Abstract
Purpose
Ataxia–telangiectasia (A–T) is an autosomal recessive disorder characterized by progressive neurological deficits, including prominent oculomotor dysfunction. We report 5 cases of eye movement assessment in children 9–15 years old with A–T.
Methods
Three different oculomotor tasks (gaze holding, visually guided saccades and visual search) were used, and video-oculography was performed. Additionally, the scale for the assessment and rating of ataxia (SARA) score was used to assess severity of the cerebellar ataxia.
Results
Unstable gaze holding, nystagmus and saccadic intrusions were found. In addition to psychophysiological assessment results, we provide quantitative analysis of oculomotor activity, revealing a specific abnormal oculomotor pattern, consisting of (i) marked saccade hypermetria, (ii) unstable gaze holding, and (iii) gaze-evoked nystagmus.
Conclusion
Our study opens the prospect to evaluate efficacy and safety of alternative methods for supporting the patient and improving his/her life quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ataxia–telangiectasia (A–T) is a multisystem autosomal recessive disorder linked to the A–T mutated gene (ATM) on chromosome 11q22–23 and is characterized by progressive neural degeneration, immunodeficiency, and progressive ocular motor dysfunction. In previous studies, the qualitative description of the ocular motor deficits from clinical examination was limited to various disturbances in saccade and gaze control, dysmetric saccades, impairments of smooth pursuit, gaze holding, convergence, vestibular and optokinetic nystagmus slow phases, and vestibulo-ocular reflex disappearance [1,2,3]. The aim of our research was to expand existing findings by providing quantitative description of oculomotor patterns in A–T patients using video-oculography (VOG).
Methods
Case report
5 children 9–15 years old (4 boys and 1 girl) with diagnosed ataxia–telangiectasia participated in the study. Clinical history and other parameters are shown in Table 1. The definitive diagnosis of A–T was reached through the detection of mutation of the ATM gene via Sanger sequencing method. The test was carried out when the patient was in the immunology department at the Dmitry Rogachev National Research Center or at the hospital in their hometown. The current investigation was conducted at the Clinical Research Rehabilitation Center “Russkoe Pole”, where medical observation and therapy correction were carried out for the patients. Thus, patients came to the “Russkoe Pole” already with a confirmed diagnosis. Scale for the assessment and rating of ataxia (SARA) was used to assess the level of ataxia. All procedures were in compliance with the principles of the Helsinki Declaration. Informed consent was obtained from patients over 15 years old and from legal representatives of children under 15 years old.
Video-oculography (VOG) recording and analysis
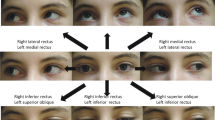
VOG was implemented to register eye movements. Eye movements were recorded at 60 samples/s monocularly (60 Hz sample rate) with an Arrington eye tracking system (Arrington Research Inc., Scottsdale, Arizona, USA). The subject’s head was placed on a chin support throughout the experiment. The head of the participants was softly attached to the chinrest. The center of vision was calculated from the location of the center of the pupil. Calibration was performed using the standard nine-point algorithm. Visual stimuli were presented on a 23′′ Samsung monitor (at a 1920 × 1080 pixel resolution); the active area of the monitor was located at 60 cm, in front of a participant’s eyes, thus forming a field with a 45° × 26° visual angle. The images were shown on a monitor using built-in ViewPoint EyeTracker® 2.9.2.5.
The patients were presented with 3 oculomotor tasks [4]. The first task was a gaze holding task. The goal of the task was to determine the stability of gaze fixation. The black central fixation point was initially shown at the center of the screen for 4–5 s. The test sequence consisted of presentation of a green dot (~ 1° in diameter; 15° left/right and 8° up/down from the center of the screen), 20 s each, at four eccentric locations on the screen. The participant was instructed: “Look at the green dot and follow it when it reappears at another place. The dot will stay at the same location for a while.” The areas of the ellipses that fitted the gaze positions were obtained. Gaze holding stability was measured for each target by approximating gaze position coordinates by an ellipse using the least squares method (fit_ellipse) in MATLAB 2013 [5]. The areas of the resulting ellipses, reflecting the gaze position coordinate dispersion, were determined for each of the four target positions in each subject. Areas were expressed in square visual degrees (sq. deg.). Mean gaze holding score reflects the arithmetic mean of the four obtained areas corresponding to the four target positions. Incorrect coordinates due to false pupil detection were excluded from analysis.
