Abstract
Introduction
Previous studies have demonstrated that Dual-specificity phosphatase 4 (DUSP4) plays an important role in the progression of different tumor types. However, the role and mechanism of DUSP4 in colorectal cancer (CRC) remain unclear.
Aims
We investigate the role and mechanisms of DUSP4 in CRC.
Methods
Immunohistochemistry was used to investigate DUSP4 expression in CRC tissues. Cell proliferation, apoptosis and migration assays were used to validate DUSP4 function in vitro and in vivo. RNA-sequence assay was used to identify the target genes of DUSP4. Human phosphokinase array and inhibitor assays were used to explore the downstream signaling of DUSP4.
Results
DUSP4 expression was upregulated in CRC tissues relative to normal colorectal tissues, and DUSP4 expression showed a significant positive correlation with CRC stage. Consistently, we found that DUSP4 was highly expressed in colorectal cancer cells compared to normal cells. DUSP4 knockdown inhibits CRC cell proliferation, migration and promotes apoptosis. Furthermore, the ectopic expression of DUSP4 enhanced CRC cell proliferation, migration and diminished apoptosis in vitro and in vivo. Human phosphokinase array data showed that ectopic expression of DUSP4 promotes CREB activation. RNA-sequencing data showed that PRKACB acts as a downstream target gene of DUSP4/CREB and enhances CREB activation through PKA/cAMP signaling. In addition, xenograft model results demonstrated that DUSP4 promotes colorectal tumor progression via PRKACB/CREB activation in vivo.
Conclusion
These findings suggest that DUSP4 promotes CRC progression. Therefore, it may be a promising therapeutic target for CRC.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer, a common type of solid malignant tumor, has a high incidence and mortality rate worldwide [1, 2]. Late detection and metastasis are challenges in the clinical treatment of tumors, including colorectal cancer [3]. The discovery of new markers for early screening of colorectal cancer is an urgent issue in colorectal cancer treatment.
Dual-specificity phosphatase 4 (DUSP4), also known as MKP2, is an important member of the dual-specificity phosphatase family, located at the chromosome 8p11-12 gene locus [4]. It contains a common theme of MKP-pTXpTY, in addition to an N-terminal MAPK-binding (MKB) structural domain (1–192), a DUSP catalytic structural domain (193–336), and a C-terminal tail (337–394) [5]. The DUSP4 catalytic domain contains 24 subunits that form a hollow spherical structure [6]. When all catalytically active sites are exposed to the solvent, the N-terminal residues containing the MKB structural domain are buried in this hollow sphere according to the N-terminal orientation of the catalytic structural domain [7]. DUSP4 inactivates the MAPK pathway (ERK, P38, and JNK) through dephosphorylation [8], thus playing an important role in cellular, physiological, and pathological processes, including senescence [9, 10], stress-induced apoptosis [11] and malignancy [12,13,14]. Indirect evidence suggests that DUSP4 is associated with the development of hepatocellular carcinoma (HCC) [12], ovarian cancer [15, 16], esophageal cancer with rib metastases [17, 18] and pancreatic tumors [4, 19]. In addition, DUSP4 is considered a candidate tumor suppressor gene, and its deletion is associated with the development of breast [13, 20], lung [21, 22] and prostate tumors [23]. However, the specific functions and molecular mechanisms of action of DUSP4 in colorectal cancer have not yet been reported.
CREB (cAMP response element binding protein) is a nuclear protein that selectively binds CRE isolated by Yamamoto from nuclear extracts of the PCI2 cell line and rat brain tissue [24]. CREB is composed of 341 amino acids and is a member of the leucine zipper family of transcription factors. It contains a basic region and leucine zipper modules, collectively known as CREB, which achieve its regulatory gene transcriptional function through phosphorylation and dephosphorylation [25]. Recent studies have revealed that CREB is closely related to cellular biological functions such as cell growth, proliferation, differentiation, and cell cycle regulation [26, 27]. Studies have shown that CREB plays an important role in the development, progression, and metastasis of melanoma [28], prostate cancer, hepatocellular carcinoma, breast cancer, and other cancers [29]. However, the role of CREB in DUSP4-mediated tumor progression has not yet been reported.
PKA C-beta, also named PRKACB, is an important member of the protein kinase superfamily [30, 31]. PRKACB mediates cAMP-dependent signal transduction when its receptor binds to G protein-coupled receptors (GPCRs). The activation of PKA kinase is closely associated with various important cellular processes, such as the regulation of chromatin condensation and depolymerization, cell proliferation, cell differentiation, and the cell cycle [32]. There are few studies on the role of PRKACB in tumors, and most have focused on its feasibility as a molecular marker for different tumors using bioinformatics methods. For example, low PRKACB expression in colorectal cancer can predict poor overall survival [33]. However, the role of PRKACB in the DUSP4-mediated CRC progression remains unclear.
In the present study, we investigated DUSP4 expression in CRC. We explored the effects of DUSP4 overexpression and knockdown on CRC cell proliferation, apoptosis and migration in vitro and in vivo. We also performed a detailed analysis of the potential mechanisms by which DUSP4 affects CRC malignant features. In addition, we validated the DUSP4-mediated signaling pathway using small-molecule inhibitors in vitro. Based on these results, we proposed a model in which DUSP4 promotes CRC progression. These findings provide a promising target for the treatment of CRC.
Materials and Methods
Online Database Analysis
The TIMER online database (http://timer.comp-genomics.org/) and the TNMplot online database (https://tnmplot.com/analysis/) were used to analyze DUSP4 expression in normal colon and colon cancer tissues.
Tumor Sample Tissues
We used CRC tissues from patients who underwent surgery at the Affiliated Hospital of Jining Medical University between 2015 and 2019; detailed information is shown in Supplementary Table 1. This study was approved by the Institutional Ethics Committee of the Jining Medical University.
