Abstract
Background
Notoginsenoside R1 (NG-R1) is the predominant active ingredient and a novel triterpene saponin compound extracted from the roots of Panax notoginseng. To date, to the best of our knowledge, there are no previous studies concerning the effect of NG-R1 on hepatocellular carcinoma (HCC).
Aims
To investigate the effects of NG-R1 on HCC cell growth, apoptosis, and invasion and to explore the underlying mechanisms.
Methods
Cell viability and lactate dehydrogenase (LDH) release were evaluated by cell counting kit-8 and LDH assay, respectively. Apoptosis was assessed using flow cytometry analysis and caspase-3/7 activity assay. Cell invasion was detected by Transwell invasion assay and western blot analysis of matrix metallopeptidase (MMP)-2 and MMP-9. The effects of NG-R1 on miR-21 expression and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway were examined by qRT-PCR and western blot, respectively.
Results
NG-R1 inhibited the viability, increased LDH release and caspase-3/7 activity, induced apoptosis, and suppressed invasion in HCC cells. NG-R1 reduced miR-21 expression in HCC cells. miR-21 overexpression significantly attenuated the effects of NG-R1 on the viability, LDH release, apoptosis, caspase-3/7 activity, and invasion of HCC cells. We further demonstrated that NG-R1 inhibited the activation of the PI3K/Akt pathway in HCC cells, which was abolished by miR-21 overexpression.
Conclusions
NG-R1 exerted anti-hepatoma activity through inactivation of the PI3K/Akt pathway by downregulating miR-21, contributing to further understanding of the anti-tumor activities of NG-R1 in HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most commonly occurring malignancies with high morbidity and mortality, ranking as the third commonest cause of digestive system cancer-associated deaths globally [1]. Despite considerable advances in the early diagnosis and treatments of HCC, the prognosis of advanced HCC patients still remains far from satisfactory due to lack of available treatments, high recurrence and distant metastasis, with a 5-year survival rate for HCC of only about 18% [2, 3]. Many risk factors including alcoholism, consumption of aflatoxins, hepatitis B/C virus infection, liver cirrhosis, and environmental pollution are well documented to contribute to the pathogenesis of HCC [4, 5]. Chemotherapy is one of the major conventional treatments for HCC, but the clinical therapeutic outcomes are still not satisfactory because of strong side effects and emergence of drug resistance [6]. In this regard, it is of great necessity to develop innovative and effective therapeutic agents with limited side effects for HCC.
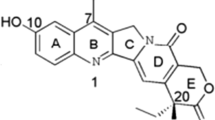
Panax notoginseng is a well-known traditional Chinese medicinal herb which has been widely used for over thousands of years in China for the prevention and treatment of cardiovascular and cerebral vascular diseases [7]. Notoginsenoside R1 (NG-R1; Fig. 1) is the predominant active ingredient and a novel triterpene saponin compound extracted from the roots of Panax notoginseng. A wide range of documents have shown that NG-R1 possesses multiple pharmacological activities, including neuroprotective, anti-oxidative, anti-inflammatory, anti-proliferative, and pro-apoptotic properties [8, 9]. Moreover, it was previously reported that NG-R1 exerted anticancer effects on colorectal cancer [10, 11]. To date, to the best of our knowledge, there are no previous studies concerning the effect of NG-R1 on HCC.
MicroRNAs (miRNAs) are a group of evolutionary conserved, small, endogenous noncoding RNAs with approximately 18–25 nucleotides in length, which suppress the mRNA translation of related genes or promote their mRNA degradation at the posttranscriptional level [12]. Modern pharmacological studies have demonstrated that deregulated miRNAs are highly correlated with the carcinogenesis and progression of various types of cancer, exerting cancer-promoting or tumor-suppressive effects, suggesting the potential of miRNAs to be diagnostic and prognostic biomarkers in tumors [13]. Specifically, miR-21, a well-characterized miRNA located at the chromosome of 17q23.2, has been documented to be overexpressed in several human malignancies including HCC [14]. The upregulated miR-21 is therefore considered as a potential prognostic biomarker and therapeutic target in HCC [15]. miR-21 overexpression promotes invasion and migration [16], while miR-21 downregulation induces apoptosis and inhibits proliferation in HCC cells [17]. Interestingly, a previous report demonstrated that NG-R1 exerted the anti-atherosclerotic effects on atherosclerosis in part through downregulation of miR-21 [18]. Therefore, we supposed whether NG-R1 could exert its effects via regulating miR-21 expression in HCC.
