Abstract
Background
Tight junction (TJ) injuries induced by pepsin–trypsin-resistant gliadin (PT–G) play an important role in the pathogenesis of celiac disease. Previously, 1,25-dihydroxy vitamin D3 (VD3) was reported to be a TJ regulator that attenuates lipopolysaccharide- and alcohol-induced TJ injuries. However, whether VD3 can attenuate PT–G-induced TJ injuries is unknown.
Aim
The aim of this study was to evaluate the effects of VD3 on PT–G-induced TJ injuries.
Methods
Caco-2 monolayers were used as in vitro models. After being cultured for 21 days, the monolayers were treated with PT–G plus different concentrations of VD3. Then, the changes in trans-epithelial electrical resistance and FITC-dextran 4000 (FD-4) flux were determined to evaluate the monolayer barrier function. TJ protein levels were measured to assess TJ injury severity, and myeloid differentiation factor 88 (MyD88) expression and zonulin release levels were determined to estimate zonulin release signaling pathway activity. Additionally, a gluten-sensitized mouse model was established as an in vivo model. After the mice were treated with VD3 for 7 days, we measured serum FD-4 concentrations, TJ protein levels, MyD88 expression, and zonulin release levels to confirm the effect of VD3.
Results
Both in vitro and in vivo, VD3 significantly attenuated the TJ injury-related increase in intestinal mucosa barrier permeability. Moreover, VD3 treatment up-regulated TJ protein expression levels and significantly decreased MyD88 expression and zonulin release levels.
Conclusions
VD3 has protective effects against PT–G-induced TJ injuries both in vitro and in vivo, which may correlate with the disturbance of the MyD88-dependent zonulin release signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Celiac disease (CD) is a chronic autoimmune disease with a prevalence of approximately 1% in the Western world. The major cause of this disease is ingestion of gluten-containing foods [1]. Gluten causes CD because gliadin, one of the major components of gluten, contains large amounts of proline and glutamine, which cannot be completely digested by digestive enzymes [2]. Consequently, the undigested gliadin forms several types of peptide fragments, such as pepsin–trypsin-resistant gliadin (PT–G), in the small intestine lumen. Some types of PT–G, such as P31-43 peptide, have immunogenic effects and can be recognized as food-derived antigens by intestinal dendritic cells. This recognition results in activation of the intestinal immune system and ultimately induces CD in susceptible individuals carrying the HLA-DQ2 or HLA-DQ8 gene [3, 4]. Therefore, gluten and its components, especially PT–G, are believed to be triggers for CD, and avoiding gluten intake remains the only effective treatment for CD to date. However, strictly adhering to a lifelong gluten-free diet is extremely challenging. Thus, more effective and convenient treatments for CD are urgently needed.
Intestinal epithelial integrity plays an important role in maintaining health by preventing harmful materials from passing through intestinal mucosa barrier. Under normal conditions, PT–G cannot pass through the barrier, nor can it reach the lamina propria (LP), where it attracts intestinal immune cells because of its high molecular weight. However, in patients with CD, PT–G disrupts small intestinal epithelial integrity through a complex mechanism. CXCR3, a chemotactic factor receptor that is overexpressed on the surface of small intestinal epithelial cells in CD patients, participates in PT–G-mediated intestinal epithelial disruption [5]. PT–G was reported to bind to CXCR3, and the PT–G-CXCR3 complex further activates the zonulin release signaling pathway [6]. Zonulin, a tight junction (TJ) regulator, plays an important role in PT–G-induced intestinal epithelial disruption by inducing TJ injuries directly [7,8,9,10]. TJs are believed to be the major junctions responsible for regulating intestinal mucosa barrier permeability and are composed of several types of proteins, including occludins, claudins, and zonula occludens (ZOs) [11]. TJ injuries are believed to be an initial process that enables PT–G to enter the LP and activate intestinal immune cells. Moreover, a recent study confirmed that PT–G-induced zonulin release is a myeloid differentiation factor 88 (MyD88)-dependent process [12]. MyD88 is an intracellular signaling molecule that is recruited and activated by PT–G-CXCR3. In the study, MyD88 depletion failed to induce zonulin release and had no influence on intestinal permeability in both in vitro and in vivo experiments. Therefore, the MyD88-dependent zonulin release signaling pathway plays a critical role in PT–G-induced TJ injuries, and agents designed to inhibit MyD88 expression may be useful for CD treatment.