Plasmids Construction
Human DUSP4 shRNA oligos were synthesized, annealed to form double-stranded oligos, and ligated into a linearized pLV-shRNA-puro vector (cat. #B19, Biosettia) to construct circled pLV-shRNAs-Puro. The human-amplified DUSP4 CDS fragment was inserted into the pLV-MCS-puromycin vector (cat. # pLV-03, Biosettia, CA, USA) to construct pLV-Flag-DUSP4-Puro. The related primers and oligonucleotide sequences are listed in Supplementary Table 2.
Immunohistochemistry (IHC)
Immunohistochemical assays were performed according to previously described protocols [34]. CRC tissue microarrays (Avilabio, cat. # col11011, col11034, col1002b) were purchased from Avila Biotechnology (Xi’an, China). Primary antibodies used to target the corresponding proteins are listed in Supplementary Table 3. The score was based on the percentage of protein-positive cells in each tissue. Photographs were taken with a panoramic viewer under a 50 um or 100 um objective (3DHistech, Hungary).
Cell Culture
SW620 cells were cultured in L15 medium supplemented with 10% FBS (Gibco), 1% NEAA (Gibco), and 1% penicillin/streptomycin (Gibco). LOVO and RKO cells were cultured in DMEM (HyClone) supplemented with 10% FBS, 1% sodium pyruvate (Gibco), 1.5 g/L NaHCO3 (Gibco), and 1% NEAA (Gibco). SW620 was grown in a 0% CO2 incubator at 37 °C, and LOVO and RKO cells were grown in a 5% CO2 incubator at 37 °C. All cell lines were purchased from ATCC.
Western Blotting
Western blotting was conducted according to our previously described established protocol [35]. All primary antibodies against the antigens used in this study are listed in Supplementary Table 3.
Cell Counting Kit-8 (CCK8)
3,000 CRC cells per well were incubated in 96-well plates for 0, 12, 24, 48, 72, or 96 h. To detect CCK8, 10 uL of CCK8 reagent was added to each well and incubated for 2 h, and the absorbance at 450 nm was read automatically with a multi-scanner.
Colony Formation
CRC cells were counted, seeded into 12-well plates at a density of 500 cells/mL, cultured for 10–14 days, sequentially fixed in paraformaldehyde for 10 min, stained with the Gimsa staining solution for 5 h, rinsed twice with PBS, dried, photographed, and counted using Photoshop.
Flow Cytometry
The cell cycle was detected using a Cell Cycle Assay Kit (Cat. # KGA512, KeyGEN Biotech, Nanjing, China) according to the manufacturer's protocol. The apoptosis assay was performed using a PI/Annexin V Double Staining Kit (Cat. # KGA108, KeyGEN Biotech), following the manufacturer's protocol.
EdU Assay
For the EdU cell proliferation assay, EdU was detected using a BeyoClick EdU-488 Kit (Cat. # C0071S, Beyotime, Shanghai, China) according to the manufacturer’s instructions.
Cell Migration Assay
For trans-well assays, 2 × 105 CRC cells were resuspended in 1% serum medium and seeded into Boyden chamber inserts with 8 µm pore membranes (Corning Inc., Corning, NY, USA). Medium containing 10% FBS was added to the lower chamber. After 24 h, the chambers were removed for fixation, washing, and 0.1% crystalline violet staining. Images were collected for statistical analysis.
For the wound healing assay, 5 × 105 CRC cells were seeded and grown to 90% confluence. Scratch wounds were created using sterile pipette tips. Images were captured at 0 and 24 h (LOVO: 24 h, RKO: 48 h, SW620: 72 h) using an inverted microscope (Olympus, Tokyo, Japan).
Human Phospho-Kinase Array
Human Phospho-Kinase Array Kit (cat. # ARY003C, R&D Systems, Minneapolis, MN, USA) was used to assess the phosphorylation levels of relative kinases and proteins according to the manufacturer’s instructions.
RNA Sequence
Different cell lines were cultured, digested, washed in PBS, and resuspended in TRIzol, and RNA sequencing was performed by Beijing Biomarker Technologies.
Inhibitor Treatment
CREB inhibitor 666-15 was purchased from MCE and dissolved in DMSO for preservation. Cell lines were treated with 666-15 for 24 h at concentrations of 0.1 uM and 0.2 uM, respectively, and proteins were collected, or other experiments were performed.
Dual Luciferase Assay
The luciferase reporter vectors, the PRKACB gene promoter fragments were amplified from human genomic DNA and cloned into the firefly luciferase plasmid pGL3-basic-IRES. For reporter assays, above constructs along with pRL-TK plasmid (internal reference), pLV-DUSP4-puro and pCMV-CREB were co-transfected into RKO cells. Then cells lysates were collected and analysed with Dual-luciferase reporter assay system following its manual (Promega, E1910). And the reporter activity was measured using a microplate reader.
Immunoprecipitation
Cell protein lysates were incubated with protein A/G agarose beads (Santa Cruz, sc-2001) and DUSP4 antibody overnight at 4 °C. Then centrifuge at 2500 rpm for 30 s and wash the pellets carefully pre-chilled PBS buffer. Finally, binding proteins were boiled to proceed SDS-PAGE. Immunoprecipitates were analyzed with the specific antibody by Western blot. Antibodies used in this assay are listed in Supplementary Table 3.
In Vivo Xenograft Model
Immunodeficient mice (nude mice, 6–8 weeks) were purchased from GemPharmatech and randomly assigned to different groups of six mice each. 2 × 106 of RKO-MCS and RKO-DUSP4 cells were injected into the nude mice subcutaneously. When a palpable tumor was generated, the tumor volume was measured and recorded. After 24 days of injection, mice were anesthetized and sacrificed, and tumors were isolated and fixed, dehydrated, embedded, and sectioned to obtain tumor tissue sections.
