Abstract
Purpose To compare conventional visual evoked potential (cVEP) and multifocal visual evoked potential (mfVEP) methods in patients with optic neuritis/multiple sclerosis (ON/MS). Methods mfVEPs and cVEPs were obtained from eyes of the 19 patients with multiple sclerosis confirmed on MRI scans, and from eyes of 40 normal controls. For the mfVEP, the display was a pattern-reversal dartboard array, 48° in diameter, which contained 60 sectors. Monocular cVEPs were obtained using a checkerboard stimulus with check sizes of 15′ and 60′. For the cVEP, the latency of P100 for both check sizes were measured, while for the mfVEP, the mean latency, percent of locations with abnormal latency, and clusters of contiguous abnormal locations were obtained. Results For a specificity of 95%, the mfVEP(interocular cluster criterion) showed the highest sensitivity (89.5%) of the 5 monocular or interocular tests. Similarly, when a combined monocular/interocular criterion was employed, the mfVEP(cluster criterion) had the highest sensitivity (94.7%)/specificity (90%), missing only one patient. The combined monocular/interocular cVEP(60′) test had a sensitivity (84.2%)/specificity (90%), missing 3 patients, 2 more than did the monocular/interocular mfVEP(cluster) test. Conclusion As the cVEP is more readily available and currently a shorter test, it should be used to screen patients for ON/MS with mfVEP testing added when the cVEP test is negative and the damage is local.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Optic Neuritis (ON), the inflammation and demyelinization of the optic nerve, typically presents with unilateral periorbital pain exacerbated by movement, decreased visual acuity, and reduced light and color sensitivity. Most patients present with abnormal appearing discs and a relative afferent pupillary defect (RAPD). In 38% of patients diagnosed with multiple sclerosis (MS), ON is the first clinical manifestation of the disease [1]. A rapid diagnosis of ON/MS is beneficial to the patient, as early treatment for both ON and MS may reduce the deteriorative effects of this disease. Oftentimes, patients with ON recover their visual acuity, sensitivity to light, and color vision within weeks of the acute attack. Electrophysiological evidence of demyelinization may improve, worsen or remain the same (e.g. [2, 3]). Thus, without electrophysiological testing a diagnosis of non-acute ON rests on an expensive MRI test. Further, in about 13% of the cases, ON/MS can present without pain on eye movement [4]. Under these circumstances, acute ON/MS can be confused with non-arteritic ischemic optic neuropathy (NAION). However, electrophysiological testing can distinguish these conditions.
The conventional visual evoked potential (cVEP) is the most commonly used electrophysiological diagnostic method for diagnosing ON/MS. The cVEP is relatively easy to record with electrodes placed over the occipital cortex. Three decades ago, Halliday et al. reported that VEPs are delayed in patients with ON/MS as compared with control subjects [2, 5].
Hood et al. [6, 7] recorded mfVEPs from patients with ON/MS and argued that the multifocal visual evoked potential (mfVEP) should be superior to the cVEP in detecting local damage to the optic nerve and retina. The mfVEP technique enables the simultaneous recording of local responses from many regions of the visual field. It has the advantage of sampling regions of the central 48° (diameter) of the field, as opposed to the cVEP, which is dominated by the macular region. In addition, Hood et al. [6, 7] have noted two additional reasons why the mfVEP might be superior to the cVEP in detecting changes in latency. First, the cVEP sums responses from a wide region of the field. Thus, local delays may be lost in the process [8, 9]. Further, since the lower field can contribute more to the cVEP than the upper field [6, 10–13], the cVEP is less likely to detect upper field defects. It is therefore not unreasonable to assume that although the cVEP may detect widespread areas of demyelinization, it may fail to detect localized changes. Using the sparse stimulus mfVEP paradigm, developed by James and colleagues [14–16], Ruseckaite et al. [16] estimated a 92% sensitivity, with a specificity of 100%, for detecting MS. However, their results were not compared to cVEP recordings.
Since the cVEP, as compared to the mfVEP technique, is more readily available and faster to administer, it is important to compare these techniques in the same study. The purpose here was to test a group of patients, referred by neuro-ophthalmologists to rule out a diagnosis of ON/MS, with both cVEP and mfVEP techniques.
Methods
Subjects
All subjects underwent an ophthalmologic examination (including slit lamp and dilated fundus exams) as well as achromatic static automated perimetry (SAP). The procedures adhered to the tenets of the Declaration of Helsinki and the Committee of the Institutional Board of Research Associates of Columbia University approved the protocol. Informed consent was obtained from all subjects prior to their participation.
