP, 9 patients including grade 3 (n = 7) and 4 (n = 2) IIP were permanently discontinued immunotherapy, 6 patient with grade 1 IIP continued to receive ICI therapy, and 4 patients with grade 2 IIP received a reduced dose of ICI therapy. Thirteen patients were treated with corticosteroids, including 2 cases of high-dose, 7 cases of moderate-dose, 4 cases of low-dose. 7 patients were treated with intravenous combined with oral corticosteroids, 4 patients with grade 2 IIP were treated with oral corticosteroids only,2 patients were additionally treated with immunoglobulin, and 6 patients with grade 1 IIP were untreated (Table 3). After several weeks, 15 patients were improved, including complete remission (n = 3) and partial remission (n = 12) cases, their CT findings indicated that the IIP lesion had absorbed or dissipated; 3 patients were stable, their CT findings showed that the IIP lesion did not change, and clinical symptoms were relieved; 1 patient with grade 3 IIP was progressive, presenting new pneumonia infiltration lesions on CT images. Six untreated patients with grade 1 IIP were asymptomatic, and then their re-examination result of lung CT indicated that IIP lesions were absorbed or reduced after several weeks (Table 4). No patients died of IIP.
Analysis of representative cases
The onset, development, treatment and prognosis of three representative patients with IIP were briefly described and analyzed as follows.
Case 1
a 62-year-old male patient with stage IV quamous cell lung carcinoma, smoker, Eastern Cooperative Oncology Group (ECOG) score 1, no history of basic lung disease. After 98 days of treatment with Pembrolizumab (Keytruda, America, Merk company, 100 mg/4 ml per bottle) by intravenous infusion dose of 2 mg/kg for once every 3 weeks, he developed grade 1 IIP that the percentage of the area involved by infiltration lesions was less than 25% of homolateral lung lobe. At this time, strip-like and patches of high-density shadows were seen in both paramediastinal lung lobes on his lung-window CT images. And he held immunotherapy, and did not use glucocorticoid treatment. When he reexamined chest CT scan two weeks later, the previous IIP infiltration lesion had dissipated (Fig. 1).
Lung-window CT images of a 62-year-old male patient with grade 1 IIP. Strip-like high density shadows (arrow) in the left upper lung lobe (a) and patch of high density shadows (arrow) in the right lower lung lobe (c) were presented. And he did not use glucocorticoid treatment. When he reexamined chest CT scan two weeks later, the previous IIP infiltration lesions had dissipated (b and d). IIP, immunotherapy-induced pneumonitis
Case 2
a 65-year-old male patient with stage IV lung adenocarcinoma, smoker, ECOG score 2, no history of basic lung disease. After 120 days of treatment with Nivolumab (Opdivo, America, BMS company, 100 mg/10 ml per bottle) by intravenous infusion dose of 3 mg/kg for once every 2 weeks, she developed grade 2 IIP that the percentage of the area involved by infiltration lesions was 25%∼50%. At this time, irregular flaky high density shadow and GGO were seen in both lung lobes on his lung-window CT images. And he permanently discontinued immunotherapy, and use oral glucocorticoid treatment for three weeks. When he reexamined chest CT scan, the previous IIP infiltration lesions had absorbed or reduced and dissipated (Fig. 2).
Lung-window CT images of a 65-year-old male patient with grade 2 IIP. Irregular flaky high density shadow (arrow) in the left upper lung lobe, GGO (arrow) in the right upper lung lobe (a) and GGO (arrow) in the left upper lung lobe (c) were presented; and he use oral glucocorticoid treatment for three weeks. When he reexamined chest CT scan, the previous IIP infiltration lesions had absorbed or reduced (b) and dissipated (d). IIP, immunotherapy-induced pneumonitis; GGO, ground-glass opacity
Case 3
a 67-year-old female patient with stage IV lung adenocarcinoma, non-smoking, ECOG score 2, no history of basic lung disease. After 80 days of treatment with Sintilimab (China, 100 mg/10 ml per bottle) by intravenous infusion dose of 200 mg for once every 3 weeks, she developed grade 3 IIP that the percentage of the area involved by infiltration lesions was 50%∼75%. At this time, large patches of high-density shadow were seen in both lung lobes on her lung-window CT images. And she permanently discontinued immunotherapy, and use oral and intravenous glucocorticoid treatment for three weeks. When she reexamined chest CT scan, the previous IIP infiltration lesions had absorbed or reduced (Fig. 3).