Statistical Analysis
The GraphPad Prism5 software (GraphPad Software, San Diego, CA, USA) was used to analyze the data. Values are expressed as mean ± standard error (SEM). Student's t-test (two groups) and one-way ANOVA (more than two groups) were used to calculate P-values for comparisons across groups, unless otherwise stated. P-values below 0.05 were considered statistically significant.
Results
DUSP4 Was Highly Expressed in Human CRC Tissues
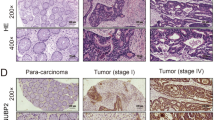
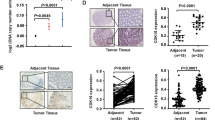
To investigate the expression patterns of DUSP4 in colorectal cancer, we first analyzed the expression of DUSP4 in normal colon tissues and colorectal cancer tissues using the online databases TIMER (Fig. 1A) and TNM plot (Fig. 1B, C). We found that DUSP4 was more highly expressed in colorectal tissues than in normal tissues. To further confirm these results, we performed immunohistochemical staining on tissue microarrays containing 260 tissues (normal: 50, cancer: 210) (Fig. 1D). We found that DUSP4 expression was significantly higher in colorectal cancer tissues than in normal tissues, and that high expression of DUSP4 represents a higher clinical stage of colorectal cancer (Fig. 1E). In addition, we examined the expression pattern of DUSP4 by western blotting in 14 pairs of CRC tissues and paraneoplastic tissues collected clinically and found that CRC tissues have a higher expression pattern of DUSP4 than paraneoplastic tissues in most cases (Fig. 1F). Microsatellite instability (MSI) is closely related to tumor development, and MSI type is more commonly seen in KRAS wild-type and BRAF-mutant tumors. In order to investigate the relationship between DUSP4 expression and microsatellite instability in colorectal cancer, we detected the correlation between DUSP4 expression and KRAS (Wild type) and BRAF V600E by IHC using tissue microarrays of serial sections. The results showed that DUSP4 expression was not correlated with KRAS expression and BRAF mutation expression (Fig. S1A, B). We also confirmed the correlation of DUSP4 gene expression with KRAS expression using the GEPIA and TNMplot. The results also showed that DUSP4 expression was not correlated with KRAS expression (Fig. S1C). Collectively, these results indicate that DUSP4 is a potential tumor mediator that is highly expressed in human CRC tissues.
DUSP4 was highly expressed in human CRC tissues. A–C TIMER and TNMplot online databases were used to analyze the transcriptional expression of DUSP4 in colon cancer (Tumor versus Normal), N means Number, M means Median. D Images of DUSP4 stained in an array of human CRC tissues were shown. (Scale bar: 50 μm) E Quantification results of DUSP4 expression in the CRC tissue array were shown. F The correlation of DUSP4 expression and tumor TNMs was quantified. G Analysis of DUSP4 expression in fresh colon cancer tissues and paracancerous tissues by Western blotting
Knockdown Expression of DUSP4 Suppresses CRC Cell Proliferation In Vitro
To investigate whether the findings observed in clinical samples are reflected in colorectal cancer cell lines, we performed western blotting to detect DUSP4 expression in normal colon cells and colorectal cancer cells, which showed an increase in DUSP4 expression in CRC cell lines, particularly in SW620 and LOVO cells (Fig. 2A). To explore the effects of DUSP4 in colorectal cancer progression, we silenced the expression of DUSP4 by specific shRNAs. As shown in Fig. 2B, western blotting confirmed the knockdown efficiency of shRNAs in SW620 and LOVO cells. A CCK-8 assay was performed, and the results showed that silencing DUSP4 inhibited the growth rate of CRC cells at the indicated time points (Fig. 2C). The colony formation tests were conducted, and the results displayed that silencing of DUSP4 reduced the ability of CRC cell colony formation (Fig. 2D, E). Moreover, cell cycle progression analysis by FACS revealed that DUSP4 silencing reduced the number of cells in the S phase, which further inhibited the proliferative capacity of the cells (Fig. 2F, G). Additionally, EdU assays were conducted and the results suggested that DUSP4 silencing reduced the proliferative capacity of the cells (Fig. 2H). Consistent with the above results, silencing DUSP4 reduced the expression of the proliferation-associated protein Ki-67 (Fig. 2J). Taken together, these results suggested that DUSP4 silencing suppressed CRC cell proliferation in vitro.
Knockdown expression of DUSP4 suppresses CRC cell proliferation in vitro. A DUSP4 expression in CRC cell lines and normal colon cell line was detected by Western blot. B DUSP4 knockdown efficiency in SW620 and LOVO cell lines was detected by Western blot. C Cell viability curve of SW620 and LOVO cells with DUSP4 knockdown or shCtrl was plotted by CCK8 assay. D and E Cell proliferation ability in SW620 and LOVO cells with DUSP4 knockdown or shCtrl was checked by colony formation. The statistical results of each group were shown. F and G Cell cycle progression in SW620 and LOVO cells with DUSP4 knockdown or shCtrl was checked by FACS (PI staining). The statistical results of each group were shown. H and I Cell proliferative capacity in SW620 and LOVO cells with DUSP4 knockdown or shCtrl was tested by EdU fluorescence staining assay. The statistical results of EdU + cells in each group were shown. J The expression of Ki-67 in each group was analysed by western blot
Silencing of DUSP4 Facilitates CRC Cell Apoptosis and Inhibits CRC Cell Migration In Vitro
To explore the role of DUSP4 in CRC cell apoptosis, a preliminary double-staining assay was performed using PI and Annexin V-FITC. FACS results showed that DUSP4 silencing promoted the apoptotic capacity of LOVO and SW620 cell lines (Fig. 3A, B). Furthermore, the expression of Bax, Bcl-2, and cleaved caspase 3 was detected using western blotting. As shown in Fig. 3G, DUSP4 silencing increased the expression of the pro-apoptotic proteins Bax and cleaved caspase 3 and reduced the expression of the anti-apoptotic protein Bcl-2.
