Abstract
Colorectal cancer (CRC) is a common malignant tumor of the gastrointestinal tract with increasing morbidity and mortality. Exploring the factors affecting colorectal carcinogenesis and controlling its occurrence at its root is as important as studying post-cancer treatment and management. Establishing ideal animal models of CRC is crucial, which can occur through various pathways, such as adenoma-carcinoma sequence, inflammation-induced carcinogenesis, serrated polyp pathway and de-novo pathway. This article aims to categorize the existing well-established CRC animal models based on different carcinogenesis pathways, and to describe their mechanisms, methods, advantages and limitations using domestic and international literature sources. This will provide suggestions for the selection of animal models in early-stage CRC research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer (CRC) not only ranks second in terms of cancer-related mortality but the third most common type of cancer globally [1]. In recent years, the incidence rate of CRC is still increasing and getting younger [2]. Researchers estimated that about 160,000 people will fall ill with CRC in the US by 2023, and nearly 60,000 people will die of CRC; interestingly, about 4000 individuals are under the age of 50 [1]. Therefore, it is crucial to early identify CRC and to explore factors affecting the malignant phenotypic transition of tumors are crucial. While establishing animal models is a common method to address the challenges of CRC research and serves as an important bridge between experimental and clinical studies. Normal intestinal epithelium can develop colon cancer through various pathways, including the adenoma-carcinoma pathway, the inflammatory bowel disease-carcinoma pathway, the serrated polyp-carcinoma pathway, and the de novo pathway [3]. Most reviews focus on classification based on modeling methods and different animal strains, but few articles describe CRC animal models surrounding different carcinogenic pathways. So, this paper aims to review existing animal models of colon cancer under different carcinogenic pathways and provide recommendations for researchers to choose the most suitable experimental animal models.
Adenoma-Carcinoma Pathway
Most colorectal tumors originate from precancerous polyps. Changes in genes that regulate DNA repair and cell proliferation lead to the formation of adenomas. As reported, APC gene mutation is the first step in traditional adenomas and subsequent the accumulation of gene mutation, such as Kras, p53 and others. Adenomas continue to grow, showing increasingly dysplastic characteristics and eventually acquiring invasive potential [3]. Common models are categorized in the table.
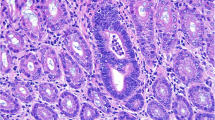
The pathological type of CRC is mostly adenocarcinoma. In the development of the majority of human colorectal adenocarcinomas, it follows the pattern of “aberrant crypt foci (ACF)–adenoma–adenocarcinoma”. Different stages of cancer progression have different histopathological manifestations. ACF is a cluster-like abnormal tubular gland in the inner layer of the colon and rectum. Under chromoendoscopy, it appears as crypts darker than the surrounding tissue, slightly enlarged, with crypt openings often fissure-like. Histologically, the crypt clusters of ACF vary in size and appearance. Adenoma appears as atypical hyperplasia of the intestinal epithelium under the microscope, with enlarged, deeply stained cell nuclei that are spindle-shaped to varying degrees, with loss of polarity and stratification. In histology, adenomas can be divided into tubular adenomas (tubular component > 75%), villous adenomas (villous component > 75%), and tubulovillous adenomas (both tubular and villous components between 25% and 75%). Among them, adenomas with a diameter of 1 cm, villous structure, or high-grade dysplasia may progress to adenocarcinoma.
Carcinogen-Induced CRC Models
Carcinogen-induced CRC models have a long history that can highly reproduce the initiation and progression of human CRC and, therefore, are commonly used in research of the etiology, pathogenesis, and related therapies for CRC [4]. Carcinogens are commonly classified as direct and indirect ones. Unlike direct carcinogens, indirect carcinogens must to be converted into active substances by enzymes in the body to exert effects. There are four categories of carcinogens widely used, including (1) azoxymethane (AOM) and dimethylhydrazine (DMH); (2) heterocyclic amines (HCAs) like PhIP; (3) aromatic Amines like Dmab; (4) alkylating agents like MNNG and MNU. All of them can be used alone or in combination, and they are administered by free feeding, gavage, intraperitoneal injection, subcutaneous injection, muscle injection and rectal administration.
Carcinogenic Mechanisms
The commonly used carcinogen DMH is oxidized to AOM in hepatic cells, and the latter is hydroxylated to form MAM through cytochrome P450-dependent pathway. MAM reaches the intestine directly through bile and intestinal lumen or enters through the circulatory system [5]. It is unstable in the body and can be decomposed to produce alkylating methyl diazonium ions [6], resulting in O-6 or N-7 position guanine methylation in DNA and causing gene mutations during the replication process [7]. ACF arise in the normal gut as a result of DNA alkylation damage by activating oncogenes and inactivating tumor suppressor genes. ACF is a precursor lesion of CRC, with the tendency to further become colorectal adenomas and cancer [8]. Research has shown that treating C57BL/6J mice with AOM can lead to the presence of ACF in the colonic mucosa after 2 weeks, and the occurrence of CRC can be seen with continued feeding [9]. HCAs, found in grilled meat and fish, are genotoxic compounds that also cause DNA mutation damage after metabolism in the liver, eventually leading to cancer [10]. MNNG and MNU are direct carcinogens and are often used to induce CRC in rats through gavage or rectal administration.
