Abstract
Background
In Japan, laser light source (Laser) endoscopy is widely available, and the characteristics of light-emitting diode light source (LED) endoscopy have not been clarified.
Aims
We assessed the visibility of early gastric cancers (EGCs) and Helicobacter pylori (H. pylori)-associated gastritis for LED endoscopy compared with laser endoscopy using white-light imaging (WLI) and linked color imaging (LCI).
Methods
We assessed 99 lesions between February 2019 and March 2020. The visibility was scored from four (excellent visibility) to one (poor visibility) by evaluating videos including EGCs and gastric mucosa captured using WLI and LCI with LED endoscopy (LED-WLI and LED-LCI, respectively) and laser endoscopy (Laser-WLI and Laser-LCI, respectively). The primary end point was the non-inferiority of the visibility of EGCs and H. pylori-associated gastritis between LED-/Laser-WLI and LED-/Laser-LCI.
Results
The visibility scores of EGCs for LED-/Laser-WLI and LED-/Laser-LCI were 3.14/2.97 and 3.39/3.35, respectively. The visibility scores of H. pylori-associated gastritis [intestinal metaplasia (IM), diffuse redness (DR), regular arrangement of collecting venules (RAC) and map-like redness (MR)] for LED-/Laser-WLI and LED-/Laser-LCI were 3.05/2.85 and 3.60/3.50 (IM), 2.76/2.50 and 2.96/2.86 (DR), 2.69/2.44 and 2.77/2.62 (RAC) and 2.97/2.75 and 3.39/3.27 (MR). Non-inferiority was demonstrated for visualizing EGCs and H. pylori-associated gastritis.
Conclusions
LED-WLI and LED-LCI can be used to visualize EGCs and H. pylori-associated gastritis with non-inferiority to Laser-WLI and Laser-LCI. Furthermore, even with LED, LCI was more effective than WLI for evaluating EGCs and H. pylori-associated gastritis. Therefore, LED endoscopy can be used to detect EGCs and evaluate H. pylori-associated gastritis accurately.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gastric mucosal atrophy and intestinal metaplasia caused by Helicobacter pylori (H. pylori) noted on endoscopy are generally considered risk factors for gastric cancer (GC) [1,2,3]. The endoscopic findings of H. pylori-associated gastritis according to the Kyoto classification of gastritis are used to diagnose the H. pylori infection status accurately and identify risk factors of early gastric cancer (EGC) [4,5,6].
In Japan, Laser-based image-enhanced endoscopy (IEE), including blue-laser imaging (BLI), BLI-bright and linked color imaging (LCI), have proven useful for evaluating the endoscopic findings according to the Kyoto classification of gastritis [7,8,9]. Furthermore, these IEE approaches have been reported to be superior to conventional white-light imaging (WLI) for the early detection and accurate diagnosis of EGCs [10,11,12,13,14,15]. We previously reported that LCI identified map-like redness (MR), a positive risk finding, and regular arrangement of collecting venules (RAC), a negative risk finding of EGC detection after H. pylori eradication, more frequently than WLI [16]. Furthermore, LCI visualized EGCs after H. pylori eradication significantly better than WLI [15].
Since the current BLI and LCI devices use a laser as a light source, there are regions and countries where obtaining approval for use of these devices as medical endoscopes is restricted based on pharmaceutical and safety standards in those countries. Recently, the ELUXEO endoscopic system (Fujifilm Co., Tokyo, Japan) with light-emitting diodes (LEDs) was developed as a novel endoscopic system between 2016 and 2017 in the United States and European countries, where laser endoscopes have not been approved for use. This system enables the development of BLI devices with LED light sources (LED-BLI) and LCI devices with LED light sources (LED-LCI) instead of laser light sources, and these devices are now being used worldwide including in areas where laser endoscopes are not available [17]. In addition, the new system is suitable for worldwide use from an economic standpoint, as it reduces running costs by saving power consumption and extending the service life of the light source compared to the conventional xenon light source.