Silencing of DUSP4 inhibits cell migration and promotes CRC cell apoptosis in vitro. A & B Cell apoptosis in SW620 and LOVO cells with DUSP4 knockdown or shCtrl was checked by FACS (PI-Annexin V). The statistical results of apoptosis cells % in each group were shown. C & D Representative images of trans-well assay and statistic results on migrated cells in shDUSP4 or shctrl were shown. E & F Representative images of wound healing assay and statistic results on migrated cells in shDUSP4 or shctrl were shown. G The expression level of cleaved caspase 3, Bax, Bcl-2, E-cadherin, N-cadherin and β-catenin in SW620 and LOVO cells with DUSP4 knockdown or shCtrl was analysed by western blot
To investigate the functional role of DUSP4 in CRC cell migration in vitro, transwell assay was performed in LOVO and SW620 cell lines, the results showed that the knockdown of DUSP4 expression inhibited the migratory ability of both cell lines (Fig. 3C, D). Similar results were obtained in wound healing assays (Fig. 3E, F, where DUSP4 knockdown inhibited the migration ability of both cell lines. Moreover, western blotting was performed to detect the expression of EMT markers. The results showed that DUSP4 knockdown decreased the expression of mesenchymal-like markers N-cadherin, β-catenin, and increased the expression of epithelial-like markers E-cadherin (Fig. 3G).
These results suggest that silencing of DUSP4 facilitates CRC cell apoptosis and inhibits cell migration in vitro.
Ectopic Expression of DUSP4 Enhances CRC Cell Proliferation, Migration and Represses Apoptosis In Vitro
To further confirm these results, we ectopically expressed Flag-DUSP4 in RKO cells, and western blotting showed that both Flag and DUSP4 were overexpressed in RKO and SW620 cells (Figs. 4A, S2A). A series of functional experiments were conducted, and the CCK-8 assay showed that the ectopic expression of DUSP4 accelerated the growth of CRC cells at the indicated time points (Figs. 4B, S2C). The colony formation assay results indicated that the ectopic expression of DUSP4 increased the colony formation ability of CRC cells (Figs. 4C, D, S2D). Furthermore, FACS results demonstrated that the ectopic expression of DUSP4 increased the number of cells in the S phase, further promoting cell proliferation (Figs. 4E, F, S2E). Additionally, EdU assay results indicated that DUSP4 ectopic expression enhanced the cell proliferative capacity (Figs. 4G, H, S2F, S2G). Consistent with these results, western blotting showed that DUSP4 ectopic expression increased the expression of the proliferation-associated protein Ki-67 (Figs. 4I, S2N).
Ectopic expression of DUSP4 enhances CRC cell proliferation and represses apoptosis in vitro. A DUSP4 overexpression efficiency in RKO cell line was analysed. B Cell viability curve of RKO cells with overexpressing DUSP4 or Ctrl was plotted by CCK8 assay. C & D Cell proliferation ability in RKO cells with overexpressing DUSP4 or Ctrl was checked by colony formation. The statistical results of each group were shown. E & F Cell cycle progression in RKO cells with overexpressing DUSP4 or Ctrl was checked by FACS (PI staining). The statistical results of each group were shown. G & H Cell proliferative capacity in RKO cells with overexpressing DUSP4 or Ctrl was tested by EdU fluorescence staining assay. The statistical results of EdU + cells in each group were shown. I The expression of Ki-67 in each group was analysed by western blot. J & K Cell apoptosis in RKO cells with overexpressing DUSP4 or Ctrl was checked by FACS (PI-Annexin V). The statistical results of apoptosis cells % in each group were shown. L The expression level of cleaved caspase 3, Bax, Bcl-2 in RKO cells with overexpressing DUSP4 or Ctrl was analysed by western blot. M & N Representative images of trans-well assay and statistic results on migrated cells in RKO expressing DUSP4 or Ctrl were shown. O & P Representative images of wound healing assay and statistic results on migrated cells in RKO expressing DUSP4 or Ctrl were shown. Q The expression of E-cadherin, N-cadherin and β-catenin in each group was analysed by western blot
Changes in apoptotic capacity following exogenous expression of DUSP4 were also detected. FACS (PI-Annexin V assay) results disclosed that DUSP4 ectopic expression increased the rate of apoptotic cells (Figs. 4J, K, S2H, S2I). Western blotting also detected the expression of Bax, Bcl-2, and cleaved caspase 3, and the results indicated that DUSP4 ectopic expression reduced Bax and cleaved caspase 3 levels and increased Bcl-2 levels (Figs. 4L, S2N).
Furthermore, the results of the transwell experiments showed that ectopic expression of DUSP4 promoted cell migration in RKO cells compared with that of control cells (Figs. 4M, N, S2L, S2M). Similar results were obtained in the wound healing assay (Figs. 4O, P, S2J, S2K), where ectopic expression of DUSP4 promoted the migration ability of RKO cells. Western blotting for EMT markers showed that ectopic expression of DUSP4 increased the expression of N-cadherin, β-catenin, and decreased the expression of E-cadherin (Figs. 4Q, S2N).
These findings collectively suggest that ectopic expression of DUSP4 enhances CRC cell proliferation, migration and represses apoptosis in vitro.