In the present study, we demonstrated that NG-R1 exerted anti-hepatoma activity by inhibiting cell growth and invasion and promoting apoptosis in HCC cells, which was mediated through inactivation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway via downregulation of miR-21.
Materials and Methods
Cell Culture and Treatments
Human normal liver cell line HL-7702 and human HCC cell lines MHCC97H, BEL7402, Huh7, HepG2, PLC/PRF/5 (PLC), and SMMC7721 were purchased from American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (Gibco, Rockville, MD, USA) containing 10% fatal bovine serum (HyClone, Logan, UT, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin (Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C in a humidified atmosphere of 95% and 5% CO2. miR-21 mimics (miR-21) and its scrambled control (miR-ctrl) were obtained from RiboBio Co., Ltd. (Guangzhou, China). In some experiments, MHCC97H and BEL7402 cells were treated with a series of concentrations of NG-R1 (0, 5, 10, 20, 40, 80, and 160 μM) (purity > 98%, Shanghai Yuanye Biotechnology, Shanghai, China) for 48 h, or transfected with miR-21 or miR-ctrl, followed by exposure to 40 μM NG-R1 for 48 h.
Cell Viability Assay
Cell viability was evaluated by cell counting kit-8 (CCK-8) assay. Briefly, MHCC97H and BEL7402 cells were seeded into 96-well plates at 2 × 103 cells/well and treated as mentioned above. After 48 h, 10 μL of CCK-8 reagent (Beyotime, Beijing, China) was added to each well and incubated for 1 h. The optical density at a wavelength of 450 nm was recorded using an Elx800 microplate Reader (Bio-Tek Instruments Inc., Winooski, VT, USA). The 50% growth inhibition concentration (GI50) was calculated using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA).
Lactate Dehydrogenase (LDH) Release Assay
After indicated treatments, the supernatants of MHCC97H and BEL7402 cells were collected for the measurement of LDH release by a commercial LDH Activity Assay Kit (Sigma, St. Louis, MO, USA). The absorbance at 490 nm was read using a microplate reader (Bio-Tek Instruments Inc.).
Flow Cytometry Analysis
Cell apoptosis was detected by an Annexin V-fluorescein isothiocyanate (FITC) Cell Apoptosis Detection kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. In brief, MHCC97H and BEL7402 cells were collected by centrifugation after treatment and washed twice with ice-cold PBS. Subsequently, these cells were resuspended in 100 µL of 1 × binding buffer containing 10 µL Annexin V-FITC and 5 µL propidium iodide (PI) and incubated for 15 min in the dark at room temperature. Finally, the proportion of apoptotic cells was determined by a FACS Calibur flow cytometer (BD Biosciences).
Caspase-3/7 Activity Assay
Caspase-3/7 activity was detected using a commercially available Caspase-Glo 3/7 assay kit (Promega, Madison, WI, USA). The treated MHCC97H and BEL7402 cells were cultured into 96-well plates, and then 100 μL of Caspase-Glo 3/7 reagent was added into each well. After incubated for 2 h, the luminescence was measured with the FLUOstar OPTIMA plate reader (BMG Labtech, Germany).
