Summary
Background The efficacy and safety of bevacizumab in elderly patients with non-small cell lung cancer remain controversial. This study focused on both selecting fit elderly patients and overcoming interpatient variability with respect to pharmacodynamics. Methods Elderly (age: ≥70 years) patients with advanced non-squamous non-small cell lung cancer were enrolled. Patients with uncontrolled congestive heart failure and uncontrolled diabetes were excluded. The treatment regimen comprised carboplatin at an area under the curve of 5 mg/ml/min on day 1, paclitaxel at 90 mg/m2 on days 1 and 8, and bevacizumab at 15 mg/kg on day 1 every 21 days for up to 4 cycles, followed by maintenance bevacizumab. Dose reduction due to side effects was performed, with a wide range of doses of paclitaxel from 23 mg/m2/week to 60 mg/m2/week. Results Of the 36 patients entered, 38.9% required a dose reduction or cancellation of paclitaxel administration on day 8, and 75% patients were able to complete 4 cycles of triplet therapy. The response rate, primary endpoint, was 69.4% (95% confidence interval [CI]: 51.9–83.7). The median progression free survival and overall survival were 8.4 months and 29.2 months, respectively. The most common adverse events included neutropenia, hypertension, anemia, and infection. Although Grade ≥ 3 adverse events were observed in 24 patients (66.7%), there were no deaths due to toxicity. Conclusion Carboplatin plus weekly paclitaxel with bevacizumab is a feasible, effective first-line regimen for elderly non-small cell lung cancer patients. (UMIN00006622).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most common cancer worldwide and the leading cause of cancer-related deaths [1]. Most patients with non-small cell lung cancer (NSCLC) are elderly, and about half of NSCLC patients are more than 75 years old in Japan. Although age itself is a contraindication to treatment, this parameter has important implications for the management and treatment of disease. Aging is associated with decreased physiologic reserve and polypharmacy, which lead to reduced tolerance of cancer chemotherapy and interpatient variability with respect to pharmacodynamics [2]. Furthermore, comorbidities are frequently present in elderly populations. Therefore, under-treatment is also an additional risk for older individuals, and elderly patients tend to be underrepresented in clinical trials [3, 4].
There are a few published reports based on studies of elderly patients with NSCLC. The first randomized phase III trial dedicated to elderly patients with advanced NSCLC employed a single-agent chemotherapy [5]. The Elderly Lung cancer Vinorelbine Italian Study compared single-agent vinorelbine to best supportive care (BSC) alone in elderly patients aged more than 70 years with advanced NSCLC. Although the primary end point of the study was health-related quality of life (QoL), a benefit in overall survival (OS) was demonstrated in vinorelbine-treated patients. More recently, a French phase III trial of elderly NSCLC patients (aged 70–89 years, performance status 0–2) was conducted by the intergroup Francophone de Cancerologie Thoracique (IFCT; IFCT-0501 trial). In this trial, a combination of weekly paclitaxel and carboplatin yielded survival benefits when compared with vinorelbine or gemcitabine monotherapy [6]. However, there was increased toxicity, particularly neutropenia, and the rate of treatment-induced death was 4.4%, indicating that interpatient variability in pharmacodynamics was critical.
Vascular endothelial growth factor (VEGF) is a cytokine that plays pivotal roles in tumor angiogenesis, growth, and metastasis. Accordingly, VEGF targeted therapies comprise an important cancer therapeutic modality [7]. A phase III trial (E4599) found that both OS and progression-free survival (PFS) in patients with non-squamous NSCLC were significantly prolonged when bevacizumab (a humanized monoclonal antibody that targets VEGF) was added to a first-line combination therapy of carboplatin and paclitaxel [8]. However, a retrospective analysis of the E4599 trial, which involved an elderly population (age > 70 years) raised some cautions regarding bevacizumab use [9]. A post-hoc subset analysis did not indicate an obvious improvement in survival relative to the standard platinum doublet, and the frequency of grade ≥ 3 adverse events (AEs) related to bevacizumab use, which included neutropenia, bleeding, and proteinuria, was significantly higher among elderly patients. In addition, treatment-related deaths tended to occur more frequently in the bevacizumab arm. To date, the clinical benefit of bevacizumab in elderly NSCLC patients remains controversial.