Modeling Methods
Animal models of carcinogen-induced colon cancer can be established differently according to different animal strains, dosing regimens, and routes of administration. Currently, multiple injections of carcinogens are used. The animal strains can be selected according to their sensitivity to the carcinogens, and the administration methods are mostly subcutaneous and intraperitoneal. Izumi et al. [11] injected 30 mg/kg of DMH subcutaneously into 85 Balb/c mice, and tumors developed in the distal colon of all mice after 24 weeks. Turusov et al. [12] gave subcutaneous injections of 8 mg/kg DMH to Balb/c and C3HA mice for 25 weeks and found that the colonic tumors incidence was higher in Balb/c mice compared to C3HA mice (93.3% vs 30.9%). Nambiar et al. [13] gave A/J, AKR/J and Balb/c mice intraperitoneally with 10 mg/kg AOM for 6 weeks, after 24 weeks, they found A/J mice had the highest sensitivity to AOM (9.2 tumors per mouse). Dikeocha et al. [14] gave weekly subcutaneous injections of 7 mg/kg AOM to 10-week-old male SD rats for 3 weeks, and large amounts of ACF could be seen at 8 weeks. Reddy et al. [15] subcutaneously injected male F344 rats with 50 mg/kg DMAB 20 times per week. Finally about 70% of rats with a high-fat diet were induced with multiple colonic tumors.
Advantages and Disadvantages
Carcinogen-induced colon cancer models have specific advantages, including (a) Easy to operate, copy, and inexpensive. (b) Induced-onset colon tumors are mostly located in the distal colon, where human colon cancers typically occur [16]. (c) The tumor initially grows with a polypoid feature and often exhibits features similar to those of male CRC in histopathological aspects [17]. (d) the induced tumors are molecularly similar to human CRC, showing mutations in APC and Kras, activation of the wnt signaling pathway, and upregulation of COX-2 levels [18,19,20]. Meanwhile, CRC models also have some drawbacks, e.g., (a) Although most studies indicate that AOM and DMH have some organ affinity, tumor formation occurs mainly in the small intestine, and a considerable amount of alkylated DNA adducts can form in the liver and kidney [21]. (b) There is a very long latency period from carcinogen application to tumorigenesis, which depends on the kind of carcinogens, dosing regimens and mouse strain. The latency period ranges from 24 to 50 weeks [13, 22, 23]. (c) Indirect carcinogens have to be metabolized by the liver, tumor formation is affected if it is blocked. (d) Tumors usually lack mucosal invasiveness, as well as metastasis, so the model is not applied to study advanced colon cancer.
This model is consistent with the progress of the CRC adenoma–carcinoma sequence, which can be used to study factors that play a regulatory role in the development of sporadic colon tumors. It is also suitable to predict the effects of chemotherapy in human colon cancer [24,25,26]. Nevertheless, future improvements in modeling techniques based on the shortcomings are still needed. For example, further confirm the specific details of each scheme, discovery of better novel colon cancer inducers, and, in order to solve the problems of long modeling time and the difficulty of metastasis, transgenic mice treated with oncogenes can accelerate the tumor progression [27].
Genetically Engineered Colon Cancer Models
Transgenic technology refers to the use of modern biotechnology to artificially isolate the target genes that people need, recombine and introduce into the chromosome, then integrate and stably inherit them by the next generation. At present, most of the applications of microinjection technology, that is, through the microinjector, will be injected into the male nucleus of the fertilized egg of the exogenous genes to obtain transgenic animals. The knockout colon cancer model artificially knocks out the relevant oncogenes so as to induce colon polyps to become cancerous. APC gene encodes a tumor suppressor protein that can be directly involved in inhibiting the adverse effects of the Wnt signal pathway on G1 to the S phase transition of the cell cycle. The APC gene mutation can affect the growth of intestinal epithelial cells and promote the occurrence of adenomas. The Apc Min (multiple intestinal neoplasia) mutant mouse is a classic model for studying intestinal tumorigenesis and development. Adenomatous phenotypes of APC Min/+ mice and C57 mice were obtained by crossing APC Min male adult mice with C57 female adult mice in a 1:2 ratio after three months. Then full-sibling mating was performed to expand the population. This mouse model develops intestinal tumors when fed either high-fat chow or normal chow [28]. The pattern of intestinal adenomas during the life cycle of APC Min mice is as follows: adenomas are formed at 9 weeks, followed by the number of small intestinal adenomas remaining constant and increasing in size. Then adenomas grow rapidly after 15 weeks. 24 weeks later, the mice died of anaemic due to the large size of the glands and excessive nutrient depletion.