The second task was a visually guided saccade task. A square (10° × 10°) was presented on the screen, and the subject was to follow a red circle (diameter ~ 1°) appearing subsequently in different corners of the square (clockwise) direction for 1500 ms, evoking visually guided saccades (Fig. 1). The participant was instructed: “Follow the red circle and jump when the circle moves to the next position.” Performance evaluation parameters in this task included total number of saccades completed, proportion of relatively isometric (amplitude (A) between 8.5 and 11.5°), hypometric (short, A < 8.5°), and hypermetric (long, A > 11.5°), and corrective saccades (occurring only after dysmetric saccades, 1.5° < A < 5°).
The third task was a visual search task, in which the subject was asked to count to him/herself (silently) targets appearing on the screen (10 black circles, diameter ~ 1°, arranged in pseudo-random order). The participant instruction was as follows: “Count the number of dots and state it aloud.” We analyzed task execution time, number of fixations, their average duration, scanpath, and average saccade amplitude. Patients’ experimental data were compared to mean values obtained from our age-matched healthy control group \(\pm\) 2SD. The control group included fifty-four age-matched (26 boys and 28 girls; age groups: 9–11 (n = 15); 12–14 (n = 22); 15–17 (n = 13)) healthy children without a history of neurological diseases [4].
Results
Gaze holding task
Patients showed impaired gaze fixation: horizontal and vertical gaze-evoked nystagmus, voluntary high-amplitude saccades, and involuntary intrusive saccades in comparison with a healthy child (Fig. 2).
Impaired gaze holding manifesting as a centrifugally directed nystagmus in eccentric gaze positions was present in all subjects but not in healthy children. Comparison of gaze holding stability in all A–T patients to the control group showed that ellipse areas were greater in patients (Table 2), pointing to the impairment of gaze-holding control in children with A–T.
A visually guided saccade task
An example of visually guided saccades in one A–T patient #1 (Fig. 3) illustrates abnormalities—hypermetric saccades, followed by a correction saccade, nystagmus, macrosaccadic oscillations. The visually guided saccade task revealed horizontal hypermetria but not vertical (see Table 2).
Visual search task
A–T patients showed larger number of fixations and a longer scanpath, likely reflecting return of gaze to objects which had already been counted (Table 2). Durations of fixations and saccade amplitudes were within normal limits.
Descriptive analysis
Due to the statistical inadequacy of studying correlations with a small group of participants (n = 5), we conducted a descriptive analysis of clinical cases from Table 2 by combining SARA data and oculomotor characteristics. For example, consider patient #3, scoring 40 points on the SARA scale. He exhibited a relatively stable gaze holding score (21.76) compared to other patients and hypometria of voluntary saccades (5.42°) in the visual search task. Trials for visually guided saccades were not available. The patient coped with the visual search task apparently due to his age (15 years old) with a relatively short duration of fixations (291 ms). This patient had the highest degree of cerebellar ataxia on the SARA scale and displayed indications of apraxia of the gaze, as identified either during neurological examination. In contrast, patient #4 had the lowest score on the SARA scale (20). He exhibited the most stable gaze fixation (13.42), he coped best with the visual search task. However, he demonstrated severe hypermetria of horizontal visually guided saccades (1.48), a characteristic absent in the vertical direction (0.87), similar to patient #2 who scored 30 points on the SARA scale. Furthermore, patient #2 exhibited the most unstable gaze fixation (64.98), had the most difficulty with the visual search task and had high amplitude of voluntary saccades (8.57°). For patient #5, all trials, except for the gaze holding task (42.30), were unavailable due to severe motor artifacts. The low score on the SARA scale (22.5) in this patient can be attributed to the preservation of other evaluated domains (speech, etc.). Patient #1, scoring 32 points on the SARA scale, demonstrated intermediate values in oculomotor tests compared to other patients.