Transwell Invasion Assay
Cell invasive ability was evaluated using the 24-well Transwell plate (Corning Incorporated, Corning, NY, USA) pre-coated with Matrigel (BD Biosciences). Following treatments, a total of 2 × 105 MHCC97H and BEL7402 cells resuspended in 200-µL serum-free culture medium were placed on the upper chamber, while 500 µL of culture medium containing 20% FBS was inoculated to the lower chamber as a chemoattractant. After culturing for 48 h, the non-invaded cells were wiped off with a cotton swab and invaded cells in the lower chamber were fixed with 4% paraformaldehyde and then stained with 0.1% crystal violet at room temperature. Finally, the invaded cells were photographed and counted in five randomly fields using an optical microscope (Olympus Corporation, Tokyo, Japan).
Quantitative Real-Time PCR (qRT-PCR)
A miRNeasy kit (Qiagen GmbH, Hilden, Germany) was used to extract miRNA from treated MHCC97H and BEL7402 cells. cDNA was synthesized from total RNA using the RevertAid™ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). miR-21 expression was detected using a Taqman Universal Master Mix II (Applied Biosystems, Foster City, CA, USA) on a Step one plus system (Roche Molecular Diagnostics, Pleasanton, CA, USA). The relative gene expression was calculated using 2−ΔΔCT method and normalized to U6 small nuclear RNA (snRNA).
Western Blot Analysis
Total protein from treated MHCC97H and BEL7402 cells was extracted using a ProteoPrep® Total Extraction Sample kit (Sigma), and protein concentration was measured by a BCA Protein Assay Kit (Thermo Fisher Scientific). Protein samples (20 μg/lane) were separated on 10% SDS-PAGE, followed by transfer to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). After being blocked with 5% non-fat dry milk at room temperature for 1 h, the membranes were incubated overnight at 4 °C with primary antibodies including matrix metallopeptidase (MMP)-2, MMP-9, phosphorylated PI3K (p-PI3K), PI3K, phosphorylated Akt (p-Akt), Akt and β-actin (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA). Then, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature. Protein bands were visualized by an Enhanced Chemiluminescence Detection System (Amersham, Little Chalfont, Buckinghamshire, England) and analyzed using the Image J software (NIH, Bethesda, MD, USA).
Statistical Analysis
Results are shown as mean ± standard deviation (SD). All statistical analyses were carried out using SPSS 19.0 (SPSS IBM, Armonk, NY, USA). The statistical significance was analyzed by one-way analysis of variance (ANOVA). P < 0.05 was considered to be statistically significant. Each experiment was performed at least three times.
Results
NG-R1 Inhibited the Viability and Increased LDH Release in HCC Cells
Firstly, the expression of miR-21 in human normal liver cell line HL-7702 and human HCC cell lines. As shown in Fig. 2a, miR-21 was highly expressed in human HCC cell lines SMMC7721, Huh7, PLC, HepG2, BEL7402, and MHCC97H compared with that in human normal liver cell line HL-7702. MHCC97H and BEL7402 cell lines were selected for further investigation since the miR-21 expression level was higher in the two cell lines than that in the other HCC cell lines. To determine the cytotoxicity of NG-R1 on HCC cells, MHCC97H, and BEL7402 cells were suffered from different concentrations of NG-R1 for 48 h, and cell viability and LDH release were evaluated by CCK-8 and LDH assay, respectively. CCK-8 assay showed that cell viability was impeded following exposure to NG-R1 at 20, 40, 80, and 160 μM in a dose-dependent manner in MHCC97H (Fig. 2b) and BEL7402 (Fig. 2c) cells. The GI50 values for NG-R1 were 88.7 and 63.0 μM in MHCC97H and BEL7402 cells, respectively. Moreover, LDH assay revealed that NG-R1 at 20, 40, 80, and 160 μM concentration-dependently enhanced LDH release in MHCC97H (Fig. 2d) and BEL7402 (Fig. 2e) cells. Therefore, we concluded that NG-R1 inhibited the viability and increased LDH release in HCC cells.