Carcinogenic Mechanism
Knockdown of the APC gene leads to the blockage of APC protein synthesis and reduces the degradation of β-catenin protein in the cytoplasm of intestinal epithelial cells. The free β-catenin protein aggregates in the cytoplasm, further transfers to the nucleus and binds to TCF4/L4F to initiate the activation of downstream proto-oncogenes. APC gene mutation is the early factor inducing CRC which mainly plays a role in the nucleus and cytoplasm, resulting in the gradual increase of abnormal lesions in the CRC until they develop into multiple adenomatous polyps or adenocarcinoma.
Advantages and Disadvantages
Studies have shown that 80% of the initial sporadic colorectal carcinogenesis is related to APC mutations, so APC Min mice have many advantages as a classical and widely used model for studying intestinal tumor occurrence and development, including (a) Mature technology, simple application method, and low cost. (b) Convenient case evaluation, for example, can find out the regularity of adenoma occurrence by comparing the number and size of adenomas in mice of different weekly ages. (c) The changes in the expression of β-catenin as determined by immunohistochemical staining can help to elucidate the pathogenesis. At the same time, this model also has several shortcomings. Firstly, the induced tumor mainly found in the small intestine, while fewer occurred in the colorectum, so the obtained samples were insufficient. Secondly, the proliferation of intestinal tumors was slower, but the apoptosis level was higher, which required a longer experimental time. In addition, although the larger adenomas had obvious cellular anisotropy, the submucosal layer did not have invasive infiltration, which indicated that the adenomas had not yet progressed to carcinoma. So, the process of adenoma to cancer is more difficult to observe in this model. Many other animal models have been improved from the APC Min mouse model.
Improvements and Developments
Cdc20loxp/+ APC min/+ villin-cre+/− Mutant Mouse Model
Cell division cycle 20 (Cdc20) homolog is one of the major cofactors of the late-promoting Anaphase-promoting complex/cyclosome (APC/C). It activates the APC/C by binding to specific elements of the substrate and involves in the process of mitotic termination. It has been shown that the spindle assembly checkpoint (SAC) is a monitor of the duplicated chromosome bipolar segregation during the mid- to late-phase transition. When the sister chromatid monomer segregation process occurs incorrectly, the mitotic checkpoint complex (MCC) is activated, resulting in the inactivation of Cdc20 and failure of mitosis to terminate in time. Therefore, abnormal or dysfunctional expression levels of Cdc20 may eliminate mitotic arrest by deregulating the activation of the APC, which in turn leads to the generation of cancer cells [29]. The Cdc20loxp/+ mutant mice prepared using gene targeting technology were mated in cages with villin-cre+/− gene mice and APC Min/+ gene mutant mice to obtain Cdc20loxp/+ villin-cre+/− transgenic mice and APC Min/+ villin-cre+/− transgenic mice respectively. Then, Cdc20loxp/+ APC Min/+/− villin-cre+/− mutant mice were obtained by mating the two mice in cages. This model promotes colon cancer development and accelerates tumor malignancy in APC Min/+ mice but has no effect on tumor number or size [30].
Tiam1/C1199-CopGFP Transgenic Mice
The Tiam1 gene, an invasive metastasis inducer in T lymphoma, is also found to be upregulated in CRC and tightly linked to the invasion and metastasis of CRC [31]. Silencing Tiam1 in CRC cell lines inhibits cell migration, invasion and metastasis in vitro. The full-length cDNA of Tiam1 in C1199 is cloned into the lentiviral vector pCDF1-CopGFP, and transgenic mice are prepared using the prokaryotic micromolecule method. Superovulatory and pseudo-pregnant ICR mice are used for the modeling. Lentiviral microinjections containing the genes of Tiam1 and EGFP are injected into the prokaryotic nucleus of each embryo. Then, it was put in the culture medium for 72 h, and the embryos were transferred into the oviducts of pseudo-pregnant mice. The mice deliveries pups at 20–21 days. Then we can obtain genomic DNA from the tail biopsies of the mice. Yu et al. [32] injected the carcinogen DMH (20 mg/kg) weekly intraperitoneally to 4-week-old Tiam1 transgenic or non-transgenic mice for 24 weeks, and pathological sections confirmed that Tiam1 promoted CRC invasion and metastasis.
Inflammatory Bowel Disease (IBD)-Cancer Pathway
Colitis-associated colon cancer (CAC) is the result of the further development of IBD. Its prognosis is poor due to late detection and local development or metastasis [33]. The pathogenesis of IBD is unclear, but its pathology is characterized by localized persistent inflammation. Ulcerative colitis (UC) and Crohn’s disease (CD) are the most common types. Chronic recurrent intestinal mucosal inflammation leads to tumor formation through a variety of mechanisms, including induction of genetic mutations, immune responses, and alteration of the tumor microenvironment. The immunological features of CD are the presence of activated Th1 cells in the intestinal tract, as well as high expression of interferon and tumor necrosis factor, whereas UC exhibits activation of Th2 cells [34, 35].
