Abstract
Background and Aims
Standard endotherapy for pancreatic duct (PD) disruption is pancreatic stenting and sphincterotomy. In patients refractory to standard treatment, treatment algorithm is currently not standardized. This study aims to report the 10-year experience with the endoscopic treatment of postoperative or traumatic PD disruption and to share our algorithmic approach.
Methods
This retrospective study was conducted on 30 consecutive patients who underwent endoscopic treatment for postoperative (n = 26) or traumatic (n = 4) PD disruption between 2011 and 2021. Standard treatment was initially applied to all patients. Endoscopic modalities used with a step-up approach in patients unresponsive to standard treatment were stent upsizing and N-butyl-2-cyanoacrilate (NBCA) injection for partial disruption, and the bridging of the disruption with a stent and cystogastrostomy for complete disruption.
Results
PD disruption was partial in 26 and complete in 4 patients. Cannulation and stenting of PD was successful in all patients and sphincterotomy was performed in 22 patients. Standard treatment was successful in 20 patients (66.6%). The resolution of PD disruption in 9 of 10 patients refractory to standard treatment was achieved with stent upsizing in 4, NBCA injection in 2, the bridging of the complete disruption in one, and cystogastrostomy after spontaneously and intentionally developed pseudocyst in one patient each. Overall, therapeutic success rate was 96.6% (100% for partial, 75% for complete disruption). Procedural complications occurred in 7 patients.
Conclusions
Standart treatment for PD disruption is usually effective. In patients refractory to standard treatment, the outcome may be improved by step-up approach using alternative endoscopic modalities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic duct (PD) disruption occurs when the integrity of PD is lost due to different etiologies and may be partial or complete according to the degree of disruption. The main causes of PD disruptions are abdominal surgery, trauma, and acute or chronic pancreatitis [1]. Leakage of pancreatic secretions due to PD disruption regardless of etiology may result in development of ascites, peripancreatic fluid collection, fistula formation, pseudocyst and pancreatic abscess [2, 3].
Postoperative PD disruption is one of the most severe complications after pancreatic surgery and is a major concern for surgeons. Despite technical advances in the perioperative setting to prevent postoperative PD disruption, the incidence of this complication still ranges from 3 to 45% even in high-volume centers [4] and clinically significant postoperative PD disruption account for the majority of this rate [5]. This complications can lead to significant morbidity, increased hospital stay and cost, and may be life-threatening [6].
Pancreatic trauma occurs in 5% of patients with blunt trauma and 8% of patients with penetrating injuries and can result in PD disruption [7]. Main pancreatic duct (MPD) injury as a result of pancreatic trauma is the main determinant of prognosis and is associated with high morbidity (45%) and mortality (30%) [8].
PD disruption may be managed by conservative, endoscopic or surgical methods. Conservative tretment consisting of bowel rest, total parenteral nutrition, and somatostatin analogues is used initially in the treatment of PD disruption and has a 50–60% success rate [9]. In the last decades, endoscopic treatment has become the preferred method in patients refractory to conservative treatment, because of the increased risk of morbidity and mortality due to surgical treatment. The mainstay of endoscopic treatment is pancreatic stent placement and sphincterotomy which facilitate drainage of pancreatic juice through the papilla by decreasing the transpapillary pressure gradient. The management of PD disruption refractory to this standard endoscopic treatment can be difficult and challenging and requires the use of a variety of endoscopic modalities, depending on whether the disruption is partial or complete. Furthermore, a treatment algorithm for refractory PD disruption is currently not standardized.
The aims of this study were to report the 10-year experience with the endoscopic treatment of PD disruption caused by surgery or trauma in a tertiary referral center and to share our algorithmic approach according to the degree of disruption.
Materials and Methods
The current study was designed as a retrospective study conducted in a single tertiary referral center and approved by the ethics committee of the Ankara City Hospital (E1/1808/2021) on 26 May 2021. Informed consent was taken for each procedure from patients. Procedures were performed in Ankara Türkiye Yüksek İhtisas Teaching Hospital from January 2011 till February 2019. Since this hospital was incorporated into Ankara City Hospital in February 2019, procedures after this date were conducted in Ankara City Hospital by the same medical staff.