Discussion
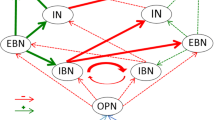
In this work, we studied the saccadic system functioning in children with A–T. We found unstable gaze holding, horizontal hypermetric saccades and impaired visual search. A–T is characterized by neurodegeneration of the Purkinje cell layer [6, 7] in the cerebellar cortex with relative preservation of the deep cerebellar nuclei. It leads to the loss of inhibitory control over the fastigial oculomotor region (FOR) and vestibular nuclei (VN) [1, 8], involved in precise control of saccade amplitudes and gaze holding.
Increased gaze holding in our patients results from the following abnormalities: gaze-evoked nystagmus (GEN), downbeat nystagmus (DBN), saccadic intrusions as identified by a neurophysiologist while recording a video-oculography [1, 9, 10]. Gaze holding deficits are common and occur in 46–85% of A–T patients [11]. In case of nystagmus, inherited leakiness of velocity-to-position neural integrator that include medial VN and nucleus prepositus hypoglossi for horizontal position commands, and superior VN and interstitial nucleus of Cajal for vertical eye position commands cannot be properly moderated from cerebellar regions such as flocculus/paraflocculus [12] and oculomotor vermis [13]. In case of saccadic intrusions, disinhibition of FOR leads to the loss of suppression of omnipause neurons on excitatory and inhibitory burst neurons, providing spontaneous saccadic movements [14]. We did not observe periodic alternating nystagmus (PAN) in our patients. Also, we observed ocular motor apraxia only in the oldest patient #3, although it is considered to be a well-known hallmark of A–T. The most likely reason for this is that only patients with relatively stable eye movement parameters met the inclusion criteria for eye tracking experiment and were therefore selected for the study.
In a visually guided saccade task we observed horizontal hypermetria in patients. Lesions in the cerebellum are known to cause saccadic dysmetria [12, 15]. In some studies, it was shown that A–T patients have predominantly hypometric saccades although we did not find them in our patients [3, 14]. These abnormalities usually appear after 5 years of age and tend to develop from deficits in saccadic component of smooth pursuit eye movements into to hypometric saccades with increased latency and thereafter to horizontal oculomotor apraxia [2, 9, 17].
It was hypothesized that dysfunction in the omnipause neurons might cause abnormal saccadic amplitudes [14, 16]. These neurons receive regulatory inputs from superior colliculi and oculomotor cerebellum. Damage to the superior colliculus does not lead to constant impairments of the precise trajectory of saccades, but damage in the cerebellum, as seen in A–T, can cause saccadic dysmetria [14]. Considering this, our results look ambiguous. Observed hypermetria is unlikely to be due to additional head movement because our patients were fixed in the chinrest. It has been found that bilateral damage of fastigial nuclei can cause horizontal hypermetria in both ipsi- and contraversive direction [8], whereas damage of posterior interpositus nuclei can lead to vertical hypermetria [18]. Thus, we assume bilateral damage to the fastigial nuclei and or its connections in our patients.
In the visual search task such parameters as tasks completion times, numbers of fixations, and scanpath lengths were different in comparison with data obtained from healthy children (Table 2). We found no difference in the mean duration of fixations (except for patient #1) or mean saccade amplitude between patients and healthy children (Table 2). Task completion times, numbers of fixations, and scanpath length parameters reflect the visuospatial organization of visual scanning in children performing the visual search task. Higher values of oculomotor parameters seen in patients arise due to recurrent oculomotor pattern consisting in the patient’s gaze returning to previously counted objects, indicating difficulties in perception, integration, and visuospatial memory [19]. These abnormal oculomotor patterns may point to malfunction of spatial “ambient” vision relying on rapid scanning of the visual scene with the purpose of primary object identification [20]. These impairments are associated with disturbance to spatial orientation, attention, and working memory resulting from damage to the cerebellar-cortical projections.