Effects of NG-R1 on cell viability and LDH release in HCC cells. a The expression of miR-21 was determined by qRT-PCR in human normal liver cell line HL-7702 and human HCC cell lines SMMC7721, Huh7, PLC, HepG2, BEL7402, and MHCC97H. MHCC97H and BEL7402 cells were exposed to various doses of NG-R1 (0, 5, 10, 20, 40, 80, and 160 μM) for 48 h, followed by assessment of cell viability (b, c) and LDH release (d, e) by CCK-8 and LDH assays, respectively. *P < 0.05 compared with 0 μM NG-R1. Statistical analysis was conducted using one-way ANOVA with Dunnett’s posttest
NG-R1 Promoted Apoptosis of HCC Cells
To investigate the effect of NG-R1 on apoptosis in HCC cells, flow cytometry analysis and caspase-3/7 activity assay were performed. As shown in Fig. 3a and b, NG-R1 treatment induced apoptosis in MHCC97H and BEL7402 cells. Meanwhile, caspase-3/7 activity was increased in MHCC97H (Fig. 3c) and BEL7402 (Fig. 3d) cells in response to NG-R1. These results suggested that NG-R1 promoted apoptosis of HCC cells.
Effect of NG-R1 on apoptosis of HCC cells. MHCC97H and BEL7402 cells were administrated with 0, 20, and 40 μM NG-R1 for 48 h, and then cell apoptosis (a, b) and caspase-3/7 activity (c, d) were evaluated by flow cytometry analysis and caspase-3/7 activity assay, respectively. *P < 0.05 compared with 0 μM NG-R1. Statistical analysis was conducted using one-way ANOVA with Dunnett’s posttest
NG-R1 Suppressed Invasion of HCC Cells
We further explored the effect of NG-R1 on cell invasion in HCC cells by Transwell invasion assay. The results implicated that cell invasive ability was impeded after treatment with NG-R1 in MHCC97H (Fig. 4a) and BEL7402 (Fig. 4b) cells. MMP-2 and MMP-9 are well known to play crucial roles in cancer cell metastasis and invasion [19]. The influence of NG-R1 on the expression of MMP-2 and MMP-9 was detected by western blot, and the results presented that 20 and 40 μM NG-R1 both effectively restrained the protein levels of MMP-2 and MMP-9 in MHCC97H (Fig. 4c) and BEL7402 (Fig. 4d) cells. These results suggested that NG-R1 suppressed invasion of HCC cells.
Effect of NG-R1 on cell invasion in HCC cells. MHCC97H and BEL7402 cells were exposed to 0, 20, and 40 μM NG-R1 for 48 h. Transwell invasion assay was performed to detect cell invasion in the treated MHCC97H (a) and BEL7402 (b) cells. Western blot was applied to determine the protein levels of MMP-2 and MMP-9 in the treated MHCC97H (c) and BEL7402 (d) cells. *P < 0.05 compared with 0 μM NG-R1. Statistical analysis was conducted using one-way ANOVA with Dunnett’s posttest
NG-R1 Downregulated miR-21 Expression in HCC Cells
To explore whether miR-21 was involved in the regulation of NG-R1, we determined the expression of miR-21 in HCC cells following NG-R1 treatment. qRT-PCR analysis revealed that miR-21 expression was dose-dependently inhibited in response to NG-R1 in MHCC97H (Fig. 5a) and BEL7402 (Fig. 5b) cells, suggesting that NG-R1 downregulated miR-21 expression in HCC cells.
Effect of NG-R1 on miR-21 expression in HCC cells. a, b MHCC97H and BEL7402 cells were exposed to 0, 20, 40, and 80 μM NG-R1 for 48 h, followed by qRT-PCR analysis of miR-21 expression. *P < 0.05 compared with 0 μM NG-R1. Statistical analysis was conducted using one-way ANOVA with Dunnett’s posttest
miR-21 Overexpression Attenuated the Effects of NG-R1 on the Viability and LDH Release of HCC Cells
To explore whether miR-21 could affect the effects of NG-R1 on HCC cells, MHCC97H and BEL7402 cells were transfected with miR-21 or miR-ctrl, followed by treatment with 40 μM NG-R1 for 48 h. CCK-8 and LDH assays showed that delivery of miR-21 reversed NG-R1-induced inhibition of cell viability (Fig. 6a and b) and increase in LDH release (Fig. 6c and d) in MHCC97H and BEL7402 cells.