LED endoscopy was developed to have the same degree of visibility as conventional laser endoscopy. However, the efficacy of the observation with a LED endoscope has not been validated.
Therefore, we conducted the present study to validate the efficacy of LED endoscopy compared with laser endoscopy by evaluating the endoscopic findings of EGCs and H. pylori-associated gastritis using LED and laser endoscopy with IEE.
Methods
Patients
This was a single-center non-inferiority study conducted at Kyoto Prefectural University of Medicine between February 2019 and March 2020. Patients ≥ 20-years-old with gastric tumors who were scheduled to undergo endoscopic submucosal dissection (ESD) were considered eligible [18] for enrollment in this study. The inclusion criterion was as follows: patients with EGC diagnosed as a gastric adenocarcinoma pathologically. The exclusion criteria were as follows: patients < 20-years-old, those with non-cancerous lesions pathologically from ESD specimens, those in whom informed consent could not be obtained, those with recurrent lesions and those with a history of gastrectomy. In addition, we excluded lesions with erosion and white coat or ulcer, cases with marks (e.g., clips or India ink around the lesion) that could affect lesion recognition and cases diagnosed as gastric adenoma or gastritis pathologically. We enrolled patients prospectively to minimize the selection bias whenever possible.
The study was approved by the Ethical Review Committee of the Kyoto Prefectural University of Medicine (ERB-C-1345) and was conducted in accordance with the Declaration of HELSINKI. All patients provided their written informed consent to undergo esophagogastroduodenoscopy (EGD) using LED endoscopy and laser endoscopy with IEE at the University Hospital, Kyoto Prefectural University of Medicine.
End Points
The primary end point of this study was the non-inferiority of the endoscopic findings of EGCs and H. pylori-associated gastritis using LED endoscopy versus laser endoscopy with IEE. The secondary end point was the interobserver agreement of the endoscopic findings for EGC using LED endoscopy versus laser endoscopy.
Endoscopic System and Devices
Laser endoscopic images were obtained using an EG-L600ZW or EG-L600ZW7 endoscope and the LASEREO endoscopic system, consisting of a VP-4450HD processor and a LL 4450 light source (Fujifilm Co.). LED endoscopic images were obtained using an EG-6600R endoscope, and an endoscopic system consisting of an EP-6000 combined with three LED light source and a processor (Fujifilm Co.).
Participating Endoscopists
All endoscopic videos acquired using LED and laser endoscopes were obtained by one expert endoscopist (O.D.) who had diagnosed > 500 cases using LED and laser endoscopes. The evaluation was performed retrospectively by 5 endoscopists (T.Y., T.Y., Y.A., H.K. and S.M.) who had experienced LCI observation for more than 50 patients in our institution.
Video Recording
Videos obtained with laser endoscopy were first recorded for the lesion using WLI following the use of LCI. On different days, videos with LED endoscopy were recorded for the same lesion using WLI following the use of LCI. The period between the two examinations was less than one month. No biopsy specimens were collected from the lesions during the period between the two examinations. Non-magnifying high-definition videos obtained using LED, and laser endoscopes with WLI and LCI were recorded in 99 gastric lesions with background gastric mucosa. After washing the gastric mucosa to remove mucus, an endoscopist captured a video of the entire gastric mucosa for one minute and tumors for about 5 s. We took special care to keep the shooting speed as constant as possible. All endoscopic images had key information removed (i.e., examination date, endoscopic number and endoscopic system) to reduce the observer bias.
Definition of Endoscopic Findings
In this study, the definitions of endoscopic findings were as follows [7]: diffuse redness (DR), a uniform redness on the non-atrophic mucosa of the fundic gland in the corpus; intestinal metaplasia (IM), multiple ashen nodular or cobblestone-like lesions observed typically on the atrophic mucosa; MR, an erythematous lesion with a shallow depression and distinct boundaries from background mucosa, which had a variety of sizes and color tones; and RAC, a collecting venule was observed as a fine vessel with a starfish-like pattern using non-magnifying endoscope. If an orderly arrangement of collecting venules was observed in the remaining fundic gland area, RAC was determined to be positive.