Lung-window CT images of a 67-year-old female patient with grade 3 IIP. An irregular tumor (arrow) in the left upper lung lobe was presented (a); After 98 days of treatment with Pembrolizumab by intravenous infusion dose of 2 mg/kg for once every 3 weeks, the mass shrunk (b). And she developed grade 3 IIP, presenting large patches of high-density shadow (arrows) in both lungs, and the bronchial inflation sign (arrows) was seen in the lesions (c). She used oral and intravenous glucocorticoid treatment for three weeks. When she reexamined chest CT scan, the previous IIP infiltration lesions had absorbed or reduced (d). IIP, immunotherapy-induced pneumonitis
Discussion
PD-1/PD-L1 inhibitors can block the interaction between PD-1 receptor on T cells and its ligand PD-L1 on tumor cells, so as to restore T cell function and enhance T cell killing ability to tumor cells [14]. However, overactivated T cells can cause autoimmune-mediated adverse reactions, produce immune damage to various system tissues of the body, such as skin and gastrointestinal reactions, fatigue, endocrine toxicity, hepatotoxicity, neurotoxicity, cardiotoxicity, as well as rare IIP. In this study, proportions of CD3+ and CD4+ of T lymphocyte subset in patients with IIP were significantly higher than those in patients without IIP. IIP is a relatively rare but fatal immune related adverse events (irAEs). In a phase I study of Nivolumab in the treatment of NSCLC, 3 (2%) patients died of IIP [15], and similar studies had been reported one after another. Therefore, the special adverse event of IIP deserved great attention. Some studies had shown that the incidence of IIP after PD-1 inhibitor monotherapy was 3–5% [16,17,18]. In general, clinical studies excluded patients with poor ECOG scores for performance status (PS), interstitial lung disease and chronic obstructive pulmonary disease, which was inconsistent with real-world reports of IIP.
Our study summarized and analyzed the incidence, severity, treatment, and prognosis of IIP in NSCLC patients after immunotherapy with single PD-1 or PD-L1 inhibitors. The results showed that the overall incidence of IIP was 19.4% (19/98), the percentage of grade 1–2 IIP was 52.6% (10/19), and the percentage of grade 3–4 IIP was 47.4% (9/19). The onset time of IIP was 42–210 days after immunotherapy, and the median time was 97 days. The incidences of IIP in the Checkmate 017 study and Checkmate 057 study were 4.6% and 1.4% respectively [16, 19], and the incidence of IIP in the Keynote 010 study was 5% [17]. Yamaguchi et al. [20] reported that the incidence of IIP in NSCLC patients treated with PD-1 inhibitors was 14.6%, the percentage of IIP ≥ grade 3 was 3.3%, and the median onset time of IIP was 60 days after immunotherapy. Fujimoto et al. [21] retrospectively analyzed the real-world study in 615 advanced NSCLC patients treated with Nivolumab. 63 patients (10.2%) developed IIP and 5% of the patients belonged to grade 3 IIP or above. Naidoo et al. [22] analyzed 915 patients with NSCLC and melanoma treated with PD-1/PD-L1 inhibitors, and the total incidence of IIP was 5%. And they thought there was no significant difference in the incidence of IIP between NSCLC and melanoma patients. Among them, 72% of the patients belonged to grade 1–2 IIP, 86% of the patients gradually relieved their symptoms after hormone therapy, and only 1 patient died of IIP. In another retrospective analysis of 64 patients with IIP, Delaunay et al. [23] found that the incidence of IIP in males and smokers was higher than that in females and non-smokers, which was consistent with some of the results in our study. In this study, the incidence of IIP was relatively high, considering that most of the previously reported data were clinical studies, and the scores for performance status (PS) of patients was better, while this study included some non-clinical studies patients. Although patients with pulmonary tuberculosis and fungal infection were excluded, some patients were still complicated with chronic interstitial lung disease, so the incidence of IIP was relatively high in this study.