Under persistent inflammation stimulation, the pathological development process of CAC follows a pattern of “inflammation–atypical hyperplasia–carcinogenesis”, with pathological manifestations similar to adenoma-cancer. In gross tissue, CAC can be observed with ulcers extending deep into the muscle layer. Histologically, there is obvious infiltration of inflammatory cells in the tissue, including basal plasmacytes, lymphocytes and neutrophils, and tend to form crypt abscess. Even inflammatory cells break through the basement membrane and infiltrate into the submucosa. The colonic crypts lose their original tubular structure, presenting as twisted, branched, atrophic crypts, with a decrease in goblet cells. Significant proliferation of crypt cells was observed by Ki67 staining. The luminal surface showed high-grade intraepithelial tumor-like changes, further showing marked cellular atypia, with a few infiltrates into the muscle layer, and these tumors were histologically diagnosed as tubular adenoma or tubular adenocarcinoma.
A combination of oncogenic and inflammatory agents is commonly used to induce the CAC model. See “Carcinogen-Induced CRC Models” section for details of oncogenic agents. Common inflammatory agents include dextran sodium sulfate (DSS) and oxazolone, both of which mimic the UC immune process, and TNBS mimics the immune process of CD [36, 37].
TNBS and oxazolidinone are semi-antigens [38, 39], which are converted to antigens and trigger a variety of immune responses when bound to tissue proteins. TNBS-induced colitis is a delayed hypersensitivity reaction to semi-antigenic proteins [38]. The use of inflammatory agents after treatment of animals with the carcinogens DMH and AOM accelerate colon carcinogenesis. TNBS and oxazolone are administered by enema and TNBS needs to be mixed with ethanol for it to be effective. Fragoso et al. [40] administered 4 doses of DMH (40 mg/kg) twice weekly to Wistar rats. Two weeks later, 10 mg of TNBS mixed with 0.25 mL of 50% ethanol was given in the rectum and executed rats after 25 weeks. 60% showed low-grade abnormal hyperplasia with the presence of ACF. Xiao et al. [41] gave a single intraperitoneal injection of AOM (10 mg/kg) followed by intrarectal administration of 2.5 mg TNBS mixed with 150 μL 50% ethanol to C57BL/6 mice, which showed extensive atypical hyperplasia and precancerous lesions in the mucosa. Currently most of the studies use AOM/DSS to establish the CAC model.
AOM/DSS Induced Model
Carcinogenic Mechanism
DSS, as a chemical inflammatory agent, its essence is a sulfated polysaccharide that directly damages the colonic epithelium, leading to an impaired mucosal barrier and the continuous entry of bacteria and related antigens to cause inflammation [42]. Ingesting DSS in mice can induce intestinal inflammation, with manifestations such as blood in the stool and ulceration of the intestinal mucosa. Multi-cycle feeding of DSS can cause chronic and recurrent inflammation in the intestinal tract, miming the process of human CAC genesis after synergistic action with carcinogens.
Modeling Method
At the beginning of the modeling process, mice or rats are given a single injection of carcinogen AOM and fed water containing DSS once a week later. Depending on the cycles DSS is given, the modeling method can be categorized as “two-step” and “four-step.” The former involves a single AOM injection and a single cycle of DSS to induce acute colonic inflammation. Studies have shown that different mouse strains can develop colonic tumors within 20 weeks [43, 44]. The latter refers to a single AOM injection followed by three rounds of using DSS administration, shortening the modeling time to around 10 weeks [45]. As the driver of CAC progression is mostly chronic inflammation, the “four-step” method is mostly used in studies. Becker et al. [46] administered a single injection of AOM (7.4 mg/kg) to FVB mice. One week later, mice cycled through three cycles of 3% DSS drinking water for 1 week and normal drinking water for 2 weeks. On day 45 endoscopy found polyps. Doulberis et al. [47] gave a single injection of AOM (10 mg/kg) to 5–6 week old Balb/c mice, followed 1 week later by three cycles of DSS (1 week of drinking 1% DSS and 1 week of normal drinking water as a cycle). Necropsy 3.5 months later revealed 20 polypoid adenomas in the colon of 70% of mice. Angelou et al. [48] found that C57BL/6 mice were induced to develop colon cancer either by three times the use of 3% DSS administrations for 5 days or three times the use of 2.5% DSS administrations for 7 days after injection of AOM (10 mg/kg).
Advantages and Disadvantages
This model is relatively quick to establish, easy to operate, reproducible, and cost-effective. However, the modeling success rate is influenced by factors such as mouse strain, diet, microbiota, the cycle number of DSS, and its concentration, with more uncertainty and low colon cancer incidence. Likewise, in carcinogen-induced CRC models, the rate of invasion and metastasis is rare. Furthermore, it does not detect common Kras or p53 gene mutations frequently seen in human CRC [49]. Since human sporadic colon carcinogenesis is not accompanied by inflammation of the colonic mucosa, this model is distinct from carcinogen-induced models and is rarely used to conduct studies related to sporadic colon cancer.
In order to improve the rate of invasion and metastasis, a model using DSS-treated APC mutation mice was developed, further increasing dysplasia and cancer formation rates as well as shortening the modeling time. Cooper et al. [50] showed that after two cycles of 4% DSS treatment in APC Min mice, the occurrence rate of CRC increased by 40% compared with the control group, and when treated for a single cycle (3 weeks), all mice developed dysplasia. Similar to carcinogen-induced models, most models also reflect the progression from ACF to adenoma and cancer well [4]. This model simulates the development process of human IBD and is suited to study the interaction of inflammation and CRC. It is particularly useful for studying tumor progression driven by chronic colonic inflammation.