DUSP4 Mediates CREB Activation to Regulate PRKACB Expression
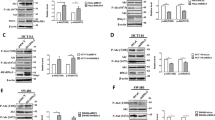
To explore the potential mechanisms of DUSP4-mediated CRC progression, we performed human phosphokinase arrays in RKO-MCS and RKO-DUSP4 cells, containing 37 kinase phosphorylation patterns and two key proteins. As shown in Fig. 5A, B, DUSP4 ectopic expression increased the expression of many phosphokinases, especially p-CREB. To further confirm these results, western blotting was performed to detect CREB activation in both DUSP4 silencing and overexpression cell lines. The results showed that the knockdown of DUSP4 expression inhibited CREB activation, and the corresponding overexpression of DUSP4 promoted CREB activation (Figs. 5C, S2B).
DUSP4 mediates CREB activation to regulate PRKACB expression. A & B Human phosphokinase array was performed to detect 39 kinases activation in RKO cells with overexpressing DUSP4 or Ctrl. C Analysis the expression of CREB, p-CREB by western blot in various cell lines. D The Volcano plot result of RNA-seq was shown. E The GO analysis result of RNA-seq was shown. F The KEGG analysis result of RNA-seq was shown. G The GSEA results of cell cycle, DNA replication were shown. H The identified 10 genes with the significant expression changes were shown. I The expression of PRKACB in each group was analysed by western blot. J The co-expression of Flag-DUSP4 and p-CREB was detected by Immunoprecipitation assay. K Dual fluorescence reporter assay was performed to detect PRKACB promoter activity in each group
To further determine the underlying mechanism of action of DUSP4, RNA sequence was performed to discover novel new target genes of DUSP4. The Volcano plot results showed that 378 DEGs were upregulated and 599 DEGs were downregulated upon ectopic expression of DUSP4 (Fig. 5D). Moreover, GO analysis revealed that DUSP4 ectopic expression altered many biological processes, especially DNA replication (Fig. 5E). The KEGG pathway enrichment results suggested that DUSP4 ectopic expression regulates many pathways, particularly the cell cycle and DNA replication (Fig. 5F). In agreement with this, the GSEA results indicated that DUSP4 ectopic expression positively regulated the cell cycle and DNA replication (Fig. 5G). To discover novel targets, we identified 10 genes with significant changes following DUSP4 ectopic expression, which contains PKA subunit (PRKACB), ATAD3C and other genes (Fig. 5H). Interestingly, combined with transcription factor-target gene activity analysis, we found that PRKACB gene was regulated by the transcription factor CREB. To verify the above results, western blotting was performed to check the expression change of PRKACB. The results showed that silencing of DUSP4 decreased the expression of PRKACB, and overexpression of DUSP4 increased the expression of PRKACB (Figs. 5I, S2N).
To further confirm the relationship between DUSP4 and CREB, we initially examined the co-expression of Flag-DUSP4 and p-CREB by immunoprecipitation assays, which showed that DUSP4 may bind CREB to play a regulatory role (Fig. 5J). Further, we examined the regulatory effects of DUSP4 and CREB on the target gene PRKACB by dual fluorescence reporter assay, and found that overexpression of both DUSP4 and CREB, respectively, was able to enhance PRKACB promoter activity, and overexpression of both DUSP4 and CREB resulted in a higher PRKACB promoter activity (Fig. 5K).
In summary, these results reveal that DUSP4 promotes CREB activation to regulate the expression of PRKACB to mediate CRC progression.
DUSP4 Mediates CRC Malignant Features Relying on CREB Activation In Vitro
Since DUSP4 expression can promote CREB activation, we further explored the role of CREB activation in DUSP4-mediated CRC progression. The specific inhibitor of CREB (666-15) was used to inhibit the phosphorylation and activation of CREB, and the results showed that 0.1 uM of inhibitor 666–15 inhibits CREB activation effectively in RKO-DUSP4 and SW620-DUSP4 cells (Figs. 6A, S2B). We initially examined the changes in PRKACB expression in different treatment groups. As shown in Fig. 6B, overexpression of DUSP4 was able to promote PRKACB expression, and 66-15-treated cells significantly decreased PRKACB expression, both in the control and overexpression of DUSP4 groups. In contrast, in 666-15-treated cells, although overexpression of DUSP4 was able to up-regulate PRKACB expression relative to the MCS group, the trend of up-regulation was significantly weaker. Then a series of functional experiments were conducted, and the results of the CCK8 assay showed that the enhanced cell proliferation after DUSP4 expression was abolished after 666-15 (0.1uM) treatment (Figs. 6C, S2C). The results of the cell colony formation assay showed that the enhanced colony formation capacity after DUSP4 expression was also diminished by 666-15 (0.1 uM) treatment (Figs. 6D, S2D). The same results were demonstrated by PI single staining experiments, which showed that cell cycle acceleration mediated by DUSP4 was decelerated by 666-15 (0.1 uM) treatment (Figs. 6E, F, S2E). In addition, changes in apoptotic capacity were detected, and FACS (PI-Annexin V) assay results disclosed that the cell apoptosis rate reduced by DUSP4 expression was raised by 666-15 (0.1 uM) treatment (Figs. 6G, H, S2H, S2I). The results of the trans-well and wound healing experiments showed that DUSP4 promoted cell migration was abolished by 666–15 (0.1 uM) treatment (Figs. 6I, L, S2J, S2M). Finally, western blot results showed that the expression of Ki-67, N-cadherin and β-catenin increased by DUSP4 expression was abolished by 666–15 (0.1 uM) treatment, the expression of cleaved caspase 3 and E-cadherin reduced by DUSP4 expression was abolished by 666–15 (0.1 uM) treatment (Figs. 6M, S2N). These findings suggest that DUSP4 mediates CRC malignant features by relying on CREB activation in vitro.