Evaluation of the Endoscopic Findings
All sets of videos were numbered randomly, and key information (i.e., examination date and type of endoscope) was removed. Videos obtained using LED and laser endoscopes with WLI or LCI were displayed on a monitor to assess the visibility of EGCs, IM, DR, MR and RAC according to the Kyoto classification of gastritis. The presentation of each video was randomized to minimize the effect, as each video with WLI or LCI may improve the observer's visibility of the other. All endoscopic findings were evaluated for visibility using the following scores: (1) excellent visibility (easy detectable or recognizable); (2) good visibility (detectable or recognizable with careful observation); (3) fair visibility (detectable or recognizable with repeated careful examination; (4) poor visibility (not detectable or not recognizable with repeated careful examination) as previously reported [19,20,21,22]. Regarding the EGC detection, if the wrong lesion was recognized, the visibility score was rated as 1. Regarding DR, IM, MR and RAC, the visibility score could not be evaluated for cases with negative findings; cases in which all endoscopists assigned scores of 1 were considered as negative findings and were excluded from the calculation. Each endoscopist scored the visibility of each lesion, and the mean and standard error of all scores for WLI and LCI were calculated and compared between LED endoscopy and laser endoscopy.
We also examined the interobserver agreement in relation to the evaluation of the images obtained with WLI and LCI among endoscopists.
Diagnostic Criteria of H. pylori Infection Status
H. pylori-positive patients were defined as follows: (1) presence of positive results for at least one of the following reliable clinical tests: a serum antibody test (E-plate; Eiken, Tokyo, Japan), a C-urea breath test (UBit; Tokyo, Japan), or stool antigen test. H. pylori-uninfected patients were defined as follows: (1) no medical history of H. pylori eradication therapy; (2) lack of endoscopic atrophy; (3) absence of positive results at least in one of the former reliable clinical tests. Successful H. pylori eradication was confirmed by at least one of the following tests: rapid urease test (PyloritTek; Serim Research Corp., Elkhart, IN, USA), UBit, stool antigen test, a histopathological evaluation and cultivation with a personal history of H. pylori eradication therapy. All patients who were performed H. pylori eradication therapy underwent endoscopy at least 1 year after successful H. pylori eradication.
Pathological Diagnosis
All lesions were resected via ESD, fixed with 10% formalin and evaluated pathologically. The pathological diagnoses were made by highly experienced clinical pathologists who were unaware of the endoscopic findings. The pathological diagnosis was categorized according to the Japanese Classification of Gastric Carcinoma proposed by the Japanese Gastric Cancer Association.
Calculation of the Sample Size
We evaluated the visibility of videos obtained via LED endoscopy with IEE compared with those obtained via laser endoscopy. We designed this controlled study as a non-inferiority test. In a pilot study, Laser-WLI and Laser-LCI achieved visibility scores of 3.14 and 3.33, respectively, with a standard deviation (SD) of 1.0 in 30 lesions. We hypothesized that the visibility scores of WLI and LCI using LEDs would be similar to those of laser endoscopy, and the visibility score of LED-LCI would be better than that of LED-WLI. Therefore, a non-inferiority margin of − 0.2 was chosen at the outset of this trial. The study required at least 82 lesions for a threshold of non-inferiority and a statistical power of 80% with statistical significance at p < 0.05.
Statistical Analyses
Regarding the analysis of the primary end point, the visibility with a LED endoscope was defined as non-inferior to that with a laser endoscope if the lower limit of the 95% confidence interval (CI) for the estimated difference in the primary outcome was higher than − 0.2 points. Data are expressed as mean ± standard error (SE). A two-tailed paired t-test was used to compare endoscopic visibilities with LED and laser endoscopy. For all analyses, a p-value of < 0.05 was considered statistically significant. Interobserver agreement was calculated using Gwet's Agreement Coefficient (AC1) [23], and associated 95% CIs were used to compare the ratings made by 5 endoscopists.