Study Population
Endoscopic database of our tertiary referral center where more than 2000 ERCPs are performed annually was retrospectively reviewed to identify patients with PD disruption from January 2011 to January 2021. All consecutive patients who have undergone ERP (endoscopic retrograde pancreatography) for symptomatic, postoperative or traumatic PD disruption unresponsive to consevative therapy were included in the study. Patients with PD disruption due to inflammatory causes (acute or chronic pancreatitis) and after whipple procedure were excluded from the study. Data collected from patients included demographic information, etiology and clinical manifestation of PD disruption, results of imaging studies prior to ERP, ERP findings, details of endoscopic treatment procedures and complications of the interventions.
Definitions
PD disruption was diognosed with the presence of amylase-rich fluid drainage from the percutaneous drain and/or the detection of pancreatic fluid collection by imaging methods, and/or extravasation of contrast material from the PD during ERP. PD disruption was defined as complete (also called disconnected PD syndrome) if the contrast material during ERP extravasated into the fluid collection and the proximal PD to the site of disruption could not be visualized. PD disruption was defined as partial if there was visualization of PD proximal to the site of disruption with contrast during ERP (lateral leak) or if there is extravasation of contrast material from the distal end of the MPD (pancreatic stump leak). Patients with intact PD in ERP but clinically pancreatic disruption (symptomatic patients with percutaneous drainage of amylase-rich fluid and associated imaging findings) were accepted to have side branch leak and these patients also were included in the partial disruption group.
The degree of postoperative PD disruption was determined according to the criteria updated by International Study Group on Pancreatic Surgery in 2016 [4]. According to this update, grade A postoperative PD disruption has no clinical importance. Grade B postoperative PD disruption requires a change in the postoperative management such as percutaneous or endoscopic drainage. Grade C postoperative PD disruption requires reoperation or leads to single or multiple organ failure.
Therapeutic success was defined as successful resolution of the PD disruption demonstrated clinically (cessation of drainage of pancreatic juice from percutaneous drain), radiologically (resolution of pancreatic fluid collection) or endoscopically (absence of contrast extravasation on last ERP).
Procedures
All the procedures were performed using a duodenoscope (TJF-260V and TJF- Q180V, Olympus, Japan) under the midazolam or propofol anesthesia by a single experienced endoscopist (Ödemiş B.) performing more than 1000 ERCP annually. Intravenous hyoscine- N-butylbromide or glucagon were used to inhibit intestinal peristalsis.
In the standard endoscopic treatment; PD was first tried to be cannulated through the major papilla with a guidewire-loaded catheter. When the PD could not be cannulated from major papilla, cannulation was attempted through the minor papilla. After cannulation, pancreatogram was obtained to detect the presence and localization of the PD disruption. Pancreatic sphincterotomy was also performed according to the endoscopist's preference. Pancreatic stent was placed as close as possible to the site of leak into the distal PD in patients with pancreatic stump leak (Fig. 1c) and in patients with complete PD disruption. In patients with lateral leak from MPD, pancreatic stent was placed across the leak site into upstream PD, whenever possible (Fig. 2b). Stent diameters were 5 French (Fr) and 7 Fr according to the diameter of the PD. Pancreatic stent exchange or removal was decided by the endoscopist based on clinical and imaging findings. The percutaneous drain in patients with partial duct disruption was clamped to accelerate fistula closure after standard endoscopic treatment if the resolution of intra-abdominal fluid collection was confirmed with cross-sectional imaging modalities. Then, it was removed if there are no clinical and radiological deterioration in the follow-ups.
Endoscopic treatment of pancreatic stump leak. a ERP indicating extravasation of contrast from the distal end of the pancreatic duct (arrow). b Endoscopic view of pancreatic sphicterotomy. c 5-Fr pancreatic stent was placed as close as possible to the leak site (arrow). d Pancreatogram showing the resolution of disruption
Standard endoscopic treatment including pancreatic stent placement with/without sphincterotomy was tried first for all patients. Representative images from two selected patients with pancreatic stump and lateral leak are provided in Figs. 1 and 2. In patients refractory to standard endoscopic therapy, alternative endoscopic treatment modalities were employed with a step-up approach according to the type and location of the leak. Alternative endoscopic treatment modalities for partial disruption were stent upsizing and endoscopic closure of pancreatic fistula with N-butyl-2-cyanoacrilate (NBCA) injection (Fig. 3). These modalities for complete disruption were the bridging of the complete disruption with a stent and endoscopic transmural drainage for spontaneously and intentionally developed pseudocyst. Technical details about the alternative methods are given in the following subheadings.