Our data contribute to the clinical picture of A–T by providing quantitative data on oculomotor performance. Assessment of these parameters may be instrumental for further clinical monitoring of a patient's state.
We also state that oculomotor impairments should be treated using a multidisciplinary approach based on intensive support from rehabilitation specialists and medical workers from adjacent fields implementing approaches focused on compensation techniques in order to support oculomotor performance [21].
Future studies are needed to unveil all mechanisms of eye movement deficits in ataxia–telangiectasia and to evaluate efficacy and safety of alternative methods for supporting the patient and improving his/her life quality.
Data availability
Data are available on request.
Abbreviations
- AT:
-
Ataxia–telangiectasia
- ATM:
-
A–T mutated gene
- VOG:
-
Video-oculography
- SARA:
-
Scale for the assessment and rating of ataxia
- A:
-
Amplitude
- FOR:
-
Fastigial oculomotor region
- GEN:
-
Gaze-evoked nystagmus
- DBN:
-
Downbeat nystagmus
- PAN:
-
Periodic alternating nystagmus
References
Shaikh AG, Marti S, Tarnutzer AA, Palla A, Crawford TO, Straumann D et al (2009) Gaze fixation deficits and their implication in ataxia–telangiectasia. Neurol Neurosurg Psychiatry 80:858–864. https://doi.org/10.1136/jnnp.2008.170522
Nissenkorn A, Banet-Levi Y, Vilozni D, Berkun Y, Efrati O, Frydman M et al (2011) Neurologic presentation in children with ataxia–telangiectasia: is small head circumference a hallmark of the disease? J Pediatr 3:466–471. https://doi.org/10.1016/j.jpeds.2011.02.005
Lewis RF, Lederman HM, Crawford TO (2002) Slow target-directed eye movements in ataxia–telangiectasia. Invest Ophthalmol Vis Sci 43:686–691
Shurupova MA, Latanov AV (2023) Oculomotor impairments in children after posterior fossa tumors treatment. Cerebellum. https://doi.org/10.1007/s12311-023-01553-1
Ohad G Fit_Ellipse. MATLAB Central File Exchange. https://www.mathworks.com/matlabcentral/fileexchange/3215-fit_ellipse Accessed 26 Dec 2021
Gatti RA, Vinters HV (1985) Cerebellar pathology in ataxia–telangiectasia: the significance of basket cells. Kroc Found Ser 19:225–232
Vinters HV, Gatti RA, Rakic P (1985) Sequence of cellular events in cerebellar ontogeny relevant to expression of neu- ronal abnormalities in ataxia–telangiectasia. Kroc Found Ser 19:233–255
Beh SC, Frohman TC, Frohman EM (2017) Cerebellar control of eye movements. J Neuro-Ophthalmol 37:87–98. https://doi.org/10.1097/WNO.0000000000000456
Farr AK, Shalev B, Crawford TO et al (2002) Ocular manifestations of ataxia–telangiectasia. Am J Ophthalmol 134:891–896. https://doi.org/10.1016/s0002-9394(02)01796-8
Riise R, Ygge J, Lindman C et al (2007) Ocular findings in Norwegian patients with ataxia–telangiectasia: a 5 year prospective cohort study. Acta Ophthalmol Scand 85:557–562. https://doi.org/10.1111/j.1600-0420.2007.00890.x
Tarnutzer AA, Straumann D, Salman MS (2018) Neuro-ophthalmologic assessment and investigations in children and adults with cerebellar diseases. In: Manto M, Huisman TAGM (eds) Handbook of clinical neurology. Elsevier, Amsterdam, pp 305–327. https://doi.org/10.1016/B978-0-444-63956-1.00019-9
Zee DS, Yamazaki A, Butler PH et al (1981) Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol 46:878–899. https://doi.org/10.1152/jn.1981.46.4.878
Hufner K, Stephan T, Kalla R, Deutschlander A, Wagner J, Holt-mannspotter M, Schulte-Altedorneburg G, Strupp M, Brandt T, Glasauers S (2007) Structural and functional MRIs disclose cerebellar pathologies in idiopathic downbeat nystagmus. Neurology 69:1128–1135. https://doi.org/10.1212/01.wnl.0000276953.00969.48