Serrated Polyps-Cancer Pathway
Intestinal dysplastic adenomas are not the only precursor lesion of CRC, as it has been shown that 15% of CRC develop from serrated polyps via the serrated neoplasia pathway [51]. Serrated polyps are a kind of heterogeneous disease, including hyperplastic polyps, sessile serrated adenomas (SSA), and traditional serrated adenomas (TSA). SSA and TSA have a tendency to malignancy, especially SSA, which has a high tendency and is closely associated with CRC, whereas hyperplastic polyps are essentially non-cancerous. Unlike traditional adenomas, serrated adenomas have unique manifestations in pathology. SSA is seen throughout the lumen and the proliferative zone is often distributed asymmetrically other than the crypt. The crypt is twisted and dilated, serrated, and may be inverted as T-shaped or L-shaped. TSA has an overall complex villous-like structure with coated columnar epithelium, and a pen-shaped, narrow nucleus with eosinophilic cytoplasm. TSA has the formation of ectopic crypts, that is, the crypts are far away from the mucosal muscle layer, and a visible serrated structure can appear locally.
A serrated mutation pathway is a unique form of colon carcinogenesis; the rate of tumor progression correlates with microsatellite instability (MSI). A striking feature of the serrated mutation pathway is the activation of the BRAF gene at the V600E site [52]. As a member of the mitogen-activated protein kinase (MAPK) pathway, BRAF mutation leads to structural activation of the MAPK-ERK pathway and uncontrolled cell division. Another feature is the high level of CpG island methylation in gene promoter regions [53]. It disrupts transcription when the upstream promoter region of the tumor suppressor gene has high CpG methylation, causing the silencing of tumor suppressor genes and, eventually, tumor formatting. Therefore, transgenic mice are commonly used to prepare this type of model. See Table 1.
Genetic Engineering Mouse Models
Carcinogenic Mechanism
Current research on the molecular mechanisms of the serrated pathway in CRC suggests that the serrated mutation pathway mainly involves BRAF mutation, KRAS mutation, and CpG island methylation. Therefore, transgenic models can be prepared by targeting specific genes for knockout using genetic engineering technology. In recent years, models based on the above gene mutations have been developed. Sakamoto et al. [54] further molecularly analyzed tumors diagnosed as serrated colon cancer and found that the loss of CDX2 gene function leads to intestinal epithelial transdifferentiation, expressing serrated changes similar to gastric epithelium, and the loss of the CDX2 gene interacts synergistically with BRAF mutation. Studies have also shown that, like the KRAS gene, the PTEN gene is associated with the occurrence of colon cancer through the regulation of the PI3K pathway [55,56,57]. Fortunately, in lung cancer, endometrial cancer, and pancreatic cancer model systems, the tumor synergistic effect between Pten and Kras mutations has been observed [58,59,60,61,62]. Therefore, targeting the knockout of PTEN and Kras can induce the occurrence of serrated adenomas and enhance tumor metastasis.
Modeling Methods
Based on the above mechanisms, the Cre–loxP recombination system, with its simple operation and high recombination rate, is widely used. This recombination system can not only be used to operate specific site-specific recombination in cells but also in tissues or entire organisms and even to knockout or express a specific gene at a specific time, achieving temporal and spatial specificity of gene operation. Cre recombinase recognizes loxP sites, producing three types of recombination events: excision, inversion and translocation. Breeding Flox mice with specific lox sites for genes that need to be excised with Cre mice can produce transgenic mice with the corresponding gene knockout. To study the gene function at specific stages of organism development, a tamoxifen (Tam)-induced Cre system has been developed, in which Cre is fused with the estrogen receptor (ER). In the presence of Tam, the interaction between ER and Tam induces nuclear translocation of Cre. In the nucleus, CreER recognizes loxP sites, leading to the inactivation of the corresponding gene in the tissue. For example, the PTENfl/flKrasLSL/+ model and the CDX2−/−/BRAF+ mouse model were reported in the literature [54, 62].
Advantages and Disadvantages
First, the genetic combined mutation model is an improvement on the model of inducing single gene mutations, summarizing the entire morphological spectrum of human colorectal serrated pathway tumors. Meanwhile, the tumors exhibit phenotypes similar to human serrated CRC, providing new insights into the molecular pathogenesis of serrated CRC. Second, there are currently no known biomarkers that can clearly distinguish serrated lesions-induced CRC from traditional adenoma-induced CRC, so the model may help further discover new phenotypic markers for the relationship between serrated precursor lesions and CRC. It is worth noting that the injuries observed in these mice were found in the small intestine rather than the colon.