Patients
A total of 19 patients with MS, based on clinical manifestations and MRI findings, were referred by neuro-ophthalmologists for VEP testing. The patients, 13 female and 6 male, ranged in age from 29 to 59 yrs., with a mean age of 43.0 ± 10.3 yrs. Four had bilateral ON. In these bilateral cases, the right eye was used in the monocular analyzes.
Control group
The control group consisted of 40 subjects with normal ophthalmic examination, visual acuity of 20/20 or better, and normal SAP fields. They ranged in age from 18 to 67 yrs., with a mean age of 44.3 ± 15.6 yrs. The right eye was used in the monocular ROC analyzes.
Stimuli and recording
cVEP stimulus
The stimulation and recording conditions followed ISCEV guidelines [17]. The stimulus was a reversing checkerboard, 48° in diameter, with a mean luminance of 70 cd/m2, and a contrast close to 100% (Espion System Software V4.0.12, 2004; Diagnosys, Boston, MA) and was displayed on a monitor (Model CPD-G4205, Sony, Tokyo, Japan) viewed at 32 cm. Two different check sizes, 60′ and 15′ were used. Both check sizes were presented at two reversals per second. A small red dot was placed at the center of the checkerboard for fixation. The stimuli were viewed through natural pupils with lenses added for the appropriate refraction. During monocular stimulation one eye was occluded.
cVEP recording
The electrodes were placed at Fz (reference) and at 4 cm above the inion (active). A ground electrode was placed on the forehead. The impedance was maintained at less than 5 k. The cVEP was recorded using cutoff frequencies of 3 and 100 Hz.
mfVEP stimulus
The dartboard stimulus array, which was produced by commercial software (VERIS Dart Board 60 with Pattern; Electro-diagnostic Imaging, Inc., (EDI, San Mateo, CA)) and displayed on a monitor (Model M20DCD2RE, Richardson Electronics Ltd, La Fox, IL), is depicted in Fig. 1a. It consists of 60 sectors, each with 16 checks: 8 white (200 cd/m2) and 8 black (>1 cd/m2), with the dartboard subtending 45° in diameter at the viewing distance of 32 cm. The central 12 sectors fall within the central 5.2° (diameter). The dartboard, approximately scaled for cortical magnification, was viewed through the appropriate refraction through natural pupils. Stimulation was monocular; the non-tested eye was occluded. A black and white monitor was used to present the stimulus array and was driven at a frame rate of 75 Hz. Each sector followed a pseudorandom sequence and had a 0.5 probability of reversing in contrast or staying the same at each frame change. For details about the mfVEP see Baseler et al. [18] and Hood and Greenstein [12].
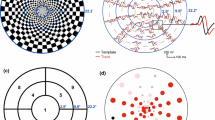
(a) The mfVEP stimulus. A dartboard stimulus array consisting of 60 sectors scaled for cortical magnification and subtending 45°. (b) Sample mfVEP responses for a patient with ON/MS. (c) Monocular mfVEP latency probability plot for the records in (b). (d) Interocular mfVEP latency probability plot for the records in (b)
mfVEP recording
The procedures were previously described in detail [12, 19–21]. In brief, using gold cup electrodes, three channels of continuous VEP (EEG) were recorded simultaneously using VERIS 4.3 software (Electro-diagnostic Imaging, San Mateo, CA). A ground electrode was placed on the forehead and a reference electrode on the inion. The midline channel consisted of the reference electrode and an electrode placed 4 cm above the inion (active). Two additional active electrodes were placed 4 cm lateral and 1 cm above the inion on either side, for an additional two channels. An additional three “derived channels” were obtained by taking the difference between pairs of channels, resulting in a total of 6 channels. The records were filtered with a high frequency cutoff at 3 Hz and a low frequency cutoff at 100 Hz (one half amplitude; Grass Instruments preamplifier P511J, Quincy, MA), and were sampled at 1200 Hz (every 0.83 ms). The resistance was less than 5 k and four monocular 7 min recordings were obtained, two for each eye, in an ABBA fashion (in a single session, beginning with the right eye). See Fig. 1b for sample responses.
Analysis of monocular latency
cVEP
The data were exported from the Espion system for both check sizes and entered into a graphics program for analysis. In most cases, P100 was easily identified as the prominent peak between 75 and 150 ms. If P100 was not easy to identify, two lines were drawn, estimating a best fit to the rising or declining phase of the wave at P100, with the point of intersection of these lines providing the latency measure [22].
mfVEP
The mfVEP data were exported from the system (VERIS; Electro-Diagnostic Imaging; San Mateo, CA) for all channels and all runs. The two runs for each channel were averaged, and further analyzed using a program written in commercial software (MatLab; The MathWorks Inc., Natick, MA). The program and methods of determining monocular and interocular latency have been previously described in detail [8, 9, 19, 21].