We had previously investigated a phase II study employing the three drug regimen consisted of carboplatin plus weekly paclitaxel with bevacizumab for elderly patients. This regimen comprised carboplatin at an concentration-time curve (AUC) of 6 mg/ml/min on day 1, paclitaxel at 70 mg/m2 on days 1, 8, 15, and bevacizumab at 15 mg/kg on day 1 every 28 days for up to 4 cycles, followed by maintenance bevacizumab. This study had terminated following enrollment of the first 5 patients, because two of five patients had experienced the treatment related deaths due to congestive heart failure (CHF) or severe diarrhea (Data has not been published). Especially, one patient having cardiac comorbidities had experienced toxic death due to CHF.
To avoid an anaphylactic reaction, paclitaxel needs to be administered concomitantly with a high dose of steroid, which elevates blood sugar level. Diabetic nephropathy, which is one of the most important long-term complications of diabetes, is often associated with proteinuria and decreasing glomerular filtration rate (GFR) even during the early stages of disease. This condition may be enhanced due to treatment with bevacizumab and due to the myelotoxicity induced by carboplatin. It was for these reasons that we used HbA1c data to exclude patients with uncontrollable DM.
Based on theses, we focused on patient selection criteria in an attempt to avoid severe or fatal toxicities due to bevacizumab. We conducted this prospective phase II trial to evaluate the three drug regimen under dose adjustment due to side effects in a selected population of elderly NSCLC patients without uncontrollable DM and CHF.
Patients and Methods
Patient eligibility
Eligible patients were 70 years or older, had histologically or cytologically confirmed stage IIIB, IV, or recurrent non-squamous NSCLC (TNM classification, 7th edition), and had never undergone cytotoxic chemotherapy. The following were required for all patients: an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; measurable lesions, as defined by the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1); adequate organ function, including an absolute neutrophil count ≥2000/μl; hemoglobin ≥10 g/dl; platelet counts ≥100,000/μl; aspartate transaminase and alanine transaminase ≤100 Units/L; bilirubin ≤1.5 mg/dl; serum creatinine ≤1.5 mg/dl; urine dipstick <2+ for protein; hemoglobin A1c (HbA1c) ≤7.0%; Left ventricular ejection fraction (LVEF) ≥50%; and B-type natriuretic peptide (BNP) ≤100 pg/ml.
Key exclusion criteria included the following: a history of hemoptysis (≥2.5 ml); regular use of aspirin (≥325 mg/day) or anticoagulants taken within 10 days prior to enrollment; a tumor in close proximity to a major vessel or with cavitation; symptomatic central nervous system metastases; uncontrollable hypertension; presence of unstable ascites, pleural effusions, or pericardial effusions; clinically unstable comorbidities, including cardiovascular disease, stroke, gastric ulcer, and interstitial pneumonitis; history of active double cancer; or a decision of ineligibility provided by an investigator.
Treatment
Eligible patients received bevacizumab at a dose of 15 mg/kg intravenously on day 1, paclitaxel at a dose of 90 mg/m2 intravenously on days 1 and 8, and carboplatin at a dose calculated to produce an area AUC of 5 mg/ml/min intravenously on day 1 every 21 days, for up to 4 cycles during the induction phase. Patients who did not exhibit disease progression after the induction phase were eligible to continue bevacizumab at a dose of 15 mg/kg, administered intravenously on day 1 of a 21-day cycle until progression or intolerance. Paclitaxel was administered on day 8 depending on toxicity recovery (i.e., PS of 0–1, absolute neutrophil count ≥1000/μl, platelet count ≥75,000/μl, and return to grade 0 or 1 non-hematological toxicities).
Carboplatin and/or paclitaxel dose reductions were performed in each subsequent cycle in the event of severe toxicities in the previous cycle, including thrombocytopenia <25,000/μl, febrile neutropenia, grade ≥ 2 sensory neuropathy or other grade ≥ 3 non-hematological toxicities. A dose reduction comprised a decrease in carboplatin dosing to an AUC of 4 mg/ml/min and a decrease in paclitaxel to 70 mg/m2. Subsequent dose increases were not allowed after a reduction in the chemotherapy dose. Bevacizumab administration was delayed in the presence of bevacizumab-related severe toxicities, such as grade ≥ 3 thrombotic events, bleeding events, hypertension, or proteinuria. Bevacizumab dose reductions were not allowed. Patients who required a treatment delay of ≥3 weeks were not permitted to restart bevacizumab.