All statistical analyses were performed using the SPSS software program, Version 25.0 (IBM Japan, Ltd., Tokyo, Japan) and R statistical software 3.6.2 (R core team).
Results
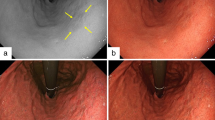
A total of 92 patients with 104 gastric lesions were prospectively enrolled in this study. All patients who participated in this study underwent endoscopy and ESD at the University Hospital of Kyoto Prefectural University of Medicine. Four patients and five lesions were excluded according to the criteria. A total of 88 patients with 99 gastric lesions histologically diagnosed as EGCs were included in the analysis that are shown in Fig. 1. Typical endoscopic images of EGC and each finding of the Kyoto classification of gastritis using Laser and LED endoscope with WLI and LCI are shown in Fig. 2. The clinicopathological features are summarized in Table 1. There were 67 men and 21 women, with a mean age of 72.1-years-old. According to the histological type, there were 92 differentiated and 7 undifferentiated adenocarcinomas. Mean video times of LED-WLI, LED-LCI, Laser-WLI and Laser-LCI for the entire gastric mucosa were 61.0, 56.0, 56.0 and 54.2 s for the entire gastric mucosa and 6.3, 6.4, 6.5 and 6.0 s for tumors.
Typical endoscopic images of early gastric cancer and each finding of the Kyoto classification of gastritis using a laser light source (Laser) and light-emitting diodes (LEDs) with white-light imaging (WLI) (left) and linked color imaging (LCI) (right). a Early gastric cancer (EGC) with Laser (arrow heads), b EGC with LED (arrow heads), c intestinal metaplasia (IM) with Laser, d IM with LED, e diffuse redness (DR) with Laser, f DR with LED, g map-like redness (MR) with Laser, h MR with LED, i regular arrangement of collecting venules (RAC) with Laser, j RAC with LED
EGC Visibility
An overview of the endoscopic visibility scores for the 99 EGCs observed using LED or laser endoscopy is shown in Fig. 3a. The visibility scores of EGCs evaluated by Laser-WLI, LED-WLI, Laser-LCI and LED-LCI were 2.97 ± 0.05, 3.14 ± 0.05, 3.35 ± 0.05 and 3.39 ± 0.04, respectively; the difference between Laser- and LED-WLI was 0.17 (p = 0.01; 95% CI 0.04–0.30), and the difference between Laser- and LED-LCI was 0.04 (p = 0.47; 95% CI − 0.07 to 0.16).
Visibility of H. pylori-associated findings
An overview of the endoscopic visibility scores for H. pylori-associated findings for the 87 patients observed using Laser or LED endoscopy is shown in Fig. 3b–e.
The visibility scores of IM on Laser-WLI, LED-WLI, Laser-LCI and LED-LCI, which was associated with the history of H. pylori infection, were 2.85 ± 0.04, 3.05 ± 0.04, 3.50 ± 0.04 and 3.60 ± 0.03, respectively; the difference between Laser- and LED-WLI was 0.20 (p = 0.0005; 95% CI 0.09–0.32), and the difference between Laser- and LED-LCI was 0.10 (p = 0.04; 95% CI 0.004–0.20). The visibility scores of DR on Laser-WLI, LED-WLI, Laser-LCI and LED-LCI, which was observed frequently in H. pylori-positive patients, were 2.50 ± 0.08, 2.76 ± 0.09, 2.86 ± 0.10 and 2.96 ± 0.10; respectively; the difference between Laser- and LED-WLI was 0.26 (p = 0.03; 95% CI 0.02–0.49), and the difference between Laser- and LED-LCI was 0.10 (p = 0.47; 95% CI − 0.17 to 0.37). The visibility scores of MR, on Laser-WLI, LED-WLI, Laser-LCI and LED-LCI, which was specifically emerged after successful H. pylori eradication, were 2.75 ± 0.06, 2.97 ± 0.06, 3.27 ± 0.06 and 3.39 ± 0.05, respectively; the difference between Laser- and LED-WLI was 0.23 (p = 0.005; 95% CI 0.07–0.39), and the difference between Laser- and LED-LCI was 0.12 (p = 0.12; 95% CI − 0.03 to 0.27). The visibility scores of RAC on Laser-WLI, LED-WLI, Laser-LCI and LED-LCI, which were identified as a characteristics of a normal stomach without H. pylori, were 2.44 ± 0.08, 2.69 ± 0.08, 2.62 ± 0.08 and 2.77 ± 0.09, respectively; the difference between Laser- and LED-WLI was 0.25 (p = 0.03; 95% CI 0.03–0.47), and the difference between Laser- and LED-LCI was 0.15 (p = 0.20; 95% CI − 0.08 to 0.38).