Improvements and Developments
Chromosome Engineering Organ System Transplantation Model
Although we can use genetic engineering technology to introduce mutations in certain genes and establish mouse models with specific gene mutations, human CRC is a process involving multiple gene mutations, so it is important to develop models to introduce multiple gene mutations in the mouse colon. The recent chromosome engineering organ system transplantation model first proposed for TSA. TSA has unique histological features, and research has found that 1%–5% of sporadic CRCs involve chromosomal rearrangements of the R-spondin gene without APC gene mutations [63]. Subsequent studies also showed that over 30% of TSA patients repeatedly had fusions of the R-spondin gene [64], but the role of this gene in TSA development remains to be elucidated. Therefore, Kawasaki et al. [65] developed a chromosome engineering organ model to study the fusion of the R-spondin gene and its role in TSA. They used CRISPR-Cas9 (chromosome engineering organ) to introduce chromosomal rearrangements involving the R-spondin gene into human colon organoids and then induced BRAFV600E mutations using transgenic technology, transplanting the organoids into the colon of NOG mice to induce the occurrence of mouse TSA.
This model elucidates the necessary steps for the initiation and progression of TSA at the histopathological level and can introduce multiple gene mutations in the colon organoids through chromosomal rearrangements, providing insights into the pathogenesis of serrated colon cancer. However, the genome editing efficiency and transplantation efficiency are issues that cannot be ignored, and Kawasaki et al. are hopeful that the use of a niche-based selection and fine culture conditions can improve this issue.
De-Novo Pathway
Most colon cancers develop from colonic polyps, but a few reports have described a special pathway through which CRC directly develops from normal colonic mucosa, bypassing the intermediate process of traditional polyp formation. This type of cancer progression is referred to the De-novo pathway. CRC occurring from this pathway is also known as De-novo cancer [61], which has characteristics such as small tumor size, rapid growth, gross morphology of shallow depressed type, absence of adenomatous components around the tumor and high malignancy. In addition to common malignant tumor pathological features such as crowded disordered crypt glands in the mucosa and submucosa and marked cellular atypia, the most prominent histological feature of de-novo cancer is well-defined border with normal mucosa, absence of adenomatous components, and invasion of cancer tissue into the superficial muscle layer.
Due to the low occurrence and low detection rate of these lesions under endoscopy, there are few reports of such lesions. Regarding animal models, back in 1978, Miyoshi et al. [62] found De-novo cancer without any adenomatous components in a rat model of CRC induced by DMH. However, there is limited research on animal models, with a focus mainly on the molecular mechanisms. Current research suggests that the occurrence of De-novo cancer may involve two mutation pathways, namely the p53-APC gene mutation pathway and the only p53 gene mutation pathway [66, 67]. However, the exact mechanism of its occurrence is still not fully understand, so further research is needed to develop animal models for this type of cancer progression.
Outlook
With the change in people’s lifestyles, the incidence of colon cancer is increasing and tends to be younger, which constantly forces us to conquer cancer. More and more animal models of colon cancer have been proposed. The classification of the existing mature models from the perspective of different pathways of colon carcinogenesis reveals that there is no animal model for the de-novo pathway. We still need to study the related mechanisms further and develop models that can simulate this mutation pathway. Additionally, existing models need to be further optimized to shorten the modeling time while ensuring animal welfare, making the animal models more representative of the actual occurrence of human CRC and better serving humanity.
Data availability
No datasets were generated or analysed during the current study.
References
Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233–254.
郑莹,王泽洲. 全球结直肠癌流行数据解读[J]. 中华流行病学杂志,2021, 42(1):149–152.
Nguyen LH, Goel A, Chung DC. Pathways of colorectal carcinogenesis. Gastroenterology. 2020;158:291–302.
De Robertis M, Massi E, Poeta ML et al. The AOM/DSS murine model for the study of colon carcinogenesis: from pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9.
Weisburger JH. Colon carcinogens: their metabolism and mode of action. Cancer. 1971;28:60–70.
Hawks A, Magee PN. The alkylation of nucleic acids of rat and mouse in vivo by the carcinogen 1,2-dimethylhydrazine. Br J Cancer 1974;30:440–447.
Venkatachalam K, Vinayagam R, Arokia Vijaya Anand M, Isa NM, Ponnaiyan R. Biochemical and molecular aspects of 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis: a review. Toxicol Res (Camb). 2020;9:2–18.
Kobaek-Larsen M, Thorup I, Diederichsen A, Fenger C, Hoitinga MR. Review of colorectal cancer and its metastases in rodent models: comparative aspects with those in humans. Comp Med. 2000;50:16–26.
Suzui M, Morioka T, Yoshimi N. Colon preneoplastic lesions in animal models. J Toxicol Pathol. 2013;26:335–341.
Yamashita K, Umemoto A, Grivas S, Kato S, Sato S, Sugimura T. Heterocyclic amine-DNA adducts analyzed by 32P-postlabeling method. Nucleic Acids Symp Ser. 1988;19:111–114.
Izumi K, Otsuka H, Furuya K, Akagi A. Carcinogenicity of 1,2-dimethylhydrazine dihydrochloride in BALB/c mice. Influence of the route of administration and dosage. Virchows Arch A Pathol Anat Histol. 1979;384:263–267.