The active form of vitamin D3, 1,25-dihydroxy vitamin D3 (VD3), is well known for its ability to regulate calcium absorption and bone formation. Recently, an increasing number of studies have shown that VD3 has many other functions. Specifically, VD3 was reported to regulate immune response, inhibit cancer cell growth, alleviate asthma, and prevent acute respiratory infections [13,14,15]. In these reports, VD3 performs its functions by binding with a nuclear ligand, namely vitamin D receptor (VDR), regulating the expression of numerous functional proteins. Notably, VD3 also has beneficial effects on gastrointestinal diseases. For example, a previous study reported that VD3 can alleviate 2,4,6-trinitrobenzenesulfonic acid-induced colitis [16]. More interestingly, several studies have reported that VD3 has a protective effect on intestinal TJ injuries induced by dextran sulfate sodium (DSS) [17], TNF-α [18], and alcohol [19]. In these studies, VD3 significantly up-regulated TJ protein expression. Moreover, many studies have also reported that VD3 is capable of inhibiting intestinal MyD88 expression [20,21,22].
Given these data, we hypothesize that VD3 attenuates PT–G-induced TJ injuries by disrupting the MyD88-dependent zonulin release signaling pathway and increasing TJ protein levels. To confirm this, we used Caco-2 monolayers and gluten-sensitized mice to evaluate the effects of VD3 on PT–G-induced TJ injuries and estimate the activity of MyD88-dependent zonulin release signaling.
Materials and Methods
PT–G Preparation
PT–G was prepared as described previously, with minor modifications [23]. A total of 10 g of gliadin from wheat (Sigma-Aldrich, St. Louis, MO, USA; G3375-25G) was dissolved in 100 mL of 0.2 mmol/L HCL and then incubated with 250 mg of pepsin (Sangon Biotech, Songjiang District, Shanghai, China; 1:250) for 4 h at 37 °C. After the pH of the solution was adjusted to 8.0 using 1 mmol/L NaOH, the pepsin-digested product was digested by 250 mg of trypsin (Sangon Biotech, Songjiang District, Shanghai, China; 1:3000) for an additional 4 h in an oscillator with a contrast temperature of 37 °C and a speed of 50 rpm. We then boiled the mixture for 30 min to inactivate the enzymes before lyophilizing the mixture and storing it at −20 °C. PT–G was suspended in PBS at a final concentration of 1 mg/mL when used.
Cell Culture Conditions
The Caco-2 cell line was purchased from ATCC and cultured in DMEM with 4.5 mg/mL glucose, 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin, according to the manufacturer’s instructions. The cells were incubated in a 37 °C incubator with 5% CO2 and were subcultured at a 1:2 ratio when they were approximately 80% confluent. Cells from passages 20 to 40 were used for the experiments. All cell culture reagents were purchased from Sigma-Aldrich, St. Louis, MO, USA.
Trans-Epithelial Electrical Resistance (TEER) Measurements
A trans-well system with 12 inserts (Corning Incorporated, Corning, NY, USA; 0.4 μm pore size) was used for these experiments. Caco-2 cells were seeded in the apical chambers at a high density (1 × 105 cells/chamber) and bathed with 1.0 mL of medium, which was changed every 24 h. The TEER of each chamber was measured by an ERS-2 m (Merck Millipore, Kennebunk, ME, USA), which measures paracellular resistance on a daily basis, as previously described [24]. When the TEER reached 300 Ω cm2, we concluded that the Caco-2 monolayers had been successfully established. We then randomly divided all the chambers into the following five groups and treated each group with the indicated reagent(s) for 24 h: control medium, 1 mg/mL PT–G, 1 mg/mL PT–G + 10−7 M VD3 (Sigma-Aldrich, St. Louis, MO, USA), 1 mg/mL PT–G + 10−8 M VD3, and 1 mg/mL PT–G + 10−9 M VD3. The changes in TEER in each group were measured to assess the changes in monolayer permeability induced by the indicated treatments.