Primary End Point
Because the lower limit of the 95% CI was higher than the non-inferiority margin of − 0.2 in EGCs and H. pylori-associated findings, LED endoscopy was deemed not inferior to laser endoscopy for detecting EGCs, evaluating findings associated with H. pylori and assessing H. pylori infection status. Furthermore, the visibility scores of LED-LCI were significantly higher than those of LED-WLI in EGCs (p < 0.001), IM (p < 0.001), and MR (p < 0.001).
Difference in Visibility Scores Between LED and Laser Endoscopy
To examine in which tumors visibility improvement was obtained, we investigated the case specific difference in the mean visibility score for each EGC. The visibility score variations in each case using LED endoscopy compared with that of laser endoscopy with WLI and LCI are shown in Fig. 4. The evaluation of the images showed that an improved visibility was observed in 17% of cases, equivalent visibility was observed in 82% of cases, and worsened visibility was observed in 1% of cases in LED-WLI compared with Laser-WLI. On LED-LCI, an improved visibility was observed in 4% of cases, equivalent visibility was observed in 95% of cases, and worsened visibility was observed in 1% of cases compared with Laser-LCI. The results of the case specific investigation showed that the mean visibility scores of LED endoscopy were the similar to or improved over those with laser endoscopy in more than 95% of both WLI and LCI cases, regardless of lesion characteristics.
Secondary End Point
We evaluated the interobserver agreements by calculating the visibility scores of LED-/Laser-WLI and LED-/Laser-LCI. The interobserver agreements for evaluating EGCs with LED-WLI, Laser-WLI, LED-LCI and Laser-LCI were 0.73 (95% CI 0.62–0.84), 0.68 (95% CI 0.56–0.96), 0.80 (95% CI 0.71–0.89) and 0.80 (95% CI 0.71–0.89), respectively. LED-/Laser-WLI showed a substantial interobserver agreement, while LED/Laser-LCI showed an almost perfect interobserver agreement (Table 2).
Discussion
This comparative study was conducted to compare LED and laser endoscopy procedures in terms of the visibility of EGCs and H. pylori-associated findings. In this study, the visibility of LED-WLI and LED-LCI was shown to be similar to that of Laser-WLI and Laser-LCI for EGCs and findings associated with H. pylori according to the Kyoto classification of gastritis. Furthermore, the visibility scores of LED-LCI were significantly higher than those of LED-WLI in EGCs, IM, and MR. Even with LED, LCI for detecting EGCs and evaluating H. pylori-associated gastritis was more effective than WLI. In addition, the interobserver agreement between endoscopists was substantial with LED-/Laser-WLI and almost perfect with LED-/Laser-LCI. These results suggest that endoscopy with LED-WLI and LED-LCI has the potential to be a more valid alternative than conventional Laser-WLI and Laser-LCI for the detection of EGCs and H. pylori-associated findings and for the diagnosis of the H. pylori infection status.