At present, there are still some difficulties in the diagnosis of IIP, because some patients’ clinical symptoms and CT manifestations are neither obvious nor typical, showing diversity. When a patient received immunotherapy, and there were new respiratory symptoms and new infiltration lesions in the lung, IIP should be considered in the diagnosis of lung disease. A study thought that the pathogenesis of IIP was that inflammatory factors led to inflammatory response by relieving the regulation of immune effector factors and T cells in the pulmonary interstitium [24]. In addition, a significant increase in the proportion of lymphocytes was found in bronchoalveolar lavage fluid and bronchoscopic biopsy specimens of patients with IIP. Compared with the normal control group, the number of activated T cells in bronchoalveolar lavage fluid of patients with IIP increased, indicating that T cells may play an important role in the pathogenesis of IIP [24]. In this study, proportions of CD3 + and CD4 + of T lymphocyte subset in patients with IIP were significantly higher than those in patients without IIP. Naidoo et al. [22] reported that the main clinical symptoms of IIP were cough, dyspnea, hypoxia, and even respiratory failure. Chest CT examination before the appearance of clinical symptoms could find lung-related lesions, and it was possible to find asymptomatic patients with IIP. Nishino et al. [25] reported CT findings of IIP in 2 patients with advanced NSCLC treated with Nivolumab. Most of them were ground glass opacity (GGO) in both lungs, as well as consolidation of the lower and peripheral lung lobe; they found that patients presented the symptoms of cough, expectoration, chest tightness, shortness of breath, and the symptoms were relieved after hormone therapy. In this study, the main CT manifestations of IIP revealed GGO and patchy infiltration shadow in the peripheral and lower lung lobes. Of the 13 IIP patients treated with hormone, 12 were improved, 1 was progressive, and there was no death caused by IIP. Nishino et al. [26] reported that among the 10 clinical studies, 20 (11.8%) patients developed IIP, 85% of the patients received hormone therapy, and 19 patients were relieved.
This study had some limitations and shortcomings. First, this study was a retrospective study, which could produce IIP evaluation bias; Second, the sample size of this study was small and needed to be further verified by large sample data.
In conclusion, IIP caused by PD-1/PD-L1 inhibitors was a relatively rare but fatal adverse event with various CT findings, which could be significantly relieved after early detection and hormone therapy.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34. https://doi.org/10.3322/caac.21551
Chen WQ, Zheng RS, Baade PD et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–132. https://doi.org/10.3322/caac.21338
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Khanna P, Blais N, Gaudreau PO, Corrales-Rodriguez L (2017) Immunotherapy comes of age in lung cancer. Clin Lung Cancer 18(1):13–22. https://doi.org/10.1016/j.cllc.2016.06.006
Allenbach Y, Anquetil C, Manouchehri A (2020) Immune checkpoint inhi-bitor-induced myositis, the earliest and most lethal complication among rheumat-ic and musculoskeletal toxicities. Autoimmun Rev 19(8):102586. https://doi.org/10.1016/j.autrev.2020.102586
Sławiński G, Wrona A, Dąbrowska-Kugacka A, Raczak G, Lewicka E (2020) Immune checkpoint inhibitors and cardiac toxicity in patients treated for non-small lung cancer: A review. Int J Mol Sci 21(19):7195. https://doi.org/10.3390/ijms21197195
Suresh K, Naidoo J, Lin CT, Danoff S (2018) Immune checkpoint immunotherapy for non-small cell lung cancer (benefits and pulmonary toxicities). Chest 154(6):1416–1423. https://doi.org/10.1016/j.chest.2018.08.1048
Rossi E, Schinzari G, Tortora G (2020) Pneumonitis from immune checkpoint inhibitors and COVID-19: current concern in cancer treatment. J Immunother Cancer 8(2):e000952. https://doi.org/10.1136/jitc-2020-000952
De Velasco G, Je Y, Bosse D et al (2017) Comprehensive meta analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res 5(4):312–318. https://doi.org/10.1158/2326-6066.CIR-16-0237
Mitropoulou G, Daccord C, Sauty A et al (2020) Immunotherapy-induced airway disease: A new pattern of lung toxicity of immune checkpoint inhibitors. Respiration 99(2):181–186. https://doi.org/10.1159/000504968