FITC-Dextran 4000 (FD-4) Flux Measurements
We also evaluated the changes in Caco-2 monolayer permeability induced by the indicated treatments by measuring the changes in FD-4 (Sigma-Aldrich, St. Louis, MO, USA) flux. After the monolayers were treated with the indicated reagents for 24 h, as described above, we removed the medium from each apical chamber, washed the monolayers twice with pre-cooled PBS, and then placed the trans-well system in a 37 °C incubator for 10 min to stabilize the monolayers. We then added 200 μL of FD-4 (10 mg/mL) to each apical chamber and 1 mL of PBS to each basal chamber. The trans-well system was subsequently incubated for an additional 2 h, after which FD-4 flux was assessed by removing 100 μL of PBS from each basal chamber and evaluating the fluorescence intensity of the solution with a microplate reader. The excitation wavelength was 490 nm, and the emission wavelength was 520 nm. We then determined the final concentrations of FD-4 using a standard curve generated by serial dilution.
Quantitative PCR (qPCR) for TJ Protein and MyD88 mRNA Expression
The effects of treatment with PT–G and VD3 on TJ protein and MyD88 mRNA expression in the Caco-2 monolayers were evaluated by qPCR. Briefly, cells were collected by trypsin digestion, and total RNA extraction was performed using a total RNA extraction kit (Sangon Biotech, Songjiang District, Shanghai, China), according to the manufacturer’s instructions. Two micrograms of total cell RNA was subsequently used to synthesize cDNA using a Revert Aid First-strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. Real-time qPCR was subsequently performed in a reaction system with a final volume of 25 μL. The reaction mixture comprised 2.5 μL of cDNA, 12.5 μL of Fast Start Universal SYBR Green Master Mix (Roche Life Science, Indianapolis, IN, USA), 2.0 μL of paired primers (Invitrogen Biotechnology, Carlsbad, CA, USA), and 8 μL of ddH2O. The reaction comprised the following steps: 95 °C for 5 min, followed by 40 cycles of 15 s at 95 °C and 60 s at 60 °C. CT values were used to calculate the relative mRNA expression levels of each target gene (occludin, claudin-1, ZO-1, and MyD88) using the 2−ΔΔCT method. The following paired primers were used for the experiment: occludin (forward): 5′-CCCCCATCTGACTATGTGGAAAG-3′ and occludin (reverse): 5′-AACCACCGCTGCTGTAACGAG-3′; claudin-1 (forward): 5′-CTGTGGATGTCCTGCGTGTC-3′ and claudin-1 (reverse): 5′-ACTGGGGTCATAGGGTCATAGAAT-3′; ZO-1 (forward): 5′-ATTTGGCGAGAAACGCTATGA-3′ and ZO-1 reverse: 5′-TGGGTCTGGTTTGGACACTAAG-3′; MyD88 (forward): 5′-TCCTCCACATCCTCCCTTCC-3′ and MyD88 (reverse): 5′-TCCGCACGTTCAAG AACAGA-3′; and GAPDH (forward): 5′-ACTTTGTATCGTGGAAGGACTCAT-3′ and GAPDH (reverse): 5′-GTTTTTCTAGACGGCAGGTCAGG-3′.