Briefly, for the monocular analysis each of the 60 “best” responses (from the channel with the largest SNR) were cross-correlated with a template created from a normative group (n = 100), and the mean deviation from the template in ms was calculated. Records with SNRs (<0.23 log unit) or with cross-correlation values less than 0 were excluded from the analysis.
Probability plots were constructed as seen in Fig. 1c, which provide spatial information about the latency changes. Each point in the plot corresponds to one of the 60 sectors on the dartboard display. Black points indicate that the response latency was within normal limits. Responses that did not meet the minimum SNR criterion for latency analysis are marked in gray. The colored circles demarcate responses that are significantly delayed at the 5% level (desaturated red and blue) or the 1% level (saturated red and blue). The right eye is indicated by blue and the left eye is indicated by red. The percentage of abnormal points (mfVEP(percent abnormal)) was calculated by dividing the total number of abnormal points (at the 5% and 1% level) by the total number of points meeting the SNR criterion. For example, in Fig. 1c, 14 (number of blue points) out of 55 (total number of points meeting SNR criterion) or 25.4% of the points were abnormal. The mean latency (mfVEP(latency)) was calculated as the mean of the latency of all points meeting the SNR criterion.
In addition, for each monocular plot, the presence of clusters of contiguous points at the 5% and 1% level were noted. For example in Fig. 1c, there are 2 clusters of contiguous points, one with 5 points made up of 4 points at 5% and one point at 1% and a second cluster with greater than 6 points at 5%. For the cluster criterion (mfVEP(cluster)), we counted all the eyes with clusters equal to, or more extreme than, some criterion. For example, a criterion of 551 would include all those eyes with 3 or more contiguous significant points with at least one at the 1% level (i.e. 551, 511, 111, 5551, 5511, etc.). For the ROC curve, a determination was made of the number of eyes meeting the criteria from 5 to 1111.
Relative interocular latency
cVEP
In order to determine the relative interocular latency for the cVEP, the difference between the two P100 values was calculated for both the large check size (cVEP(60′)) and small check size (cVEP(15′)).
mfVEP
Relative interocular latency was measured as the temporal shift producing the best cross-correlation value between the corresponding responses of each eye for each of the 60 locations and its corresponding best channel [9]. As in the monocular analysis, mfVEP(latency), mfVEP(percent abnormal), and mfVEP(cluster) were measured. For example in Fig. 1d, 45 (number of blue points) out of 56 (total number of points meeting the SNR criterion, including the red point) or 80.3% were abnormal. As for the monocular analysis, a mfVEP(cluster) criteria were also defined, in this case using the interocular plots (Fig. 1d). For the ROC curve, a determination was made out of the number of eyes meeting the criteria from 5 to 1111.
Results
Monocular
The ability of 5 tests/criteria to discriminate between normal controls and patients confirmed with ON/MS was assessed. The 5 tests/criteria compared were cVEP(60′) and cVEP(15′), the two check sizes on the cVEP, and mfVEP(latency), mfVEP(percent abnormal) and mfVEP(cluster), the 3 criteria for the mfVEP. The results from the 19 patients (19 eyes) confirmed with ON/MS and from the 40 individuals (40 eyes) in the control group were used to construct receiver operating characteristic (ROC) curves as in Fig. 2a. Here the proportion of patient eyes classified as abnormal (true positive rate or sensitivity) is plotted against the proportion of control eyes classified as abnormal (false positive rate or (1 − specificity)). The curves were constructed by varying the cutoffs defining an abnormal VEP latency.
(a) Receiver operator characteristics (ROC) curves for monocular tests/criteria of cVEP(60′), cVEP(15′), mfVEP(latency), mfVEP(percent abnormal), and mfVEP(cluster). (b) ROC curves for interocular tests/criteria of cVEP(60′), cVEP(15′), mfVEP(latency), mfVEP(percent abnormal) and mfVEP(cluster). (c) ROC curves for monocular tests/criteria for region of good specificity (10%), within rectangle in (a). (d) ROC curves for interocular tests/criteria for region of good specificity (10%), within rectangle in (b)
The ROC curves for the 5 tests/criteria, shown in Fig. 2a are fairly similar. For the monocular test, the areas under the ROC curve ranged from 0.86 to 0.95 with the monocular cVEP(60′) having the greatest areas (0.95) (Table 1).