DUSP4 mediates CRC progression relying on CREB activation in vitro. A The inhibition efficiency of 666-15 to CREB activation was checked in RKO-DUSP4 cells, the treatment concentration was 0uM, 0.1 uM, 0.2 uM, respectively. B QPCR and western blot were performed to check PRKACB expression in various groups. C Cell viability curve of RKO cells with or without 666-15 treatment was plotted by CCK8 assay. D Cell proliferation ability in RKO cells with or without 666-15 treatment was checked by colony formation. The statistical results of each group were shown. E & F Cell cycle progression in RKO cells with or without 666-15 treatment was checked by FACS (PI staining). The statistical results of each group were shown. G & H Cell apoptosis in RKO cells with or without 666-15 treatment was checked by FACS (PI-Annexin V). The statistical results of apoptosis cells % in each group were shown. I & J Representative images of trans-well assay and statistic results on migrated cells in RKO expressing DUSP4 with or without 666-15 treatment were shown. K & L Representative images of wound healing assay and statistic results on migrated cells in RKO expressing DUSP4 with or without 666-15 treatment were shown. M The expression level of cleaved caspase 3, Bax, Bcl-2, E-cadherin, N-cadherin and β-catenin in RKO expressing DUSP4 with or without 66-15 treatment was analysed by western blot
Ectopic Expression of DUSP4 Promotes CRC Tumor Progression In Vivo
Since DUSP4 mediates CRC tumor progression by enhancing cell proliferation and suppressing cell apoptosis in vitro, we examined these findings in a mouse model in vivo. We subcutaneously injected stable RKO-MCS and RKO-DUSP4 cells into the fourth fat pad of nude mice. As shown in Fig. 7A, B, the tumor growth and tumor volume in the RKO-DUSP4 group displayed an obvious increase compared to those in the RKO-MCS group. Moreover, HE staining of mouse lungs showed an increase in lung metastatic nodules and areas in the RKO-DUSP4 group relative to the RKO-MCS group (Fig. 7C, D). The IHC staining results also showed that ectopic expression of DUSP4 increased the expression of Ki-67, p-CREB, PRKACB, N-cadherin, β-catenin and reduced the expression of cleaved caspase 3 and E-cadherin, which was consistent with the in vitro findings (Fig. 7E, F). These findings indicate that ectopic DUSP4 expression promotes CRC tumor progression in vivo.
Ectopic expression of DUSP4 promotes CRC tumor progression in vivo. A The tumor growth curves of RKO cells with overexpressing DUSP4 or Ctrl were measured. B The tumors separated from different group mice were shown. C Representative images of H&E staining on lungs from RKO-MCS and RKO-DUSP4 groups were shown. D Statistic results of metastatic nodules in the lungs from RKO-MCS and RKO-DUSP4 groups were shown. E The expression of DUSP4, Ki-67, cleaved caspase 3, p-CREB, PRKACB, E-cadherin, N-cadherin and β-catenin in various group mice tumors was checked by IHC staining. F The statistical results of the DUSP4, Ki-67, cleaved caspase 3, p-CREB, PRKACB, E-cadherin, N-cadherin and β-catenin positive cells in various group mice tumors were shown
Proposed Model of DUSP4 Promotes CRC Progression
We propose the following model based on the findings of our study (Fig. 8): DUSP4, a specific phosphatase, is mainly localized in the nucleus. DUSP4 binds and activates CREB in an unknown way, stabilizing the transcriptional regulation of CREB on the downstream target gene PRKACB, mediating the acceleration of the cell cycle, promoting cell proliferation, migration and inhibiting apoptosis. PRKACB, as the catalytic subunit of PKA kinase, increases its expression to recruit more cAMP, further enhancing the activation of CREB, thus forming a positive feedback regulatory loop and ultimately mediating CRC progression.
Discussion
The microenvironment in which tumors, including colorectal cancer, grow is a complex system composed of tumor cells, stromal cells, and the extracellular matrix, with biological characteristics such as hypoxia, low pH, high interstitial pressure, multiple growth factors and protein hydrolase production, inflammatory response, and immunosuppression, which are considered important for tumor proliferation, invasion, migration, adhesion, and neovascularization [36]. Identifying targets for the early diagnosis and treatment of tumors from different perspectives may provide new strategies for tumor treatment. In our previous work, we constructed a siRNA library of 1027 inflammatory genes and performed a high-throughput screening of inflammatory genes capable of regulating tumor stemness using breast cancer stem cells as a model and the stemness molecule OCT4 promoter-linked luciferase system as a tool, and found many candidate genes capable of regulating both tumor inflammation and tumor stem cell stemness, and DUSP4 was one of them [35].
DUSP (dual-specificity phosphatase) is a newly discovered subclass of the protein tyrosine phosphatase (PTP) family [37]. As a member of the DUSP family, DUSP4 inactivates the MAPK pathway (ERK, P38, and JNK) through dephosphorylation, thereby playing an important role in cellular, physiological, and pathological processes, including senescence, stress-induced apoptosis, and malignancy. DUSP4 appears to play a dual role in tumor progression. However, it plays the role of a tumor suppressor oncogene by dephosphorylating MAPK; yet, it has been reported to be closely associated with the progression of various malignancies, such as hepatocellular carcinoma [12], ovarian cancer [15], pancreatic cancer [19], and esophageal cancer [18]. Recent studies have demonstrated that dual inactivation of DUSP4 and DUSP6 could excessively activate MAPK signaling, further selectively impairing the growth of NRAS- and BRAF-mutant cells [38]. Similarly, some studies have reported that DUSP4 plays an important role in the regulation of colorectal cancer proliferation and metastasis, such as DUSP4 directly deubiquitinates and stabilizes Smad4 protein, which promotes the proliferation and metastasis of colorectal cancer cells [39], as well as the down-regulation of dual-specificity phosphatase 4 enhances the proliferation and invasiveness of colorectal cancer cells [40]. However, the above studies were performed only in in vitro cells and did not involve in vivo experiments in mice. And no comprehensive research has been done on the mechanism study. In our study, we found that DUSP4 functions as an oncogene that is highly expressed in colorectal cancer, promotes cell proliferation, and inhibits apoptosis in colorectal cancer in vitro and in vivo. Furthermore, we identified a novel mechanism by which DUSP4 directly or indirectly activates CREB to exert its pro-oncogenic effects by different ways, providing novel evidence for the identity of the DUSP4 oncogene and a new target for colorectal cancer treatment.