Turusov VS, Lanko NS, Krutovskikh VA, Parfenov YD. Strain differences in susceptibility of female mice to 1,2-dimethylhydrazine. Carcinogenesis. 1982;3:603–608.
Nambiar PR, Girnun G, Lillo NA, Guda K, Whiteley HE, Rosenberg DW. Preliminary analysis of azoxymethane induced colon tumors in inbred mice commonly used as transgenic/knockout progenitors. Int J Oncol. 2003;22:145–150.
Dikeocha IJ, Al-Kabsi AM, Chiu HT, Alshawsh MA. Faecalibacterium prausnitzii ameliorates colorectal tumorigenesis and suppresses proliferation of HCT116 colorectal cancer cells. Biomedicines. 2022;10:1128.
Reddy BS, Ohmori T. Effect of intestinal microflora and dietary fat on 3,2′-dimethyl-4-aminobiphenyl-induced colon carcinogenesis in F344 rats. Cancer Res. 1981;41:1363–1367.
Druckrey H, Preussmann R, Matzkies F, Ivankovic S. Selektive Erzeugung von Darmkrebs bei Ratten durch 1,2-Dimethyl-hydrazin [Selective production of intestinal cancer in rats by 1,2-dimethylhydrazine]. Naturwissenschaften. 1967;54:285–286.
Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004.
Maltzman T, Whittington J, Driggers L, Stephens J, Ahnen D. AOM-induced mouse colon tumors do not express full-length APC protein. Carcinogenesis. 1997;18:2435–2439.
Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K. Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21:1117–1120.
Vivona AA, Shpitz B, Medline A et al. K-ras mutations in aberrant crypt foci, adenomas and adenocarcinomas during azoxymethane-induced colon carcinogenesis. Carcinogenesis. 1993;14:1777–1781.
Sohn OS, Fiala ES, Requeijo SP, Weisburger JH, Gonzalez FJ. Differential effects of CYP2E1 status on the metabolic activation of the colon carcinogens azoxymethane and methylazoxymethanol. Cancer Res. 2001;61:8435–8440.
Panikolaou A, Wang QS, Papanikolaou D, Whiteley HE, Rosenberg DW. Sequential and morphological analyses of aberrant crypt foci formation in mice of differing susceptibility to azoxymethane-induced colon carcinogenesis. Carcinogenesis. 2000;21:1567–1572.
Clapp NK, Henke MA, London JF, Shock TL. Enhancement of 1,2-dimethylhydrazine-induced large bowel tumorigenesis in Balb/c mice by corn, soybean, and wheat brans. Nutr Cancer. 1984;6:77–85.
Baek SJ, Okazaki R, Lee SH et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131:1553–1560.
Greten FR, Eckmann L, Greten TF et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296.
Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur J Cancer. 2005;41:1911–1922.
Yang J, Wei H, Zhou Y et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. 2022;162:135–149.e2.
苗晋鑫,宋韶鹤,李秀敏. 结直肠癌小鼠模型研究进展[J]. 中国实验动物学报, 2020,28(2):267–272.
Wu WJ, Hu KS, Wang DS et al. CDC20 overexpression predicts a poor prognosis for patients with colorectal cancer. J Transl Med. 2013;10:142.
Wu F, Sun Y, Chen J et al. The oncogenic role of APC/C activator protein Cdc20 by an integrated pan-cancer analysis in human tumors. Front Oncol. 2021;11:721797.
江海丽,刘宣,李琦. 转基因大肠癌动物模型应用的研究进展[J]. 世界华人消化杂志, 2015(10):1603–1608.
Yu LN, Zhang QL, Li X et al. Tiam1 transgenic mice display increased tumor invasive and metastatic potential of colorectal cancer after 1,2-dimethylhydrazine treatment. PLoS ONE. 2013;8:e73077.
Neto Í, Rocha J, Gaspar MM, Reis CP. Experimental murine models for colorectal cancer research. Cancers (Basel). 2023;15:2570.
Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16:26–42.
Osawa E, Nakajima A, Fujisawa T et al. Predominant T helper type 2-inflammatory responses promote murine colon cancers. Int J Cancer. 2006;118:2232–2236.
Modesto R, Estarreja J, Silva I, Rocha J, Pinto R, Mateus V. Chemically induced colitis-associated cancer models in rodents for pharmacological modulation: a systematic review. J Clin Med. 2022;11:2739.
Gadaleta RM, Garcia-Irigoyen O, Moschetta A. Exploration of inflammatory bowel disease in mice: chemically induced murine models of inflammatory bowel disease (IBD). Curr Protoc Mouse Biol. 2017;7:13–28.
Elson CO, Beagley KW, Sharmanov AT et al. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol. 1996;157:2174–2185.
Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638.
Fragoso MF, Romualdo GR, Vanderveer LA et al. Lyophilized açaí pulp (Euterpe oleracea Mart) attenuates colitis-associated colon carcinogenesis while its main anthocyanin has the potential to affect the motility of colon cancer cells. Food Chem Toxicol. 2018;121:237–245.