Endoscopic closure of pancreatic fistula with cyanoacrilate injection. a CT demonstrating peripancreatic fluid collection (asterixis). b ERP indicating extravasation of contrast from the pancreatic stump (arrow). c Pancreatic sphincterotomy was performed. d 7-Fr pancreatic stent was placed. e In the follow-ups, the fistula did not close. Pancreatic duct (PD) was cannulated with the balloon catheter and the balloon catheter (arrows) was positioned and inflated in the PD immediately downstream of the leak site. Afterwards, 2 ml of the cyanoacrilate/lipiodol mixture was injected into the fistulous tract (arrowheads). f A new pancreatic stent was placed. g CT demonstrating pancreatic stent (arrow) and the mixture filling the fistulous tract (arrowhead). h CT demonstrating complete resolution of the peripancreatic fluid collection and the mixture (arrow)
Stent Upsizing
A larger diameter stent (7 F) was placed in patients with 5 Fr stent in the first session if PD diameter is wide enough.
Endoscopic Closure of Pancreatic Fistula with NBCA Injection
In patients with contrast extravasation from pancreatic stump, NBCA (LiquiBand Standard; Advanced Medical Solutions Ltd., Plymouth, UK) was used to seal the leak. NBCA was diluted with oily contrast agent (Lipiodol, Ultra-Fluid; Guenbert GmbH, Sulz-bach, Germany) at a ratio of 1 to 1 mL to achieve the fastest possible solidification of the glue while still allowing for fluoroscopic visualization.The dead space volume of the balloon catheter (Micro-Tech Co., Ltd., Nanjing, P.R. China) was measured as 2 mL and the balloon cathater was flushed with 5% dextrose solution to avoid polymerization before glue injection. After removal of the previously placed pancreatic stent, the PD was cannulated with the balloon catheter and the balloon catheter was positioned in the distal PD immediately downstream of the site of leak. The balloon in the PD was inflated to protect the intact PD from damage by cyanoacrylate, and 2 mL of the mixture was injected into the fistulous tract under fluoroscopic control. Following injection of the mixture, 2 mL of 5% dextrose solution was immediately injected to deliver the total volume of the mixture into fistulous tract. At the end of the NBCA injection, the balloon was deflated and quickly removed as negative pressure was applied to the syringe. To prevent the glue from sticking to the duodenoscope, the balloon catheter was removed without being inserted into working channel of the duodenoscope. Afterwards, the duodenoscope was reentered and pancreatic plastic stent was placed slightly downstream of the glue mixture to decompress the PD and prevent pancreatitis. Representative images from a selected patient for endoscopic sealing of pancreatic fistula by using NBCA injection are provided in Fig. 3.
The Bridging of the Complete Disruption with a Stent (Bridging Method)
In patients with complete disruption, the retroperitoneal space between the disconnected proximal and the distal PD was filled with a contrast material injected through a previously placed percutaneous drain into this area or a cannula inserted transpapillary into the PD, thus aiming retrograde filling of the upstream disconnected PD with contrast material. Afterwards, the disconnected proximal PD was tried to be cannulated with a guidewire under fluoroscopy and bridged with a pancreatic stent.
Endoscopic Transmural Drainage of Spontaneously Developed Pseudocyst
In patients with complete duct disruption whose bridging could not be achieved with the above method, pancreatic stent was placed as close as possible to the disruption into the distal PD. During the follow-ups, if a pseudocyst developed spontaneously, cystogastrostomy was performed.
Endoscopic Transmural Drainage of Intentionally Created Pseudocyst by Clamping the Percutaneous Drain (Pseudocyst-Creating Method)
In patients with complete disruption and failure of the bridging method, if there is no spontaneously developing pseudocyst, an attempt was intentionally made to create a pseudocyst. For this purpose, a percutaneous drain in the leak area was clamped to create a pseudocyst. After the pseudocyst wall has matured and the pseudocyst has reached the appropriate size, cystogastrostomy was performed.
Complications related to ERP were eveluated based on the revised Atlanta criteria for acute pancreatitis and on the basis of consensus definitions for the major complications of ERCP for other complications [10, 11].
Post-procedure Follow-Up
After endoscopic procedures, patients were followed-up clinically (to observe the amount of pancreatic fluid from the percutaneous drain), endoscopically (to document healing of duct disruption) and by abdominal tomography (to confirm resolution of the pancreatic fluid collection).
During the follow-up after standard endoscopic treatment, pancreatic stent exchange or removal was decided by the endoscopist based on clinical and imaging findings. When standard endoscopic treatment fails, alternative endoscopic treatment modalities were used at intervals determined by the endoscopist with a step-up approach according to the type and location of the disruption.