Clinical assessment
We planned a feasibility analysis conducted by an independent data monitoring committee following enrollment of the first 6 patients. The primary endpoint of this study was the objective response rate (ORR), which was confirmed complete responses (CR) and/or partial responses (PR). Responses were assessed using the RECIST, version 1.1. Tumors were assessed every other therapy cycle to determine treatment responses. Secondary endpoints included PFS, OS, disease control rate (DCR), and AE profiles. PFS was defined as the time from registration to the date of initial disease progression or death, whichever occurred first. OS was defined as the time from registration to death from any cause. Tumor responses and PFS were assessed in an external review. AEs were assessed using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Statistical methods
The study design assumed an expected overall RR of 50% and a minimum acceptable ORR of 25%, with α and β errors of 0.05 and 0.1, respectively. Survival data were estimated using Kaplan–Meier methodology, and the differences between subgroups were analyzed by log rank. P values less than .05 were considered significant. In addition, we performed a subset analysis to correlate the epidermal growth factor receptor (EGFR) mutation status or age (70–74 years vs. ≥75 years) with efficacy and safety outcomes.
Results
Baseline patient characteristics
Our preplanned feasibility analysis was performed upon accrual of the first six patients. As no severe AEs were observed, the Independent Data Monitoring Committee accepted additional patient accrual. A total of 36 patients were enrolled in this study between February 2012 and September 2014. Table 1 shows the baseline characteristics of all patients. Nearly all patients had been diagnosed with adenocarcinoma. Fourteen and 22 patients were EGFR mutation-positive and -negative, respectively; among the EGFR mutation-positive patients, 10 (71.4%) had previously been treated with EGFR tyrosine kinase inhibitors.
Exposure to study treatment
The consort diagram is shown in Fig. 1. The 36 eligible patients received a median of 4 cycles (range: 1–4 cycles) of chemotherapy in the induction phase. Twenty-seven (75%) of 36 patients were able to complete all 4 cycles. Seven patients (19.4%) required at least 1 dose reduction and 14 (38.9%) required cancellation of paclitaxel administration on day 8; however, 22 (61.1%) of the 36 patients completed induction chemotherapy without dose reductions or cancellation of paclitaxel administration on day 8.
In addition, 25 (69.4%) of the 36 patients were eligible for the maintenance phase. A total of 11 patients discontinued maintenance with bevacizumab (3 because of disease progression, 3 because of chemotherapy-related AEs, 2 because of patient and investigator discontinuance, and 3 for other reasons). Patients who were treated with bevacizumab as maintenance therapy received a median of 6 cycles (range: 1–26 cycles).
Responses and safety profiles
Of the 36 patients, one who dropped out before the first efficacy assessment was not evaluable for responses and survival outcomes. Two patients (5.6%) achieved a complete response and 23 patients achieved a partial response, for a response rate of 69.4% (95% CI: 51.9–83.7%; Table 2, Fig. 2).
The AEs experienced by all patients during the induction and maintenance phases are summarized in Table 3. Grade ≥ 3 AEs were observed in 24 patients (66.7%). Grade ≥ 3 non-hematologic toxicities occurred in 13 patients (36.1%), and grade ≥ 3 hypertension occurred in 4 patients (11.1%). In addition, 2 patients (5.6%) had febrile neutropenia. Bleeding events were observed in 8 patients (22.2%), including Grade 3 bleeding events such as epistaxis or colonic hemorrhage in 2 patients. One patient experienced a grade 1 thromboembolic event, whereas no patients experienced a grade > 3 thrombotic or cardiovascular treatment-related AE. Importantly, although Grade ≥ 3 AEs were observed in 24 patients (66.7%) in our study, there were no toxicity-related deaths.
Survival outcomes
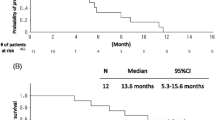
Among all enrolled patients, 24 received chemotherapy as a second-line therapy (66.7%). Among 14 patients harboring EGFR mutations, 4 patients received EGFR tyrosine kinase inhibitors after the study. And one patient with anaplastic lymphoma kinase gene rearrangement received crizotinib as a second-line therapy. The median PFS duration was 8.4 months (95% CI: 6.5–12.3 months), and the median OS duration was 29.2 months (95% CI: 17.0 to not reached). Figures 3a and b show the Kaplan–Meier curves for PFS and OS, respectively.
Subset analyses
We performed the first subset analysis to compare survival efficacy between the EGFR mutation-positive and -negative groups. Notably, the median PFS (7.4 months vs. 10.5 months, HR = 0.948; p = 0.436) and OS durations (29.2 months vs. NR, HR = 0.746; p = 0.670) did not significantly differ between the groups (data not shown). We also conducted a second subset analysis to compare patients with respect to age (<75 vs. ≥75 years). Again, we found no differences between the groups with respect to the median PFS (7.6 months vs. 10.5 months, HR = 0.739; p = 0.436) and OS durations (HR = 1.062; p = 0.918) (data not shown). The safety profiles were similar in both subsets (Table 4).