CREB, an important transcription factor, plays a vital role in the development and progression of various tumors [41]. Typically, in NSCLC, CREB regulation of proliferation- and apoptosis-related genes is significantly enhanced, and inhibition of CREB activation with RNAi induces apoptosis in NSCLC cells [42]. Studies have shown that different signaling pathways with different kinases could activate CREB, such as PKA, RSK, GSK3β, etc. In our study, we found that DUSP4 activated CREB directly and enhanced the activation of CREB by activating PKA/cAMP signaling pathway. However, we have not yet studied the specific mechanism of direct activation of CREB by DUSP4, which will be the focus of our next study.
In conclusion, we demonstrated that DUSP4 was remarkably upregulated in CRC tissues and cell lines compared to the control. We discovered that knockdown DUSP4 expression inhibited cell proliferation, migration and promoted cell apoptosis, ectopic expression of DUSP4 facilitated cell proliferation, migration and diminished apoptosis in CRC cell lines in vitro and in xenograft models in vivo. We found that DUSP4 promotes PRKACB expression and CREB activation by activating PRKACB (PKA)/cAMP signaling in vitro and in vivo. Using a CREB inhibitor in vitro, we found that CREB activation is indispensable for DUSP4-mediated CRC malignant features. Finally, we assume that DUSP4 acts as a tumor oncogene in colorectal and that DUSP4 may represent a new therapeutic target for the treatment of CRC.
Data Availability
The data are available for all study authors. The data sets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DUSP4:
-
Dual-specificity phosphatase 4
- CRC:
-
Colorectal cancer
- PRKACB:
-
Protein kinase CAMP-activated catalytic subunit beta
- PKA:
-
Protein kinase A
- CREB:
-
Cyclic-AMP response element binding protein
References
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (Lond, Engl). 2019;394:1467–1480.
Siegel RL, Giaquinto AN. Cancer statistics. J CA 2024;2024:12–49.
Cai M, Gao Z, Liao J, Jiang Y, He Y. Frailty affects prognosis in patients with colorectal cancer: A systematic review and meta-analysis. Front Oncol. 2022;12:1017183.
Yip-Schneider MT, Lin A, Marshall MS. Pancreatic tumor cells with mutant K-ras suppress ERK activity by MEK-dependent induction of MAP kinase phosphatase-2. Biochem Biophys Res Commun. 2001;280:992–997.
Hu B, Zhang D, Zhao K et al. Spotlight on USP4: structure, function, and regulation. Front Cell Dev Biol. 2021;9:595159.
Lawan A, Torrance E, Al-Harthi S et al. MKP-2: out of the DUSP-bin and back into the limelight. Biochem Soc Trans 2012;40:235–239.
Jeong DG, Jung SK, Yoon TS et al. Crystal structure of the catalytic domain of human MKP-2 reveals a 24-mer assembly. Proteins. 2009;76:763–767.
Zhang T, Mulvaney JM, Roberson MS. Activation of mitogen-activated protein kinase phosphatase 2 by gonadotropin-releasing hormone. Mol Cell Endocrinol 2001;172:79–89.
Bignon A, Régent A, Klipfel L et al. DUSP4-mediated accelerated T-cell senescence in idiopathic CD4 lymphopenia. Blood. 2015;125:2507–2518.
Tresini M, Lorenzini A, Torres C, Cristofalo VJ. Modulation of replicative senescence of diploid human cells by nuclear ERK signaling. J Biol Chem. 2007;282:4136–4151.
Cadalbert L, Sloss CM, Cameron P, Plevin R. Conditional expression of MAP kinase phosphatase-2 protects against genotoxic stress-induced apoptosis by binding and selective dephosphorylation of nuclear activated c-jun N-terminal kinase. Cell Signal. 2005;17:1254–1264.
Huang S, Ma Z, Zhou Q et al. Genome-wide CRISPR/Cas9 library screening identified that DUSP4 deficiency induces lenvatinib resistance in hepatocellular carcinoma. Int J Biol Sci. 2022;18:4357–4371.
Hanna A, Nixon MJ, Estrada MV et al. Combined Dusp4 and p53 loss with Dbf4 amplification drives tumorigenesis via cell cycle restriction and replication stress escape in breast cancer. Breast Cancer Res. 2022;24:51.
Zeng X, Zhu C, Zhu X. DUSP4 promotes the carcinogenesis of CCRCC via negative regulation of autophagic death. Biosci Biotechnol Biochem. 2021;85:1839–1845.
Chesnokov MS, Yadav A, Chefetz I. Optimized transcriptional signature for evaluation of MEK/ERK pathway baseline activity and long-term modulations in ovarian cancer. Int J Mol Sci. 2022;23:8.
Sieben NL, Oosting J, Flanagan AM et al. Differential gene expression in ovarian tumors reveals Dusp 4 and Serpina 5 as key regulators for benign behavior of serous borderline tumors. J Clin Oncol. 2005;23:7257–7264.
Wu W, Wei H, Hu S et al. Long noncoding RNA PCAT6 regulates cell proliferation and migration in human esophageal squamous cell carcinoma. J Cancer. 2022;13:681–690.