Statistical Analysis
Statistical analysis was performed with the 26th version of Statistical Package for the Social Sciences (SPSS, IBM, Armonk, NY, USA). Continuous variables were tested with Kolmogorov–Smirnov and Shapiro–Wilk tests for distribution pattern evaluation. While mean and standard deviation were used for normally scattered continuous variables, median and interquartile range were used otherwise. Number and percentage were given for categorical data.
Results
Consecutive 30 patients (20 men, mean age 52.7 years [SD: 16.9], range 16–78) who underwent endoscopic treatment for PD disruption between 2011 and 2021were included in the study. The causes of PD disruption were surgery in 26 patients and trauma in 4 patients. PD disruption etiologies of the patients are summarized in Table 1. Contrast enhanced CT was obtained in 24 patients before ERP. According to CT results, peripancreatic fluid collection was detected in 16 patients, ascites in 6, and disconnected PD syndrome in 2 patients. In only 4 patients, the exact location of the PD disruption could be determined by CT. Two of 26 patients with postoperative PD disruption underwent emergency surgical intervention due to postoperative hemodynamic instability. Twenty-five of 26 patients with postoperative PD disruption had surgically placed drains, and additional percutaneous drain was placed in 8 patients by interventional radiology due to lack of clinical improvement. Three of 4 patients with traumatic PD disruption were operated before the ERP procedure and a percutaneous drain was surgically placed. One patient was not operated before the procedure and a percutaneous drain was placed by interventional radiology.
The median time from initial clinical diagnosis of PD disruption to endoscopic therapy was 22.0 days (IQR 23.5, range 7–138). PD was cannulated from the major papilla in 28 patients. Two patients were cannulated from the minor papilla because one patient had an incomplete divisum and the other patient could not be cannulated through the major papilla. Cannulation of the PD was successful in all patients. In 27 of 30 patients, initial ERP showed overt extravasation of contrast material. In these 27 patients, ERP demonstrated pancreatic stump leak in 20 patients, lateral leak in 3 patients and complete disruption in 4 patients. In the remaining 3 patients, leakage from the MPD was not visualized during ERP and these patients were considered to have side branch leakage based on their clinical and radiological data. As a result, according to ERP findings, 26 patients had partial disruption and 4 had complete disruption. Characteristics of PD disruptions were listed in Table 2.
Twenty-four of 26 postoperative PD disruption patients were grade B and 2 were grade C. In the Grade B group, 23 patients had partial disruption and 1 patient had complete disruption. In both patients in the grade C group had partial disruption. Of the 4 patients with traumatic PD disruption, 3 patients had complete disruption and 1 patient had partial disruption.
Standard endoscopic treatment was initially applied to all patients. A pancreatic stent was successfully placed in all patients, while pancreatic sphincterotomy was performed in 22 patients. In the initial procedure, 7 Fr pancreatic stent were placed in 21 patients and 5 Fr stent were placed in 9 patients. Standard endoscopic treatment was successful in 20 of total 30 patients (66.6%).
Resolution of PD disruption could not be achieved with standart treatment in 4 of 9 patients (44.4%) with 5Fr pancreatic stent placed in the first session. In these 4 patients, the 5 Fr stent was replaced by the 7 Fr stent and leakage closed in all of these patients. Endoscopic closure of pancreatic fistula with NBCA was used in 2 patients with pancreatic stump leak and unresponsive to standard endoscopic treatment and fistula closure was achieved in both patients.
Standard endoscopic treatment was unsuccessful in all 4 patients with complete disruption. In one of these patients, resolution of PD disruption was achieved by bridging the complete disruption with a pancreatic stent. Bridging method was unsuccessful in 3 of 4 patients with complete disruption. In one of these 3 patients spontaneously developed a pseudocyst during follow-up and endoscopic cystogastrostomy was performed. In another patient, cystogastrostomy was performed after pseudocyst-creating method. Resolution of PD disruption was achieved in both patients who underwent cystogastrostomy. In last patient with complete disruption, ERP performed at the 4th month of folow-up with a 7 Fr transpapillary pancreatic stent revealed total cutoff of contrast material injected into the downstream disconnected PD. However, drainge of pancreatic fluid from the percutaneous drain did not decrease and a spontaneous pseudocyst did not develop. This patient was lost at follow-up and endoscopic treatment was considered unsuccessful.