Discussion
Our two strategies, which were constructed by selecting fit elderly patients and adjusting interpatient variability in pharmacodynamics, succeeded in a triplet treatment of carboplatin plus weekly paclitaxel with bevacizumab in elderly patients with advanced non-squamous NSCLC. The ORR was 69.4%, meeting the expectations for the primary endpoint of this study. The median PFS and OS durations were 8.4 and 29.2 months, respectively. As the median PFS and OS durations for the elderly subset of the previous E4599 trial were 4.9 and 12.1 months, respectively, the outcomes of our study were more favorable.
In this study, elderly patients with unstable CHF were excluded. The major toxicity of paclitaxel, neutropenia, did not appear related to either peak paclitaxel plasma concentration or paclitaxel areas under the curve [10]. Rather, neutropenia was related to the duration of time that plasma paclitaxel concentrations reached or exceeded a threshold value. Paclitaxel is widely distributed among body fluids and tissues after IV administration. The main changes in drug pharmacokinetics seen in patients with CHF are a reduction in the volume of distribution and impairment of clearance, which elicit strong myelotoxicity [11]. Furthermore, VEGF pathway plays an essential role in endothelial cell maintenance and cardiac physiology. Thus, anti-VEGF therapy might be associated with arteriovascular disease and CHF. Previous phase II trials of bevacizumab in elderly patients with NSCLC reported severe cardiovascular toxicities such as cardiac arrhythmia, thrombosis and embolism, and myocardial ischemia [12, 13]. To exclude the patients suffered from chronic CHF, we used LVEF data and BNP levels. The baseline LVEF (less than 50%) has been the key exclusion criteria for the prospective trials using bevacizumab in the breast cancer, because bevacizumab treatment is also associated with an increased risk of CHF in breast cancer patients [14, 15]. Additionally, the plasma concentrations of natriuretic peptides and the LVEF evaluation by echocardiogram has been useful biomarkers in diagnosis of heart failure, and the guidelines for diagnosis and treatment of heart failure has used these parameters for algorithms to screening patients with heart failure [16]. The absence of severe or fatal cardiovascular toxicity events in our study suggests that eligibility criteria based on LVEF and BNP might facilitate the selection of elderly patients fit for bevacizumab treatment.
In terms of interpatient variability in pharmacodynamics, we employed a wide range of paclitaxel dose adjustments to each patient, in addition to adjusting the dose of carboplatin according to creatinine clearance. In the IFCT-0501 phase III trial, paclitaxel was planned to be administered at 90 mg/m2 on days 1, 8, and 15 of a 4-week cycle (67.5 mg/m2/week). However, this trial also reported a paclitaxel cancellation rate on days 8 and/or 15 of 28%, yielding a relative paclitaxel dose intensity of 81.1% (54.7 mg/m2/week). Furthermore, a recent phase II trial of carboplatin and weekly paclitaxel with bevacizumab reported that nearly half of all patients required dose reduction or skipping [17]. In consideration of the risk of increased hematological toxicity caused by adding bevacizumab, we consequently scheduled paclitaxel administration at a planned dose of 90 mg/m2 on days 1 and 8 of a 3-week cycle (60 mg/m2/week). Furthermore, our dose reduction method, which permitted the suspension of paclitaxel treatment on day 8, produced the wide range of paclitaxel doses (range 23 mg/m2/week to 60 mg/m2/week). Notably, 19.4% and 38.9% of patients required dose reduction and cancellation, respectively, of paclitaxel administration on day 8 during the induction phase. Thanks to dose adjustments of paclitaxel, 75% of patients were able to complete 4 cycles of triplet therapy.