Effects of NG-R1 or together with miR-21 on the viability and LDH release in HCC cells. MHCC97H and BEL7402 cells were transfected with miR-21 or miR-ctrl, followed by treatment with 40 μM NG-R1 for 48 h. a, b Cell viability in the treated MHCC97H and BEL7402 cells was evaluated by CCK-8 assay. c, d LDH release in the treated MHCC97H and BEL7402 cells was measured by LDH assay. *P < 0.05 compared with miR-ctrl. #P < 0.05 compared with miR-ctrl + NG-R1. Statistical analysis was conducted using one-way ANOVA with Tukey’s posttest
miR-21 Overexpression Attenuated the Effects of NG-R1 on Apoptosis of HCC Cells
Flow cytometry analysis and caspase-3/7 activity assay manifested that NG-R1-induced increase of apoptotic rate (Fig. 7a and b) and caspase-3/7 activity (Fig. 7c and d) in MHCC97H and BEL7402 cells were attenuated by transfection with miR-21, suggesting that miR-21 overexpression attenuated the effects of NG-R1 on apoptosis of HCC cells.
Effects of NG-R1 or together with miR-21 on apoptosis in HCC cells. MHCC97H and BEL7402 cells were transfected with miR-21 or miR-ctrl and treated with 40 μM NG-R1 for 48 h. a, b The apoptotic rate was detected by flow cytometry analysis. c, d Casapse-3/7 activity was measured by caspase-3/7 activity assay, respectively. *P < 0.05 compared with miR-ctrl. #P < 0.05 compared with miR-ctrl + NG-R1. Statistical analysis was conducted using one-way ANOVA with Tukey’s posttest
miR-21 Overexpression Attenuated the Effects of NG-R1 on the Invasion of HCC Cells
As displayed in Fig. 8a and b, promotion of miR-21 abolished the suppressive effect of NG-R1 on the invasive ability in MHCC97H and BEL7402 cells. Meanwhile, western blot analysis proved that cotreatment with NG-R1 and miR-21 overexpression offset the suppressive effect of NG-R1 on MMP-2 and MMP-9 expression in MHCC97H and BEL7402 cells (Fig. 8c and d). Collectively, these results revealed that miR-21 overexpression attenuated the effects of NG-R1 on the invasion of HCC cells.
Effect of NG-R1 or combined with miR-21 on cell invasion in HCC cells. MHCC97H and BEL7402 cells were introduced with miR-21 or miR-ctrl and exposed to 40 μM NG-R1 for 48 h. a, b Transwell invasion assay was conducted to evaluate cell invasive ability in the treated MHCC97H and BEL7402 cells. c, d Western blot analysis was performed to detect the protein levels of MMP-2 and MMP-9 in the treated MHCC97H and BEL7402 cells. *P < 0.05 compared with miR-ctrl. #P < 0.05 compared with miR-ctrl + NG-R1. Statistical analysis was conducted using one-way ANOVA with Tukey’s posttest
miR-21 Overexpression Resisted NG-R1-Induced Inactivation of PI3K/Akt Pathway in HCC Cells
To investigate the potential regulatory mechanism of NG-R1 in HCC cells, the PI3K/Akt pathway was examined in this study. As presented in Fig. 9a and b, treatment with NG-R1 significantly inhibited the expression of p-PI3K and p-Akt, but had no impact on PI3K and Akt expression in MHCC97H and BEL7402 cells. However, delivery of miR-21 effectively abrogated the inhibitory effects of NG-R1 on the expression of p-PI3K and p-Akt in MHCC97H and BEL7402 cells. These results indicated that miR-21 overexpression resisted NG-R1-induced inactivated the PI3K/Akt pathway in HCC cells.