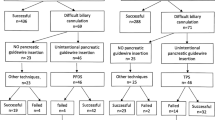
A total of 70 procedures were performed for 30 patients, including the last procedure in which the pancreatic stent was removed. The median number of endoscopic sessions was 2.0 (IQR:1.0). The median duration of pancreatic stent treatment was 41.0 days (IQR 32.5, range 11–177 days). The resolution of the PD disruption was achieved in all of 26 patients (100%) with partial disruption and 3 of 4 patients (75%) with complete disruption. Overall, therapeutic success with endoscopic treatment was achieved in 29 of 30 patients (96.6%). Flowchart of the algorithmic treatment approach according to the type of PD disruption is summarized in Fig. 4.
When we evaluate the results of our study according to the etiology of the disruption, endoscopic treatment was successful in 25 of 26 patients (96.1%) with postoperative PD disruption (95.8% for the grade B disruption and 100% for the grade C disruption) and in all 4 patients (100%) with traumatic PD disruption.
A total of 7 (23.3%) patients had procedural complications, including stent migration in 6 patients (3 proximal, 3 distal) and bleeding due to pancreatic sphincterotomy in 1 patient. Bleeding was stopped with adrenaline injection during the procedure and blood transfusion was not required. The pancreatic stent was removed in 2 of 3 patients with proximal stent migration, whereas it could not be removed in one patient with a fractured stent. The patient was asymptomatic in the follow-up. None of our patients developed procedure-related pancreatitis. Endoscopic treatment data are summarized in Table 3.
Discussion
The current study presents the 10-year experience of a single tertiary referral center about the endoscopic management strategy of postoperative and traumatic PD disruption. In our study, overall therapeutic success rate was 96.6%. In previously performed studies [1, 9], this rate was reported as 80% and 88.2% for patients with postoperative and traumatic etiology. We believe that the main cause of the high overall success rate in our study is that we use alternative methods in the endoscopic armamentarium for treatment of PD disruption with a step-up approach. Another cause of the high overall success rate may be that all procedures are performed by a single experienced endoscopist (Ödemiş B.) who performs over a thousand ERCPs annually in a single high-volume center.
Partial PD disruption unresponsive to conservative therapy is usually treated with standard endoscopic modalities, including pancreatic stent pacement with/without sphincterotomy which promote antegrade flow by decreasing the transpapillary pressure gradient. This endoscopic treatment was successful in 20 (76.9%) of 26 patients with partial duct disruption in our study. 4 out of 6 patients who failed standard endoscopic treatment had 5Fr pancreatic plastic stent placed in the first session. After the 5 Fr stent was replaced by the 7 Fr stent, the resolution of PD disruption was obtained in all 4 patients. Based on our experience and that of Rodrigues et al. [12] placement of a 7 F pancreatic stent provides higher resolution than 5 F stent, which could be explained by greater decompression of the PD. Therefore, before progressing to more aggressive endoscopic therapies in patients refractory to standard therapy, stent upsizing should be considered as the next endoscopic treatment modality if the PD diameter is suitable.
Despite the decreasing the pressure gradient between the PD and the duodenum by pancreatic sphincterotomy and stent placement, resolution of PD disruption may not achieve in all patients, even if the stent diameter is increased. NBCA injection is a relatively new treatment method with promising clinical results in sealing the leak in patients refractory to standard treatment. In the present study, the stent diameter could not be enlarged in 2 of 6 patients unresponsive to standard therapy. These two patients had leakage from the pancreatic stump and NBCA and lipiodol mixture was injected to close the fistula. Fistula closure was successful in both patients and no complications that can be attributed to NBCA injection were detected. Seewald et al. [13] reported treatment success with NBCA in 8 (66.6%) of 12 patients unsuitable for surgical therapy. In this study, no procedure-related complications were observed in any of the patients. In another study of 4 patients using cyanoacrylate for closure of pancreatic fistula, treatment was successful in all patients and there were no complications [14]. According to the results of our study and the 2 studies mentioned above, the occlusion of pancreatic fistulas by NBCA seems safe and effective. Therefore, we recommend closure of the fistula with NBCA to obviate the need for surgery in patients with stump leakage and refractory to standard treatment, if the PD is too narrow to place a larger diameter stent. In addition, in our study unlike these two studies, a balloon catheter was inflated in the distal PD immediately the downstream of the site of leak just before injection. We suggest that this approach will provide an additional benefit to protect the intact PD from damage due to NBCA.
Thus, a success rate of 76.9% with standard endoscopic treatment in patients with partial disruption in our study increased to 100% after the use of alternative endoscopic modalities including stent upsizing and NBCA injection. This finding indicates that standard treatment alone may not always be sufficient to avoid surgical treatment and additional methods may be needed.