When one looks at the area of good specificity (e.g., false positive rate ≤10%), the 5 curves are fairly similar (Fig. 2c). Table 2 shows the sensitivity for a specificity of 95%. The best sensitivity (57.9%) is given by the cVEP(60′), mfVEP(latency), and mfVEP(percent abnormal).
Interocular analysis
The ROC curves for the interocular analysis for the five tests/criteria (Fig. 2b) are fairly similar as are the areas under the ROC curves (Table 1). The areas under the ROC curve ranged from 0.89 to 0.96 with the mfVEP(cluster) and mfVEP(percent abnormal) showing the largest areas (Table 1). Two of the mfVEP criteria yielded the greatest sensitivity when the specificity was set at 95% (5% false positive rate), as seen in Table 3 and Fig. 2b, d. In particular, the mfVEP(percent abnormal) and the mfVEP(cluster) had sensitivities of 84.2 and 89.5% at a specificity of 95%.
Combined monocular and interocular criteria
Table 4 shows the results for a combined monocular/interocular criterion. To generate these results, the criteria producing a 95% specificity in Tables 2 and 3 were used. In particular, an eye was called abnormal if either the monocular or interocular criterion was met. With a specificity of 90%, the sensitivity was best for the mfVEP(percent) and mfVEP(cluster). The mfVEP detected 2 more patients than were detected based upon the cVEP(60), the best of the 2 cVEP tests.
Discussion
The purpose of this study was to compare the effectiveness of the cVEP and the mfVEP in detecting damage secondary to ON/MS. For a specificity of 95%, the interocular mfVEP(cluster) criterion showed the best sensitivity of the individual criteria. When the interocular and monocular tests were combined, the mfVEP again performed the best with a sensitivity of 94.7% and a specificity of 90%. However, while the mfVEP criteria performed best overall, the conventional test, cVEP(60′), did quite well.
None of the tests did particularly well with the monocular criteria. For a specificity of 95%, the best sensitivity was 57.9%, shown by the cVEP(60′) and two of the mfVEP criteria. The sensitivity of every interocular test (Table 3) was greater than that shown by the corresponding monocular test (Table 2). For the interocular tests, the mfVEP(cluster) had the highest sensitivity and detected 2 more patients than did the cVEP(60′). Care should be exercised in using exclusively the interocular test as it may miss individuals with both eyes showing delays as in the case of some patients with bilateral disease. However, interestingly, only 1 of the 4 patients diagnosed with bilateral ON/MS was missed on the interocular mfVEP(cluster) tests. Of course, it makes most sense to call a VEP abnormal if either the monocular or interocular test was abnormal.
When the monocular and interocular tests were combined, the mfVEP(cluster) test performed best, missing only one patient with a specificity of 90%. All mfVEP and cVEP tests failed to identify this patient as abnormal, although the diagnosis of MS was confirmed based upon both MRI and other neurological findings. On the mfVEP, both eyes of this patient showed increased, but non-significant, latencies. These increased latencies were confirmed on mfVEP tests performed on separate days. These reproducible and prolonged latencies fell just short of being significant on the monocular test and, because both eyes showed increased latency, the interocular test was normal as well.
It is not surprizing that the combined monocular and interocular mfVEP(cluster) tests outperformed other tests. Field defects in ON/MS can be relatively local and the cVEP can miss these local defects as mentioned in the Introduction. Perhaps more surprizing is how well the monocular/interocular cVEP(60′) test performed. In particular, it missed only 2 more patients than did the mfVEP(cluster) test. This can be attributed to the relatively wide spread effects of ON/MS in our patients. For example, a median of 51% of the locations were significantly delayed on the interocular plot.
Finally, it is only fair to point out that the results may vary with different criteria of significance (e.g., level of specificity for the monocular test) and/or with different definitions of an abnormal test (e.g., particular length of cluster). Further, this study should be replicated with a larger sample.
In sum, within the limits of this study, the mfVEP, as expected, did better than the cVEP in detecting ON/MS. However, the difference was less than expected. This finding has important clinical implications, as the duration of the mfVEP test used here was considerably longer than that of the cVEP. However, it should be possible to substantially shorten the mfVEP test for clinical purposes. For example, Ruseckaite et al. [16] reported a sensitivity/specificity of 92%/100% using a sparse mfVEP paradigm and under 3 min of recording. In any case, cVEP testing is more readily available than mfVEP testing. Thus, it seems advisable to first test patients suspected of ON/MS with the cVEP. If the results of the test are negative, then the clinician may want to refer the patient for mfVEP testing, especially if the damage is thought to be local.