Regarding survival and response, the triplet regimen in this study led to a RR, median PFS, and median OS of 69.4%, 8.4 months, and 29.2 months, respectively. These results were clearly favorable when compared with elderly subset data from the E4599 trial, which yielded a RR, median PFS, and median OS of 29%, 5.9 months, and 12.1 months, respectively [18]. To identify the factors associated with this favorable result, we performed a subset analysis regarding EGFR mutation status as previous studies have reported a more favorable prognosis among NSCLC patients with EGFR active mutations, compared to those without EGFR active mutations, especially in Japanese trials [19]. However, we failed to observe significant differences with respect to EGFR mutation status. This discrepancy might be attributable to the fact that most patients with EGFR active mutations in our study had been pretreated with EGFR tyrosine kinase inhibitors. Patient selection might have also affected the subset findings, as the eligibility criteria for bevacizumab (e.g., absence of hemoptysis or major blood vessel invasion by tumors) were previously identified as good prognostic factors in patients with non-squamous-cell lung cancer [20]. Accordingly, the use of these unique criteria may have led to selection bias toward patients with a good prognosis. The fact that 41.7% of patients in our study had an ECOG-PS of 0 and 27% developed a postoperative recurrence supports the possibility of selection bias, as these factors were previously identified as strong prognostic factors for survival in patients with lung cancer [21] and might thus have influenced the good survival results in this study.
We note further that the definition of “elderly” patients differed among guidelines. The ASCO guideline recommends that decisions regarding chemotherapy selection should not be based on age alone [22]. On the other hand, the Japanese guideline defines patients >75 years as “elderly”. Therefore, we performed a subset analysis with respect to age, but found no differences in survival efficacy and toxicity. This result suggests that the triplet regimen described herein could be used for elderly (>75 years) patients with NSCLC.
This trial had some limitations. First, no prospective feasibility studies had been conducted to evaluate a weekly-paclitaxel schedule on days 1 and 8 of a 3-week cycle in combination with carboplatin and bevacizumab. Therefore, we performed a feasibility analysis to evaluate AEs in the first six patients; consequently, no fatal AEs occurred in this study. Second, patients who were candidates for this study were not representative of all elderly patients, and data regarding the number of patients who matched the eligibility criteria of this study were unavailable. In addition, the unique criteria of this study, such as BNP and EF data, were unusual in a cancer treatment trial. However, these criteria are clinically available and might be useful for selecting patients fit for bevacizumab treatment.
In conclusion, we demonstrate that carboplatin plus weekly paclitaxel with bevacizumab, followed by maintenance bevacizumab is a feasible and effective first-line regimen for elderly patients with advanced non-squamous NSCLC; this is dependent on selecting ‘fit’ elderly patients based on BNP, EF, and HbA1c levels and on adjusting doses based on the monitoring of side effects. Further investigations are warranted to assess these unique criteria and the use of bevacizumab-based regimens in elderly patients.
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30. doi:10.3322/caac.21332
Wedding U, Honecker F, Bokemeyer C, Pientka L, Hoffken K (2007) Tolerance to chemotherapy in elderly patients with cancer. Cancer Control : Journal of the Moffitt Cancer Center 14(1):44–56
Jennens RR, Giles GG, Fox RM (2006) Increasing underrepresentation of elderly patients with advanced colorectal or non-small-cell lung cancer in chemotherapy trials. Intern Med J 36(4):216–220. doi:10.1111/j.1445-5994.2006.01033.x
Yee KW, Pater JL, Pho L, Zee B, Siu LL (2003) Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol 21(8):1618–1623. doi:10.1200/jco.2003.12.044
The Elderly Lung Cancer Vinorelbine Italian Study Group (1999) Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 91(1):66–72
Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavole A, Dauba J, Debieuvre D, Souquet PJ, Bigay-Game L, Dansin E, Poudenx M, Molinier O, Vaylet F, Moro-Sibilot D, Herman D, Bennouna J, Tredaniel J, Ducolone A, Lebitasy MP, Baudrin L, Laporte S, Milleron B (2011) Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 378(9796):1079–1088. doi:10.1016/s0140-6736(11)60780-0
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676. doi:10.1038/nm0603-669
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550. doi:10.1056/NEJMoa061884