In the management of complete PD disruption, surgical interventions including resection of the disconnected segment with distal pancreatectomy and drainage of the PD into a Roux-en-Y limb of jejunum has traditionally been considered as the main method of treatment; however, the surgical approach may result in high morbidity (14–20%) and even mortality (1–20%) [15]. Therefore, endoscopic treatment modalities including transpapiller stent placement and endoscopic transmural drainage have evolved as safe and non-surgical treatment options over the years. However, endoscopic transpapillary techniques are associated with a high failure rate and recurrences because it is technically difficult to place a stent that bridges the complete disruption. In addition, a pseudocyst must develop in the disconnected area to perform endoscopic transmural drainage. To overcome these challenges, we used two alternative endoscopic treatment modalities: the bridging method and pseudocyst-creating method. The bridging method was successful in one of our patients. Like to our study, a study by Varadarajulu et al. [16] showed that complete disruption can be bridged with a stent. In this study including 23 patients with complete PD disruption, the outcome was successful in only 6 (26%) patients whose complete disruption was bridged with a stent. In the 6 patients with successful outcome, the stent was placed either across the papilla or within the pancreatic fluid collection at the initial ERCP. On follow-up ERCP, the PD proximal to the disruption was opacified and a stent was inserted, which resulted in resolution of the disruption. Using the pseudocyst-creating method, we achieved successful resolution of complete disruption in one of our patients. A technique similar to this method of intentionally creating a pseudocyst has been described by Rana et al. [17] earlier. This study included 18 patients with external pancreatic fistulae and disconnected PD syndrome who were treated with various endoscopic techniques including endoscopic ultasonography (EUS)-guided transmural drainage. In five of these 18 patients, pancreatic fluid collection was created by clamping the percutaneous drain for 48 h and was treated by EUS-guided transmural drainage, leading to closure of external fistulae in 4 patients. Taking into account our as well as Rana et al.’s [17] experience, we suggest that pseudocyst-creating method can be applied as a salvage treatment in suitable patients who do not respond with other endoscopic treatments.
The success rate of endoscopic treatment for complete disruption in our study was 75% thanks to the use of alternative treatment options including the bridging and the pseudocyst-creating methods. In previous studies that did not use alternative treatment options or EUS-guided techniques, this rate was low and ranged from 20 to 42% [1, 8, 9, 16]. In a study by Lawrence et al. [18] conducted on 30 patients with complete disruption, EUS was included in the endoscopic treatment, but its usage was selective and it was generally performed for diagnostic purposes. The success rate of endoscopic treatment in this study was 72%. On the other hand, this rate was as high as 94% in a study of Rana et al. [17] using EUS-guided techniques in 13 of 18 patients with complete disruption. In another study involving 13 patients with post-traumatic pancreatic fluid collection (11 complete disruption), EUS-guided transmural drainage was used in all patients and the success rate of endoscopic treatment was 100% [19]. The results of the above-mentioned studies point out the importance of adding EUS-guided techniques to the endoscopic armamentarium for treatment of complete PD disruption. Furthermore, EUS-guided transmural drainage enables drainage of collections irrespective of bulge and avoidance of intervening vessels unlike conventional transmural drainage which requires visualization of luminal bulge and subsequent blind puncture [20].
As a result, we propose an endoscopic management algorithm according to whether the PD disruption is partial or complete. In partial duct disruption, standard endoscopic treatment should be attempted initially in all patients. In patients refractory to standard treatment, stent upsizing should be considered as the next endoscopic treatment modality, if the PD is wide enough to place a larger caliber stent. If stent upsizing is not possible or fails, closure of the fistula with NBCA can be tried as an effective salvage treatment in patients with stump leakage. In complete duct disruption, a pancreatic stent should be placed into the distal PD lying as close as possible to the site of disruption or into the pancreatic fluid collection at the initial ERCP. On follow-up ERCP, the upstream PD proximal to the disruption should be opacified either via the transpapillary route or via an existing percutaneous drain and then cannulated with a guidewire under fluoroscopy, and finally bridged with a stent. If the bridging method is not successful and a pseudocyst does not develop spontaneously during follow-up, intentionally creating a pseudocyst by clamping the percutaneous drain, and then performing transmural drainage may be a viable option.