Effect of NG-R1 or combined with miR-21 on the PI3K/Akt pathway in HCC cells. a, b MHCC97H and BEL7402 cells were introduced with miR-21 or miR-ctrl and exposed to 40 μM NG-R1 for 48 h, followed by western blot analysis of p-PI3K, PI3K, p-Akt, and Akt expression. *P < 0.05 compared with miR-ctrl. #P < 0.05 compared with miR-ctrl + NG-R1. Statistical analysis was conducted using one-way ANOVA with Tukey’s posttest
Discussion
HCC is one of the deadliest cancers with increasing incidence and poor prognosis, posing a serious threat to human health [20]. It is indispensable to identify innovative and effective therapeutic agents for the prevention and treatment of HCC. Increasing studies have focused on the anti-tumor activities of various natural or herbal compounds, which provide new avenues for the treatment of cancers. In our study, we studied the anti-tumor effects of NG-R1 on HCC cells. Our study demonstrated that NG-R1 inhibited the viability, increased LDH release, promoted apoptosis, and suppressed cell invasion in HCC cells. Additionally, we found that NG-R1 downregulated miR-21 expression in HCC cells and the anti-tumor effects of NG-R1 on HCC cells were abolished by overexpression of miR-21. Moreover, further experiments proved that NG-R1 inactivated the PI3K/Akt pathway in HCC cells by downregulating miR-21.
Recently, several lines of evidence have demonstrated that Panax notoginseng extract shows anticancer activity [21]. Treatment with Panax notoginseng root extract reduced the growth in lung carcinoma cells and a lung carcinoma xenograft mouse model, suggesting that Panax notoginseng could serve as a potent anticancer agent [22, 23]. It was demonstrated that Panax notoginseng extract exerted anti-metastatic property by inhibiting human colorectal cancer cell migration, invasion, and adhesion [24]. NG-R1, a novel phytoestrogen, is believed to be the characteristic component in the root of Panax notoginseng and has multifaceted pharmacologic activity including anticancer activity [25]. For example, NG-R1 showed anti-metastatic properties in colorectal cancer by inhibiting cell migration, invasion, and adhesion and by regulating expression of metastasis-associated signaling molecules [10]. It was also proved that Panax notoginseng root extract and its major constituents including NG-R1 inhibited cell growth, increased apoptosis, arrested cells in the synthesis phase, and enhanced the action of chemotherapeutic agents in colorectal cancer cells [11, 25]. In the present study, we showed that NG-R1 inhibited cell viability, increased LDH release, promoted apoptosis, and suppressed cell invasion in HCC cells, suggesting the anti-tumor effect of NG-R1 in HCC.
In recent years, increasing experimental data have emphasized the importance of miRNA regulation in the pharmacologic activities of drugs with herbal sources [26]. For example, alkannin exerted anti-tumor activity in HCC cells via inactivating phosphatase and tensin homolog (PTEN)/PI3K/Akt signal pathways by downregulation of miR-92a [27]. Schizandrin A inhibited cell proliferation, metastasis, and induced apoptosis in thyroid cancer through inactivation of the Wnt/β-catenin and mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling pathways by downregulating miR-429 [28]. In addition, it was demonstrated that NG-R1 prevented oxidized low-density lipoprotein-induced atherogenic response in HUVEC cells via downregulating miR-132 [29]. Interestingly, a previous study proved that NG-R1 reduced miR-21 expression in atherosclerosis [18]. Consistently, we provided the evidence that NG-R1 downregulated miR-21 expression in HCC cells. Moreover, rescue experiments manifested that miR-21 overexpression attenuated the effects of NG-R1 on the viability, LDH release, apoptosis, and invasion of HCC cells, suggesting that NG-R1 exerted anti-tumor activity in HCC cells by downregulating miR-21.