Ramalingam SS, Dahlberg SE, Langer CJ, Gray R, Belani CP, Brahmer JR, Sandler AB, Schiller JH, Johnson DH (2008) Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of eastern cooperative oncology group trial 4599. J Clin Oncol 26(1):60–65. doi:10.1200/jco.2007.13.1144
Kearns CM, Gianni L, Egorin MJ (1995) Paclitaxel pharmacokinetics and pharmacodynamics. Semin Oncol 22(3 Suppl 6):16–23
Shammas FV, Dickstein K (1988) Clinical pharmacokinetics in heart failure. An updated review. Clin Pharmacokinet 15(2):94–113. doi:10.2165/00003088-198815020-00002
Spigel DR, Hainsworth JD, Shipley DL, Ervin TJ, Kohler PC, Lubiner ET, Peyton JD, Waterhouse DM, Burris HA 3rd, Greco FA (2012) A randomized phase II trial of pemetrexed/gemcitabine/bevacizumab or pemetrexed/carboplatin/bevacizumab in the first-line treatment of elderly patients with advanced non-small cell lung cancer. J Thorac Oncol 7(1):196–202. doi:10.1097/JTO.0b013e3182307efe
Dy GK, Molina JR, Qi Y, Ansari R, Thomas S, Ross HJ, Soori G, Anderson D, Aubry MC, Meyers J, Adjei AA, Mandrekar S, Adjei AA (2014) NCCTG N0821 (alliance): a phase II first-line study of pemetrexed, carboplatin, and bevacizumab in elderly patients with advanced nonsquamous non-small-cell lung cancer with good performance status. J Thorac Oncol 9(8):1146–1153. doi:10.1097/jto.0000000000000217
Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, Bellmunt J, Burstein HJ, Schutz FA (2011) Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol 29(6):632–638. doi:10.1200/jco.2010.31.9129
Verma N, Swain SM (2011) Bevacizumab and heart failure risk in patients with breast cancer: a thorn in the side? J Clin Oncol 29(6):603–606. doi:10.1200/jco.2010.32.9060
Pfister R, Schneider CA (2009) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: application of natriuretic peptides. Eur Heart J 30(3):382–383 . doi:10.1093/eurheartj/ehn560author reply 383
Kubota T, Okano Y, Sakai M, Takaoka M, Tsukuda T, Anabuki K, Kawase S, Miyamoto S, Ohnishi H, Hatakeyama N, Machida H, Urata T, Yamamoto A, Ogushi F, Yokoyama A (2016) Carboplatin plus weekly paclitaxel with bevacizumab for first-line treatment of non-small cell lung cancer. Anticancer Res 36(1):307–312
Langer CJ, Socinski MA, Patel JD, Sandler AB, Schiller JH, Leon L, Hazard SJ, Ramalingam SS (2016) Isolating the role of bevacizumab in elderly patients with previously untreated nonsquamous non-small cell lung cancer: secondary analyses of the ECOG 4599 and PointBreak trials. Am J Clin Oncol 39(5):441–447. doi:10.1097/coc.0000000000000163
Takano T, Fukui T, Ohe Y, Tsuta K, Yamamoto S, Nokihara H, Yamamoto N, Sekine I, Kunitoh H, Furuta K, Tamura T (2008) EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol 26(34):5589–5595. doi:10.1200/jco.2008.16.7254
Takagi Y, Toriihara A, Nakahara Y, Yomota M, Okuma Y, Hosomi Y, Shibuya M, Okamura T (2013) Eligibility for bevacizumab as an independent prognostic factor for patients with advanced non-squamous non-small cell lung cancer: a retrospective cohort study. PLoS One 8(3):e59700. doi:10.1371/journal.pone.0059700
Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P (2008) The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th edition of the TNM classification of malignant tumors and the proposals for the 7th edition. J Thorac Oncol 3(5):457–466. doi:10.1097/JTO.0b013e31816de2b8
Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller JH, Smith TJ, Strawn JR, Trent D, Johnson DH (2015) Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33(30):3488–3515. doi:10.1200/jco.2015.62.1342
Acknowledgements
We thank the patients and their families for their support and participation in this study. We thank the data management staff of The Tokyo Cooperative Oncology Group (TCOG) data center, especially Hiromi Odagiri, and the data analysis staff of the Medical TOUKEI Corporation. The authors also thank Dr. Keiichi Nagao, Dr. Yushi Nakai, and Dr. Hiroshi Tanaka for their assistance as the Safety Monitoring Committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Satoru Miura, Shunichi Sugawara, Kunihiko Kobayashi, Yuichi Takiguchi and Akihiko Gemma has received lecture fees from Chugai Pharmaceutical Co. Makoto Maemondo, Akira Inoue Yuichi Takiguchi has received lecture fees from Chugai and Bristol-Myers Squibb. All other authors declare that they have no conflict of interest.
Financial support
This study was supported by the North East Japan Study Group (NEJSG).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the institutional review boards of all participating sites.
Informed consent
All study participants provided written informed consent prior to enrollment.
Rights and permissions
About this article
Cite this article
Miura, S., Maemondo, M., Iwashima, A. et al. A phase II study of carboplatin plus weekly paclitaxel with bevacizumab for elderly patients with non-squamous non-small-cell lung cancer (NEJ016). Invest New Drugs 35, 227–234 (2017). https://doi.org/10.1007/s10637-017-0436-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0436-1