When we evaluate the results of our study according to the etiology of the disruption, the success rate of endoscopic treatment was 96.1% in postoperative PD disruption (95.8% for the grade B and 100% for the grade C postoperative disruption). In the study of Reddymasu et al. [21] which evaluated the efficacy of transpapillary stent placement in 8 patients with grade B pancreatic fistula due to distal pancreatectomy, clinical success rate was 100%. Grobmyer et al. [22] reported 100% clinical success rate with pancreatic stent placement in 8 patients with refractory grade C pancreatic fistula after left-sided pancreatectomy. Based on the results of our study and these 2 studies, it can be argued that the degree of postoperative pancreatic fistula is not related to the success of endoscopic treatment. Abdominal trauma is a relatively uncommon cause of PD disruption and the integrity of the MPD is the most important determinant factor of morbidity and mortality in pancreatic injury. In our study, although three (75%) of the 4 patients with traumatic PD disruption had complete disruption, endoscopic treatment success rate was 100%. In the study of Bhasin et al. [8] evaluating the efficacy of endoscopic treatment in pancreatic injury due to abdominal trauma on 11 patients, the rate of complete duct disruption (36.3%) was lower than in our study (75%), but the success rate of endoscopic treatment remained 72.7%.
Some studies have shown that a surgically placed drain increases the risk of postoperative pancreatic fistula development [6]. In the present study, twenty-eight (93.3%) of 30 patients had a surgically placed percutaneous drain before endoscopic treatment, which outnumbers the rates of 37% and 47% reported in previous studies [9, 16]. We agree with Das et al. [9], suggesting that percutaneous drainage is not an adjunct treatment to endoscopic therapy and does not affect the recovery of PD disruption. Furthermore, we believe that percutaneous drainage may compromise the closure of the pancreatic fistula, as the flow of pancreatic juice is diverted with a larger caliber catheter to the lower pressure ex vivo environment rather than a higher pressure duodenal lumen with a smaller diameter pancreatic stent. In addition, a prospective study showed reduced secondary infection rates with early removal of the percutaneous drainage catheter [6]. Therefore, the primary function of percutaneous drainage should be to ensure the resolution of intra-abdominal fluid collections as part of conservative management before endoscopic therapy. With this regard, we recommend that the percutaneous drain be clamped first to accelerate fistula closure following endoscopic treatment and subsequent confirmation of the resolution of the intra-abdominal fluid collection with cross-sectional imaging modalities, and then removed if there are no clinical and radiological deterioration in the follow-ups.
In the present study, 7 patients (23.3%) had procedural complications. Stent migration was the most common complication and seen in 6 (20%) patients, with spontaneous internal migration of stent in 3 patients (10%). The rate of internal stent migration in previous studies was lower and ranged from 1.9 to 9% [1, 9, 23]. Although pancreatitis is the most common complication of ERCP and endoscopic intervention for the PD increases the risk of pancreatitis, procedure related pancreatitis did not develop in any patient in our study, as in other studies [1, 9]. In our opinion, the presence of a pancreatic stent already in all patients for endoscopic treatment of PD disruption and continuation of pancreatic drainage through the existing disruption may explain why procedure-associated pancreatitis does not develop.
The primary limitations of our study were its retrospective methodology and single center design. Another limitation of our study was that stent-induced ductal change were not routinely evaluated with MRCP or retrograde pancreatogram. Hovewer, we injected either minimal volume of contrast or no contrast during ERP after stent removal to prevent reopening of disruption, which prevented evaluation of the stent-induced changes. In addition, the median duration of pancreatic stent treatment was not very long (41 days) and none of our patients developed symptoms or signs compatible with chronic pancreatitis. Finally, the lack of use of EUS was an important limitation of this study, although the success rate of endoscopic treatment was high due to the use of alternative modalities with a step-up approach.
In conclusion, standart endoscopic treatment including pancreatic stent placement and sphincterotomy is safe and effective for treating of most patients with postoperative and traumatic PD disruption. In patients refractory to standard endoscopic therapy, the step-up approach using alternative endoscopic modalities may be beneficial to increase the success rate and thus avoid the need for surgery. Future studies using EUS-guided novel therapeutic options will open new horizons for best management of these patients.
References
Cicek B, Parlak E, Oguz D, Disibeyaz S, Koksal AS, Sahin B. Endoscopic treatment of pancreatic fistulas. Surg Endosc 2006;20:1706–1712.
Morgan KA, Adams DB. Management of internal and external pancreatic fistulas. Surg Clin N Am 2007;87:1503–1513.
Varadarajulu S, Rana SS, Bhasin DK. Endoscopic therapy for pancreatic duct leaks and disruptions. Gastrointest Endosc Clin N Am 2013;23:863–892.