Because LED endoscopy is a new endoscopic system, few studies have reported its efficacy for tumor characterization [24]. We previously reported the efficacy of LED-BLI for upper gastrointestinal tumors [25], however, its efficacy for assessing LED-WLI and LED-LCI for EGCs and H. pylori-associated findings has not yet been described. Therefore, it is important to evaluate the diagnostic performance of LED endoscopy for EGCs and H. pylori-associated findings.
In this study, the diagnostic ability of LED and laser endoscopy was almost the same in LCI surveillance. Laser-LCI has high intensity of short wavelengths, resulting in high color contrast between lesion and surrounding areas. LED-LCI showed similar diagnostic characteristics because LED-LCI had higher intensity of 400-nm wavelengths than 450-nm wavelengths, similar to Laser-LCI [25]. However, the visibility scores using LED-WLI were higher than those using Laser-WLI. The scope we used in this study was a fixed-focus optical system for LED endoscopy (EG-6600WR) and a magnifying optical system for laser endoscopy (EG-L600ZW and EG-L600ZW7). Because the lens of the fixed-focus optical system is brighter than that used in the magnifying optical system, the videos obtained by LED endoscopy may have been brighter than those obtained by laser endoscopy; the differing brightness between the two kinds of lenses may have led to the observed differences in visibility. Although we demonstrated the high visibility of EGCs and H. pylori-associated findings on LED endoscopy, further studies will be required to confirm the utility of LED endoscopy for detecting EGCs and H. pylori-associated gastritis.
Several limitations associated with the present study warrant mention. First, this study was performed in a single academic center in Japan. Second, this study involved a review of endoscopic videos and thus did not reflect real-time observation during surveillance endoscopy. Third, this was not a blind study. we cannot exclude observer bias completely, however, we made it as difficult as possible to identify which modality was used. Fourth, we excluded the cases diagnosed as gastric adenoma or gastritis pathologically because we enrolled prospectively the cases diagnosed as adenocarcinoma endoscopically or pathologically. Fifth, we did not confirm the findings of gastritis pathologically, there was a possibility of occult cancers.
In conclusion, LED-WLI and LED-LCI were able to visualize EGCs and H. pylori-associated findings to a non-inferior degree compared with Laser-WLI and Laser-LCI. Moreover, LED-LCI was more effective than LED-WLI for evaluating EGCs and H. pylori-associated gastritis. Therefore, LED endoscopy can be used to evaluate EGCs and H. pylori-associated findings more accurately than laser endoscopy.
References
Uemura N, Okamoto S, Yamamoto S et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789.
Masuyama H, Yoshitake N, Sasai T et al. Relationship between the degree of endoscopic atrophy of the gastric mucosa and carcinogenic risk. Digestion. 2015;91:30–36.
Spence AD, Cardwell CR, McMenamin Ú et al. Adenocarcinoma risk in gastric atrophy and intestinal metaplasia: a systematic review. BMC Gastroenterol. 2017;17:157.
Sugano K, Tack J, Kuipers EJ et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367.
Sugimoto M, Ban H, Ichikawa H et al. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern Med. 2017;56:579–586.
Yoshii S, Mabe K, Watano K et al. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig Endosc. 2020;32:74–83.
Dohi O, Majima A, Naito Y et al. Can image-enhanced endoscopy improve the diagnosis of Kyoto classification of gastritis in the clinical setting? Dig Endosc. 2020;32:191–203.
Ono S, Dohi O, Yagi N et al. Accuracies of Endoscopic Diagnosis of Helicobacter pylori-Gastritis: Multicenter Prospective Study Using White Light Imaging and Linked Color Imaging. Digestion. 2020;101:624–630.
Takeda T, Asaoka D, Nojiri S et al. Linked Color Imaging and the Kyoto Classification of Gastritis: Evaluation of Visibility and Inter-Rater Reliability. Digestion. 2020;101:598–607.