Tang SY, Shaikh AG (2019) Past and present of eye movement abnormalities in ataxia–telangiectasia. Cerebellum 3:556–564. https://doi.org/10.1007/s12311-018-0990-x
Manto M, Marmolino D (2009) Cerebellar ataxias. Curr Opin Neurol 4:419–429. https://doi.org/10.1097/WCO.0b013e32832b989
Lewis RF, Lederman HM, Crawford TO (1999) Ocular motor abnormalities in ataxia telangiectasia. Ann Neurol 46:287–295. https://doi.org/10.1002/1531-8249(199909)46:3%3c287::aid-ana3%3e3.0.co;2-0
Stell R, Bronstein AM, Plant GT et al (1989) Ataxia telangiectasia: a reappraisal of the ocular motor features and their value in the diagnosis of atypical cases. Mov Disord 4:320–329. https://doi.org/10.1002/mds.870040405
Robinson FR (2000) Role of the cerebellar posterior interpositus nucleus in saccades I. Effect of temporary lesions. J Neurophysiol 84:1289–1302. https://doi.org/10.1152/jn.2000.84.3.1289
Zihl J (1995) Visual scanning behavior in patients with homonymous hemianopia. Neuropsychologia 3:287–303. https://doi.org/10.1016/0028-3932(94)00119-a
Shurupova MA, Anisimov VN, Tereshchenko LV, Latanov AV (2016) Vliyanie kognitivnoi zadachi na parametry dvizhenii glaz pri prosmotre staticheskikh i dinamicheskikh stsen [Eye movement parameters are influenced by cognitive task in viewing of static and dynamic scenes]. Sensornye Sistemy [Sens Syst] 30(1):53–62 (in Russian)
Van Os NJH, Haaxma CA, van der Flier M, Merkus PJFM, van Deuren M, de Groot IJM, A-T Study Group et al (2017) Ataxia–telangiectasia: recommendations for multidisciplinary treatment. Dev Med Child Neurol 7:680–689. https://doi.org/10.1111/dmcn.13424
Acknowledgements
The authors would like to thank Valimukhametova I.R. pediatric doctor for supporting this study, and Kasatkin V.N., Director of the Research Institute for Brain Development and Peak Performance, RUDN University for consulting this study.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
MS, SM, AF contributed to conceptualization and resources; MS, SM contributed to methodology, software, validation, formal analysis, data curation, visualization, writing—original draft preparation, writing—review and editing and investigation; MS contributed to supervision; and MS and AF contributed to project administration. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were approved by the ethics committee of Dmitry Rogachev National Research Center of Pediatric Hematology, Oncology and Immunology (Protocol Number 8e/13-17 of 27.10.2017) and was run according to the Declaration of Helsinki. The parents of our patient provided written informed consent.
Informed consent
Informed consent was obtained from legal guardians. Written informed consent for publication of their clinical details and/or clinical images was obtained from the parents relative of the patient. A copy of the consent form is available for review by the Editor of this journal.
Human and animals rights statement
This article does not contain any studies with animals performed by any of the authors. All procedures performed in this study were approved by the ethical committee of of Dmitry Rogachev National Research Center of Pediatric Hematology, Oncology and Immunology and were in accordance with tenets of Helsinki Declaration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mironets, S.A., Shurupova, M.A. & Karelin, A.F. Videoocular assessment of eye movement activity in an ataxia–telangiectasia: a case study. Doc Ophthalmol 148, 107–114 (2024). https://doi.org/10.1007/s10633-024-09964-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-024-09964-z