Bassi C, Marchegiani G, Dervenis C et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584–591.
Volk A, Distler M, Müssle B et al. Reproducibility of preoperative endoscopic injection of botulinum toxin into the sphincter of Oddi to prevent postoperative pancreatic fistula. Innov Surg Sci 2018;3:69–75.
Nahm CB, Connor SJ, Samra JS, Mittal A. Postoperative pancreatic stula: A review of traditional and emerging concepts. Clin Exp Gastroenterol. 2018;11:105–118.
Larsen M, Kozarek R. Management of pancreatic ductal leaks and fistulae. J Gastroenterol Hepatol 2014;29:1360–1370.
Bhasin DK, Rana SS, Rao C et al. Endoscopic management of pancreatic injury due to abdominal trauma. JOP 2012;13:187–192.
Das R, Papachristou GI, Slivka A et al. Endotherapy is effective for pancreatic ductal disruption: A dual center experience. Pancreatology 2016;16:278–283.
Banks PA, Bollen TL, Dervenis C et al. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–111.
Cotton PB, Lehman G, Vennes J et al. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest Endosc 1991;37:383–393.
Rodrigues-Pinto E, Pereira P, Macedo G. Are 7-Fr caliber pancreatic stents more effective than 5-Fr stents in the endoscopic resolution of pancreatic fistulas? Digest Endosc. 2015;27:776–784.
Seewald S, Brand B, Groth S et al. Endoscopic sealing of pancreatic fistula by using N-butyl-2-cyanoacrylate. Gastrointest Endosc. 2004;59:463–470.
Labori KJ, Trondsen E, Buanes T, Hauge T. Endoscopic sealing of pancreatic fistulas: Four case reports and review of the literature. Scand J Gastroenterol. 2009;44:1491–1496.
Nadkarni NA, Kotwal V, Sarr MG, Swaroop Vege S. Disconnected pancreatic duct syndrome: Endoscopic stent or surgeon’s knife? Pancreas 2015;44:16–22.
Varadarajulu S, Noone TC, Tutuian R, Hawes RH, Cotton PB. Predictors of outcome in pancreatic duct disruption managed by endoscopic transpapillary stent placement. Gastrointest Endosc. 2005;61:568–575.
Rana SS, Sharma R, Gupta R. Endoscopic treatment of refractory external pancreatic fistulae with disconnected pancreatic duct syndrome. Pancreatology 2019;19:608–613.
Lawrence C, Howell DA, Stefan AM et al. Disconnected pancreatic tail syndrome: Potential for endoscopic therapy and results of long-term follow-up. Gastrointest Endosc. 2008;67:673–679.
Rana SS, Sharma R, Dhalarla L, Gupta R. Endoscopic ultrasound-guided transmural drainage of post-traumatic pancreatic fluid collections. Ann Gastroenterol. 2021;34:751–755.
Jearth V, Rana SS. Endoscopic step up: When and how. Surg Open Sci. 2022;10:135–144.
Reddymasu SC, Pakseresht K, Moloney B, Alsop B, Oropezia-Vail M, Olyaee M. Incidence of pancreatic fistula after distal pancreatectomy and efficacy of endoscopic therapy for its management: Results from a tertiary care center. Case Rep Gastroenterol. 2013;7:332–339.
Grobmyer SR, Hunt DL, Forsmark CE, Draganov PV, Behrns KE, Hochwald SN. Pancreatic stent placement is associated with resolution of refractory grade C pancreatic fistula after left-sided pancreatectomy. Am Surg. 2009;75:654–658.
Kim S, Kim JW, Jung PY et al. Diagnostic and therapeutic role of endoscopic retrograde pancreatography in the management of traumatic pancreatic duct injury patients: Single center experience for 34 years. Int J Surg. 2017;42:152–157.
Author information
Authors and Affiliations
Contributions
All authors above contributed the following aspects of the manuscript: conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important entellectual content, final approval of the article.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Bülent Ödemiş, Dr. Muhammed Bahaddin Durak, Dr. Ali Atay, Dr. Batuhan Başpınar and Dr. Çağdaş Erdoğan have no conflicts of interests to disclose, and there was no financial support associated with this study to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ödemiş, B., Durak, M.B., Atay, A. et al. A Step-Up Approach Using Alternative Endoscopic Modalities Is an Effective Strategy for Postoperative and Traumatic Pancreatic Duct Disruption. Dig Dis Sci 68, 3745–3755 (2023). https://doi.org/10.1007/s10620-023-07996-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07996-0