References
Swanson JW (1989) Multiple sclerosis: update in diagnosis and review of prognostic factors. Mayo Clin Proc 64:577–586
Halliday AM, McDonald EI, Mushin J (1972) Delayed visual evoked response in optic neuritis. Lancet 1:661–664
Yang EB, Hood DC, Rodarte C, Zhang X, Odel JG, Behrens MM (2007) Improvement in conduction velocity after optic neuritis measured with the multifocal VEP. Invest Ophthalmol Vis Sci 48:692–698
Optic Neuritis Study Group (1991) The clinical profile of optic neuritis. Experience of the optic neuritis treatment trial. Arch Ophthalmol 109:1673–1678
Halliday AM, McDonald EI, Mushin J (1973) Visual evoked response in diagnosis of multiple sclerosis. BMJ 1:661–664
Hood DC, Odel JG, Winn BJ (2003) The multifocal visual evoked potential. J Neuro-Ophthalmol 23:279–289
Hood DC, Odel JG, Zhang X (2000) Tracking the recovery of local optic nerve function after optic neuritis: a multifocal VEP study. Invest Ophthalmol Vis Sci 41:4032–4038
Hood DC, Ohri N, Yang EB, Rodarte C, Zhang X, Fortune B, Johnson CA (2004) Determining abnormal latencies of multifocal visual evoked potentials: a monocular analysis. Doc Ophthalmol 109:189–199
Hood DC, Zhang X, Rodarte C, Yang EB, Ohri N, Fortune B, Johnson CA (2004) Determining abnormal interocular latencies of multifocal visual evoked potentials. Doc Ophthalmol 109:177–187
Fortune B, Hood DC (2003) Conventional pattern-reversal VEPs are not equivalent to summed multifocal VEPs. Invest Ophthalmol Vis Sci 44:1364–1375
Fortune B, Goh K, Demirel S, Novitsky K, Mansberger SL, Johnson CA, Cioffi GA (2003) Detection of glaucomatous visual field loss using the multifocal visual evoked potential. In: Henson DB, Wall M (eds) Perimetry update 2002/2003; Proceedings of the XVth international perimetric society meeting. Kugler, The Hague, pp 251–260
Hood DC, Greenstein VC (2003) Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res 22:201–251
Hood DC, Odel JG, Winn BJ (2003) The multifocal visual evoked potential. J Neuro-Ophthalmol 23:279–289
James AC (2003) The pattern-pulse multifocal visual evoked potential. Invest Ophthalmol Vis Sci 44:879–890
James AC, Ruseckaite R, Maddess T (2005) Effect of temporal sparseness and dichoptic presentation on multifocal visual evoked potentials. Vis Neurosci 22:45–54
Ruseckaite R, Maddess T, Danta G, Lueck CJ, James AC (2005) Sparse multifocal stimuli for the detection of multiple sclerosis. Ann Neurol 57:904–913
Odom JV, Bach M, Barber C et al (2004) Visual evoked potentials standard. Doc Ophthalmol 108:115–123
Baseler HA, Sutter EE, Klein SA, Carney T (1998) The topographic visual evoked potential: improving objective detection of local visual field defects. Invest Ophthalmol Vis Sci 39:937–950
Hood DC, Zhang X, Greenstein VC, Kangovi S, Odel JG, Liebmann JM, Ritch R (2000) An interocular comparison of the multifocal VEP: a possible technique for detecting local damage to the optic nerve. Invest Ophthalmol Vis Sci 41:1580–1587
Hood DC, Zhang X, Hong JE, Chen CS (2002) Quantifying the benefits of additional channels of multifocal VEP recording. Doc Ophthalmol 104:303–320
Hood DC (2004) Electrophysiologic imaging of retinal and optic nerve damage: the multifocal technique. Ophthalmol Clin N Am 1:69–88
Grippo TM, Hood DC, Kanadani FN, Ezon I, Greenstein VC, Liebmann JM, Ritch R (2006) A comparison between multifocal and conventional VEP latency changes secondary to glaucomatous damage. Invest Ophthalmol Vis Sci 47:5331–5336
Acknowledgements
This work was supported by National Institutes of Health, National Eye Institute Grants: RO1-EY02115 (DCH). Adam S. Wenick was funded by a fellowship from Fight for Sight, New York, NY, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grover, L.K., Hood, D.C., Ghadiali, Q. et al. A comparison of multifocal and conventional visual evoked potential techniques in patients with optic neuritis/multiple sclerosis. Doc Ophthalmol 117, 121–128 (2008). https://doi.org/10.1007/s10633-007-9112-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-007-9112-7