To date, the PI3K/Akt signal pathway, an important intracellular signal transduction pathway, has become a research focus due to its critical role in the regulation of multiple biological processes in cancer cells, including cell proliferation, apoptosis and metastasis [30]. Activation of PI3K/Akt signaling pathway participates in the development and progression of various cancers including HCC [31]. Previously, it was reported that curcumol reduced the proliferation of colorectal cancer cells through inhibiting the PTEN/PI3K/Akt pathway by targeting miR-21 [32]. Kaempferol inhibited proliferation, migration, and invasion of HCC cells by downregulating miR-21 and inactivating the PI3K/Akt/mammalian target of rapamycin (mTOR) pathway [33]. Accordingly, we supposed whether the anti-tumor activity of NG-R1 in HCC cells was mediated via inactivation of the PI3K/Akt pathway by downregulating miR-21. As expected, our study demonstrated that NG-R1 inhibited the activation of the PI3K/Akt pathway in HCC cells, which was abolished by miR-21 overexpression. Collectively, we concluded that NG-R1 exerted anti-tumor activity in HCC cells through inactivation of the PI3K/Akt pathway by downregulating miR-21.
In summary, to the best of our knowledge, we demonstrated for the first time that NG-R1 exerted anti-hepatoma activity through inactivation of the PI3K/Akt pathway by downregulating miR-21, contributing to further understanding of the anti-tumor activities of NG-R1 in HCC. Our study may provide valuable foundation for further exploring the treatment of HCC using NG-R1.
References
Buendia MA, Neuveut C. Hepatocellular carcinoma. Cold Spring Harbor Perspect Med. 2015;5:a021444.
Cheng X, Sun P, Hu QG, Song ZF, Xiong J, Zheng QC. Transarterial (chemo)embolization for curative resection of hepatocellular carcinoma: a systematic review and meta-analyses. J Cancer Res Clin Oncol. 2014;140:1159–1170.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Dimitroulis D, Damaskos C, Valsami S, et al. From diagnosis to treatment of hepatocellular carcinoma: an epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282–5294.
Liu KY, Wang LT, Hsu SH. Modification of epigenetic histone acetylation in hepatocellular carcinoma. Cancers. 2018;10:8.
Attwa MH, El-Etreby SA. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1632–1651.
Yang X, Xiong X, Wang H, Wang J. Protective effects of Panax notoginseng saponins on cardiovascular diseases: a comprehensive overview of experimental studies. Evid Based Complement Altern Med. 2014;2014:204840.
Sun B, Xiao J, Sun XB, Wu Y. Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic mice: an insight into oestrogen receptor activation and PI3K/Akt signalling. Br J Pharmacol. 2013;168:1758–1770.
He NW, Zhao Y, Guo L, Shang J, Yang XB. Antioxidant, antiproliferative, and pro-apoptotic activities of a saponin extract derived from the roots of Panax notoginseng (Burk.) F.H. Chen. J Med Food. 2012;15:350–359.
Lee CY, Hsieh SL, Hsieh S, et al. Inhibition of human colorectal cancer metastasis by notoginsenoside R1, an important compound from Panax notoginseng. Oncol Rep. 2017;37:399–407.
Wang CZ, Xie JT, Zhang B, et al. Chemopreventive effects of Panax notoginseng and its major constituents on SW480 human colorectal cancer cells. Int J Oncol. 2007;31:1149–1156.
Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell Biol. 2007;17:118–126.
Mirnezami AH, Pickard K, Zhang L, Primrose JN, Packham G. MicroRNAs: key players in carcinogenesis and novel therapeutic targets. Eur J Surg Oncol. 2009;35:339–347.
Pfeffer SR, Yang CH, Pfeffer LM. The role of miR-21 in cancer. Drug Dev Res. 2015;76:270–277.