Han J, Ye S, Chen J et al. Lysine-specific histone demethylase 1 promotes oncogenesis of the esophageal squamous cell carcinoma by upregulating DUSP4. Biochem Biokhimiia. 2021;86:1624–1634.
Hijiya N, Tsukamoto Y, Nakada C et al. Genomic loss of DUSP4 contributes to the progression of intraepithelial neoplasm of pancreas to invasive carcinoma. Cancer Res. 2016;76:2612–2625.
Al-Mutairi MS, Habashy HO. DUSP4 silencing enhances the sensitivity of breast cancer cells to doxorubicin through the activation of the JNK/c-Jun signalling pathway. Molecules (Basel, Switzerland). 2022;27:89.
Chen M, Zhang J, Berger AH et al. Compound haploinsufficiency of Dok2 and Dusp4 promotes lung tumorigenesis. J Clin Invest. 2019;129:215–222.
Lin H, Qiu S, Xie L, Liu C, Sun S. Nimbolide suppresses non-small cell lung cancer cell invasion and migration via manipulation of DUSP4 expression and ERK1/2 signaling. Biomed Pharmacother. 2017;92:340–346.
Xue Z, Vis DJ, Bruna A et al. MAP3K1 and MAP2K4 mutations are associated with sensitivity to MEK inhibitors in multiple cancer models. Cell Res. 2018;28:719–729.
Kwok RP, Lundblad JR, Chrivia JC et al. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226.
Wang H, Xu J, Lazarovici P, Quirion R, Zheng W. cAMP Response element-binding protein (CREB): a possible signaling molecule link in the pathophysiology of schizophrenia. Front Mol Neurosc. 2018;11:255.
Lu F, Zheng Y, Donkor PO, Zou P, Mu P. Downregulation of CREB promotes cell proliferation by mediating G1/S phase transition in hodgkin lymphoma. Oncol Res. 2016;24:171–179.
Sandoval S, Pigazzi M, Sakamoto KM. CREB: A key regulator of normal and neoplastic hematopoiesis. Adv Hematol. 2009;2009:634292.
Li J, Liu X, Wang W, Li C, Li X. MSK1 promotes cell proliferation and metastasis in uveal melanoma by phosphorylating CREB. Archiv Med Sci. 2020;16:1176–1188.
Meng XY, Zhang HZ, Ren YY et al. Pinin promotes tumor progression via activating CREB through PI3K/AKT and ERK/MAPK pathway in prostate cancer. Am J Cancer Res. 2021;11:1286–1303.
Bolger GB. The cAMP-signaling cancers: Clinically-divergent disorders with a common central pathway. Front Endocrinol. 2022;13:1024423.
Taylor SS, Wallbott M, Machal EMF et al. PKA Cβ: a forgotten catalytic subunit of cAMP-dependent protein kinase opens new windows for PKA signaling and disease pathologies. Biochem J. 2021;478:2101–2119.
Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348.
Yao X, Hu W, Zhang J, Huang C, Zhao H, Yao X. Application of cAMP-dependent catalytic subunit β (PRKACB) low expression in predicting worse overall survival: a potential therapeutic target for colorectal carcinoma. J Cancer. 2020;11:4841–4850.
Shen W, Du W, Li Y et al. TIFA promotes colorectal cancer cell proliferation in an RSK- and PRAS40-dependent manner. Cancer Sci. 2022;113:3018–3031.
Shen W, Xie J, Zhao S et al. ICAM3 mediates inflammatory signaling to promote cancer cell stemness. Cancer Lett. 2018;422:29–43.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
Patterson KI, Brummer T, O’Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489.
Ito T, Young MJ, Li R et al. Paralog knockout profiling identifies DUSP4 and DUSP6 as a digenic dependence in MAPK pathway-driven cancers. Nat Genet. 2021;53:1664–1672.
Xu W, Chen B, Ke D, Chen X. DUSP4 directly deubiquitinates and stabilizes Smad4 protein, promoting proliferation and metastasis of colorectal cancer cells. Aging (Albany NY). 2020;12:17634–17646.
Ichimanda M, Hijiya N, Tsukamoto Y et al. Downregulation of dual-specificity phosphatase 4 enhances cell proliferation and invasiveness in colorectal carcinomas. Cancer Sci. 2018;109:250–258.
Zhang H, Kong Q, Wang J, Jiang Y, Hua H. Complex roles of cAMP-PKA-CREB signaling in cancer. Exp Hematol Oncol. 2020;9:32.
Wong JC, Bathina M, Fiscus RR. Cyclic GMP/protein kinase G type-Iα (PKG-Iα) signaling pathway promotes CREB phosphorylation and maintains higher c-IAP1, livin, survivin, and Mcl-1 expression and the inhibition of PKG-Iα kinase activity synergizes with cisplatin in non-small cell lung cancer cells. J Cell Biochem. 2012;113:3587–3598.
Funding
This work was supported by the Tianjin Key Medical Discipline (Specialty) Construction Project (to Gang Liu), Fundamental Scientific Research Project of Tianjin University of China (No. 2017KJ191 to Gang Liu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no potential conflicts of interest.
Animal studies
All animal experiments were performed in accordance with Jining Medical University Animal Welfare Guidelines.
Ethical approval
Approval of the research protocol by an Institutional Reviewer Board: not applicable.
Informed consent
Not applicable.
Registry and the registration no. of the study/trial
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pei, W., Yin, W., Yu, T. et al. Dual-Specificity Phosphatase 4 Promotes Malignant Features in Colorectal Cancer Through Cyclic-AMP Response Element Binding Protein/Protein Kinase CAMP-Activated Catalytic Subunit Beta Activation. Dig Dis Sci 69, 2856–2874 (2024). https://doi.org/10.1007/s10620-024-08481-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-024-08481-y