Colen RR, Fujii T, Bilen MA et al (2018) Radiomics to predict immunotherapy-induced pneumonitis: proof of concept. Invest New Drugs 36(4):601–607. https://doi.org/10.1007/s10637-017-0524-2
Haanen J, Carbonnel F, Robert C et al (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:i119–i142. https://doi.org/10.1093/annonc/mdx225
Puzanov I, Diab A, Abdallah K et al (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer 5(1):95. https://doi.org/10.1186/s40425-017-0300-z
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26): 2443–2454. https://doi.org/10.1056/NEJMoa1200690
Gettinger SN, Horn L, Gandhi L et al (2015) Overall survival and long-term safety of Nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33(18):2004–2012. https://doi.org/10.1200/JCO.2014.58.3708
Brahmer J, Reckamp KL, Baas et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135. https://doi.org/10.1056/NEJMoa1504627
Herbst RS, Baas P, Kim D et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-l1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Reck M, RodrÍguez-abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non- small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Borghaei H, Paz-ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
Yamaguchi T, Shimizu J, Hasegawa T et al (2018) Preexisting pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: A retrospective analysis. Lung Cancer 125:212–217. https://doi.org/10.1016/j.lungcan.2018.10.001
Fujimoto D, Yoshioka H, Kataoka Y et al (2018) Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: A multicenter retrospective cohort study. Lung Cancer 119:14–20. https://doi.org/10.1016/j.lungcan.2018.02.017
Naidoo J, Wang X, Woo KM et al (2017) Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand-1 therapy. J Clin Oncol 35(7):709–717. https://doi.org/10.1200/JCO.2016.68.2005
Delaunay M, Cadranel J, Lusque A et al (2017) Immunecheckpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 50:1700050. https://doi.org/10.1183/13993003.00050-2017
Barjaktarevic IZ, Qadir N, Suri A et al (2013) Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest 143(3):858–861. https://doi.org/10.1378/chest.12-1467
Nishino M, Chambers ES, Chong CR et al (2016) Anti-PD-1 inhibitor-related pneumonitis in non-small cell lung cancer. Cancer Immunol Res 4(4):289–293. https://doi.org/10.1158/2326-6066.CIR-15-0267
Nishino M, Ramaiya NH, Awad MM et al (2016) PD-1inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res 22(24):6051–6060. https://doi.org/10.1158/1078-0432.CCR-16-1320
Acknowledgements
The authors would like to acknowledge all of participants with IIP and lung cancer participants treated with immune-check-point inhibitors who allowed us to conduct this research in an effort to improve the lives of people living with IIP.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81671743), the clinical key diseases diagnosis and therapy special project of Health and Family Planning Commission of Suzhou (No. LCZX201801), the program for Advanced Talents within Six Industries of Jiangsu province (No. WSW-057), and the High-level Health Personnel “six-one” Project of Jiangsu province in China (No. LGY2016035), and was funded by the Taihu High-level Talent Training Project (Double hundred Medical Youth Professionals Program) from Health Committee of Wuxi city in China (No. HB2020046).
Author information
Authors and Affiliations
Contributions
ZQS and SW contributed to the design of study, the acquisition, analysis and interpretation of data, the draft and revision of the manuscript. DHD, HLS, JFZ and YGL gave the discussion and comments on an earlier version of the manuscript. All authors read and approved the final manuscript to be published in this Journal.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Ethics approval and consent to participate
This study was approved by the Medical Ethical Committee of the First Affiliated Hospital of Soochow University, and all participants gave written informed consent.
Consent for publication
Written informed consent for publication was obtained from all participants.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Z., Wang, S., Du, H. et al. Immunotherapy-induced pneumonitis in non-small cell lung cancer patients: current concern in treatment with immune-check-point inhibitors. Invest New Drugs 39, 891–898 (2021). https://doi.org/10.1007/s10637-020-01051-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-01051-9