Western Blot Analysis
After undergoing the above treatments for 24 h, the cells were collected and lysed in RIPA buffer on ice for 2 h. The protein concentration in the lysis buffer was subsequently measured by a BCA Protein Quantitation Kit (KeyGEN BioTECH, Nanjing, Jiangsu, China), according to the manufacturer’s instructions. The proteins (40 μg) were subsequently electrophoresed and separated on a polyacrylamide gel before being transferred onto a PVDF membrane, which was blocked for 1 h with 5% skim milk to prevent non-specific binding and then incubated with the following antibodies overnight at 4 °C: rabbit anti-occludin polyclonal antibody (CUSABIO, Wuhan, Hubei, China; final dilution 1:200), rabbit anti-claudin-1 polyclonal antibody (CUSABIO, Wuhan, Hubei, China; final dilution 1:500), rabbit anti-ZO-1 polyclonal antibody (Sigma-Aldrich, St. Louis, MO, USA; final dilution 1:200), rabbit anti-MyD88 polyclonal antibody (CUSABIO, Wuhan, Hubei, China; final dilution 1:500), and rabbit anti-β-actin polyclonal antibody (CUSABIO, Wuhan, Hubei, China; final dilution 1:1000). The membrane was subsequently incubated with goat anti-rabbit IgG (CUSABIO, Wuhan, Hubei, China; final dilution 1:5000) for 1 h at room temperature. Then, the relative concentrations of the proteins were detected by chemiluminescence, and densitometric analysis of each protein-related signal was performed using a Chemidoc Molecular Imager. The intensities of the protein bands were normalized to those of β-actin.
Zonulin Release Determination
The concentration of zonulin in the culture medium containing the Caco-2 monolayer was measured at various time points within the indicated window (from 30 min to 24 h post-treatment) using a Human Zonulin ELISA Kit (CUSABIO, Wuhan, Hubei, China), according to the manufacturer’s introductions. In addition, the serum zonulin concentration of gluten-sensitized mice was also measured using a Mouse Zonulin ELISA Kit (Shanghai Heng Yuan Biotechnology, Yangpu District, Shanghai, China), according to the manufacturer’s introductions.
Gluten-Sensitized Animal Models
According to previous studies [25,26,27], several pairs of 8-week-old BALB/c mice provided by the Animal Experimental Center of Wuhan University were cohabited and inbred for at least three generations. These mice were maintained on a strict gluten-free diet purchased from the Trophic Animal Feed High-Tech Co., Ltd, Nantong, Jiangsu, China. Four-week-old third-generation mice were used in our experiments. All the mice were randomly divided into a control group, a PT–G group, and a PT–G + VD3 group. The PT–G group and PT–G + VD3 group were sensitized by 100 μg of PT–G emulsified in 100 μL of Freund’s complete adjuvant, which was administered intragastrically. In addition, the mice in these groups also received 100 μg of PT–G orally once per week for the subsequent 30 days, while the mice in the control group continued to receive the gluten-free diet. After being sensitized, the PT–G + VD3 group was treated with 100 ng/20 g VD3 orally for 7 consecutive days, and the other two groups were treated with an equal dose of peanut oil, which was used as a control. All procedures and protocols were approved by the Animal Care Committee and Institutional Review Board at the Animal Experimental Center of Wuhan University.
Measurements of Intestinal Permeability in Gluten-Sensitized Mice
The small intestinal permeability of gluten-sensitized mice was assessed by evaluating the flux of FD-4 from the intestinal lumen to the serum. Briefly, each mouse was administered 2 mg of FD-4 (10 mg/mL) orally 2 h before being killed. After each mouse was killed, 0.5–1 mL of blood was taken from the portal vein and then stored at room temperature for 1 h before being centrifuged at 3000 rpm for 10 min. One hundred microliters of serum from each sample was subsequently used to measure the concentration of FD-4 using the assays described above.