Kaneko K, Oono Y, Yano T et al. Effect of novel bright image enhanced endoscopy using blue laser imaging (BLI). Endosc Int Open. 2014;2:E212–E219.
Fukuda H, Miura Y, Hayashi Y et al. Linked color imaging technology facilitates early detection of flat gastric cancers. Clin J Gastroenterol. 2015;8:385–389.
Yoshifuku Y, Sanomura Y, Oka S et al. Evaluation of the visibility of early gastric cancer using linked color imaging and blue laser imaging. BMC Gastroenterol. 2017;17:150.
Dohi O, Yagi N, Naito Y et al. Blue laser imaging-bright improves the real-time detection rate of early gastric cancer: a randomized controlled study. Gastrointest Endosc. 2010;89:47–57.
Gao J, Zhang X, Meng Q et al. Linked color imaging can improve detection rate of early gastric cancer in a high-risk population: a multi-center randomized controlled clinical trial. Dig Dis Sci. 2021;66:1212–1219.
Ono S, Kawada K, Dohi O et al. Linked Color Imaging Focused on Neoplasm Detection in the Upper Gastrointestinal Tract: A Randomized Trial. Ann Intern Med. 2021;174:18–24. https://doi.org/10.7326/M19-2561 (Epub 2020 Oct 20).
Majima A, Dohi O, Takayama S et al. Linked color imaging identifies important risk factors associated with gastric cancer after successful eradication of Helicobacter pylori. Gastrointest Endosc. 2019;90:763–769.
Yoshida N, Dohi O, Inoue K et al. Blue Laser Imaging, Blue Light Imaging, and Linked Color Imaging for the Detection and Characterization of Colorectal Tumors. Gut Liver. 2019;13:140–148.
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19.
Yoshida N, Hisabe T, Hirose R et al. Improvement in the visibility of colorectal polyps by using blue laser imaging (with video). Gastrointest Endosc. 2015;82:542–549.
Suzuki T, Hara T, Kitagawa Y et al. Linked-color imaging improves endoscopic visibility of colorectal nongranular flat lesions. Gastrointest Endosc. 2017;86:692–697.
Kitagawa Y, Suzuki T, Hara T et al. Linked color imaging improves the endoscopic visibility of gastric mucosal cancers. Endosc Int Open. 2019;7:E164–E170.
Kitagawa Y, Suzuki T, Nankinzan R et al. Comparison of endoscopic visibility and miss rate for early gastric cancers after Helicobacter pylori eradication with white-light imaging versus linked color imaging. Dig Endosc. 2020;32:769–777. https://doi.org/10.1111/den.13585 (Epub 2019 Dec 26).
Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61:29–48.
Yoshida N, Naito Y, Yasuda R et al. Linked color imaging improves the visibility of various featured colorectal polyps in an endoscopist’s visibility and color difference value. Int J Colorectal Dis. 2017;32:1253–1260.
Takayama S, Dohi O, Naito Y et al. Diagnostic ability of magnifying blue light imaging with a light emitting diode light source for early gastric cancer: a prospective comparative study. Digestion. 2021;102:580–589.
Acknowledgments
We thank all members of the Department of Molecular Gastroenterology and Hepatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, for helping us perform this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Osamu Dohi received a research grant from Fujifilm Co., Ltd. (J192001048 and J192001259). Naohisa Yoshida received a research grant from Fujifilm Co., Ltd. (J162001222). The other authors have no conflicts of interest to declare. Fujifilm Co., Ltd., had no role in the design, conduct, data collection, data interpretation or reporting of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-021-07235-4.
Rights and permissions
About this article
Cite this article
Ishida, T., Dohi, O., Yoshida, N. et al. Enhanced Visibility in Evaluating Gastric Cancer and Helicobacter pylori-Associated Gastritis Using Linked Color Imaging with a Light-Emitting Diode Light Source. Dig Dis Sci 67, 2367–2374 (2022). https://doi.org/10.1007/s10620-021-07234-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07234-5