Xiao Y, Dai X, Li K, Gui G, Liu J, Yang H. Clostridium butyricum partially regulates the development of colitis-associated cancer through miR-200c. Cell Mol Biol (Noisy-le-grand) 2017;63:59–66.
Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol 2017;23:6016–6029.
Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973.
Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169.
Snider AJ, Bialkowska AB, Ghaleb AM, Yang VW, Obeid LM, Hannun YA. Murine model for colitis-associated cancer of the colon. Methods Mol Biol. 2016;1438:245–254.
Becker C, Fantini MC, Schramm C et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501.
Doulberis M, Angelopoulou K, Kaldrymidou E et al. Cholera-toxin suppresses carcinogenesis in a mouse model of inflammation-driven sporadic colon cancer. Carcinogenesis. 2015;36:280–290.
Angelou A, Andreatos N, Antoniou E et al. A Novel modification of the AOM/DSS model for inducing intestinal adenomas in mice. Anticancer Res. 2018;38:3467–3470.
Thaker AI, Shaker A, Rao MS, Ciorba MA. Modeling colitis-associated cancer with azoxymethane (AOM) and dextran sulfate sodium (DSS). J Vis Exp. 2012;67:4100.
Cooper HS, Everley L, Chang WC et al. The role of mutant Apc in the development of dysplasia and cancer in the mouse model of dextran sulfate sodium-induced colitis. Gastroenterology. 2001;121:1407–1416.
Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 1999;96:8681–8686.
Davies H, Bignell GR, Cox C et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954.
Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213.
Sakamoto N, Feng Y, Stolfi C et al. BRAFV600E cooperates with CDX2 inactivation to promote serrated colorectal tumorigenesis. Elife. 2017;6:e20331.
Day FL, Jorissen RN, Lipton L et al. PIK3CA and PTEN gene and exon mutation-specific clinicopathologic and molecular associations in colorectal cancer. Clin Cancer Res. 2013;19:3285–3296.
Davies EJ, Marsh Durban V, Meniel V, Williams GT, Clarke AR. PTEN loss and KRAS activation leads to the formation of serrated adenomas and metastatic carcinoma in the mouse intestine. J Pathol. 2014;233:27–38.
Marsh V, Davies EJ, Williams GT, Clarke AR. PTEN loss and KRAS activation cooperate in murine biliary tract malignancies. J Pathol. 2013;230:165–173.
Iwanaga K, Yang Y, Raso MG et al. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Res. 2008;68:1119–1127.
Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70.
Hill R, Calvopina JH, Kim C et al. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res. 2010;70:7114–7124.
Kuramoto S, Oohara T. Minute cancers arising de novo in the human large intestine. Cancer. 1988;61:829–834.
Miyoshi T, Ohhara T, Morioka M. Studies on carcinogenesis and extension of 1,2-dimethylhydrazine induced large-bowel cancer in rats (author’s transl). Nihon Shokakibyo Gakkai Zasshi. 1978;75:284–293 ((in Japanese)).
Seshagiri S, Stawiski EW, Durinck S et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664.
Sekine S, Yamashita S, Tanabe T et al. Frequent PTPRK-RSPO3 fusions and RNF43 mutations in colorectal traditional serrated adenoma. J Pathol. 2016;239:133–138.
Kawasaki K, Fujii M, Sugimoto S et al. Chromosome engineering of human colon-derived organoids to develop a model of traditional serrated adenoma. Gastroenterology. 2020;158:638-651.e8.
Aoki T, Takeda S, Yanagisawa A et al. APC and p53 mutations in de novo colorectal adenocarcinomas. Hum Mutat. 1994;3:342–346.
Watanabe T, Muto T. Colorectal carcinogenesis based on molecular biology of early colorectal cancer, with special reference to nonpolypoid (superficial) lesions. World J Surg. 2000;24:1091–1097.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (No. 82172983).
Author information
Authors and Affiliations
Contributions
Xue Chen, the first author, is responsible for proposing research topics, querying and screening literature, listing the review outline in the early stage, and completing the writing of the abstract, part 1.2, outlook and table. Responsible for proofreading the content of each part and reference citation in the later stage. Yirong Ding, the second author, is responsible for searching and screening the literature that met the topic selection, and completing the writing of Part 1.2 in the early stage. Responsible for proofreading the content of each part and reference citation in the later stage. Yun Yi is responsible for screening literatures that met the requirements of topic selection retrieved by Chen X and Ding YR, and is responsible for the writing of the third part (Inflammatory bowel disease (IBD)-cancer pathway). Correct text grammar problems in the later stages. Zhishan Chen is responsible for screening literatures that met the requirements of topic selection and completing the writing of Part three (Serrated polyps-cancer pathway) Jiaping Fu is responsible for screening literatures that met the requirements of topic selection and completing the writing of Part four (De-novo pathway). Ying Chang, the corresponding auther, provides assistance through topic selection, outline, content writing and submission
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Ding, Y., Yi, Y. et al. Review of Animal Models of Colorectal Cancer in Different Carcinogenesis Pathways. Dig Dis Sci 69, 1583–1592 (2024). https://doi.org/10.1007/s10620-024-08384-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-024-08384-y