Yoon JS, Kim G, Lee YR, et al. Clinical significance of microRNA-21 expression in disease progression of patients with hepatocellular carcinoma. Biomark Med. 2018;12:1105–1114.
Wang J, Chu Y, Xu M, Zhang X, Zhou Y, Xu M. miR-21 promotes cell migration and invasion of hepatocellular carcinoma by targeting KLF5. Oncol Lett. 2019;17:2221–2227.
Najafi Z, Sharifi M, Javadi G. Degradation of miR-21 induces apoptosis and inhibits cell proliferation in human hepatocellular carcinoma. Cancer Gene Ther. 2015;22:530–535.
Jia C, Xiong M, Wang P, et al. Notoginsenoside R1 attenuates atherosclerotic lesions in ApoE deficient mouse model. PLoS ONE. 2014;9:e99849.
Yi X, Guo J, Guo J, et al. EZH2-mediated epigenetic silencing of TIMP2 promotes ovarian cancer migration and invasion. Sci Rep. 2017;7:3568.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Konoshima T, Takasaki M, Tokuda H. Anti-carcinogenic activity of the roots of Panax notoginseng. II. Biol Pharm Bull. 1999;22:1150–1152.
Kim B, Kim EY, Lee EJ, et al. Panax notoginseng inhibits tumor growth through activating macrophage to M1 polarization. Am J Chin Med. 2018;46:1369–1385.
Yang Q, Wang P, Cui J, Wang W, Chen Y, Zhang T. Panax notoginseng saponins attenuate lung cancer growth in part through modulating the level of Met/miR-222 axis. J Ethnopharmacol. 2016;193:255–265.
Hsieh SL, Hsieh S, Kuo YH, Wang JJ, Wang JC, Wu CC. Effects of Panax notoginseng on the metastasis of human colorectal cancer cells. Am J Chin Med. 2016;44:851–870.
Wang CZ, Xie JT, Fishbein A, et al. Antiproliferative effects of different plant parts of Panax notoginseng on SW480 human colorectal cancer cells. Phytother Res. 2009;23:6–13.
Hong M, Wang N, Tan HY, Tsao SW, Feng Y. MicroRNAs and Chinese medicinal herbs: new possibilities in cancer therapy. Cancers. 2015;7:1643–1657.
Sun B, Zhang J, Liu M, Guan L. Alkannin inhibits proliferation, migration and invasion of hepatocellular carcinoma cells via regulation of miR-92a. Biomed Pharmacother. 2019;114:108782.
Ding Q, Li X, Sun Y, Zhang X. Schizandrin A inhibits proliferation, migration and invasion of thyroid cancer cell line TPC-1 by down regulation of microRNA-429. Cancer Biomark. 2019;24:497–508.
Fu C, Yin D, Nie H, Sun D. Notoginsenoside R1 protects HUVEC against oxidized low density lipoprotein (ox-LDL)-induced atherogenic response via down-regulating miR-132. Cell Physiol Biochem. 2018;51:1739–1750.
Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198.
Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170:605–635.
Liu H, Wang J, Tao Y, et al. Curcumol inhibits colorectal cancer proliferation by targeting miR-21 and modulated PTEN/PI3K/Akt pathways. Life Sci. 2019;221:354–361.
Zhu G, Liu X, Li H, Yan Y, Hong X, Lin Z. Kaempferol inhibits proliferation, migration, and invasion of liver cancer HepG2 cells by down-regulation of microRNA-21. Int J Immunopathol Pharmacol. 2018;32:1–12.
Acknowledgment
This work was supported by Grants from the Medical Science and Technology Project of Henan Province (No. 2018020978) and Nanyang Science and Technology Development Plan (JCQY2018004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Li, Z., Jia, Y. et al. In Vitro Anti-hepatoma Activities of Notoginsenoside R1 Through Downregulation of Tumor Promoter miR-21. Dig Dis Sci 65, 1364–1375 (2020). https://doi.org/10.1007/s10620-019-05856-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05856-4