Immunohistochemistry
After the gluten-sensitized mice were killed, their small intestines were divided into 3 portions. The proximal portion was used to measure expression of MyD88 by immunohistochemistry; the middle portion was used to measure TJ protein levels by western blot analysis and MyD88 mRNA level by qPCR, according to the methods described above; and the distal portion was stored at −80 °C for later use. For the immunohistochemistry experiments, we fixed the intestines in formalin, embedded them in paraffin, and then cut them into five-micrometer-thick sections, which were subsequently mounted on glass slides and blocked with 5% BSA in PBS for 1 h at room temperature before being incubated with 5 μg/mL rabbit anti-mouse MyD88 antibodies (CUSABIO, Wuhan, Hubei, China; final dilution 1:15) overnight at 4 °C. After being washed thrice with PBS, the sections were incubated with goat anti-rabbit IgG (CUSABIO, Wuhan, Hubei, China; final dilution 1:50) for 1 h at 37 °C before being washed with PBS again and stained with DAB for 5 min. The sections were then mounted with resin, observed under a microscope, and photographed as needed.
Statistical Analysis
All data are presented as the mean ± SD. The analysis was conducted using one-way ANOVA for comparisons among different groups and using Tukey’s test for multiple comparisons. All comparisons were performed by GraphPad Prism software (version 6.0, CA, USA). P < 0.05 was considered statistically significant.
Results
Effects of VD3 and PT–G Treatment on Caco-2 Monolayer Barrier Function
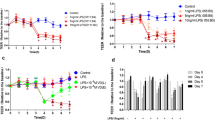
We assessed the function of the Caco-2 monolayer barrier by determining the change of TEER in each group. The results showed that TEER decreased significantly after treatment with PT–G for 24 h compared to that of the control group (190.3 ± 7.9 vs. 320.5 ± 6.8 Ω cm2, P < 0.001). However, the decrease could be attenuated by VD3, especially at a concentration of 10−8 M (295.8 ± 11.4 vs. 190.3 ± 7.9 Ω cm2, P < 0.001), as shown in Fig. 1a.
Effects of VD3 and PT–G treatments on Caco-2 monolayer barrier function. a Changes of TEER after being treated with different reagents. b Changes of FD-4 flux after different kinds of treatments. All data are pooled from six independent experiments and are shown as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. VD3 1,25-Dihydroxy vitamin D3, PT–G pepsin–trypsin-resistant gliadin, TEER trans-epithelial electrical resistance, FD4 FITC-dextran 4000
To confirm the effects of PT–G and VD3 treatments on monolayer barrier function, we further measured the changes of FD-4 flux in each group. The results showed that FD-4 flux increased significantly in the PT–G group compared to the control group (16.0 ± 0.7 vs. 5.8 ± 0.8 μg/mL, P < 0.001), a finding consistent with the changes in TEER. However, this increase was significantly attenuated by dual treatment with PT–G and different concentrations of VD3. In addition, this effect of VD3 seemed to be strongest at a concentration of 10−8 M (6.7 ± 0.4 vs. 16.0 ± 0.7 μg/mL, P < 0.001) as shown in Fig. 1b.
Effects of VD3 and PT–G Treatment on TJ Protein Expression
The TJ complex plays a critical role in maintaining monolayer barrier function. Therefore, we evaluated whether treatment with PT–G and VD3 can influence the TJ complex by measuring Caco-2 cell monolayer TJ proteins at both the mRNA and protein levels by qPCR and western blot analysis, respectively. We found that both mRNA (Fig. 2a) and protein (Fig. 2b, c) levels were slightly decreased in the PT–G-treated group compared with the control group after only 4 h of treatment. These decreases were enhanced in the experiments in which treatment time was extended to 24 h (Fig. 2d, e). However, all these decreases were attenuated by treatment with different concentrations of VD3, and this effect was more significant when the treatment was extended to 24 h. Moreover, VD3 at a concentration of 10−8 M showed stronger effects than the other two concentrations (10−7 and 10−9 M, as shown in Fig. 2).
Effects of VD3 and PT–G treatments on TJ protein expression. a qPCR analysis of TJ protein (occludin, claudin-1, and ZO-1) mRNA expression levels after being treated with different reagents for 24 h. b, c Western blot analysis of TJ protein (occludin and claudin-1) expression levels after being treated with different reagents for 4 h. d, e Western blot analysis of TJ protein (occludin, claudin-1, and ZO-1) expression levels after being treated with different reagents for 24 h. All data present the results of more than three independent experiments and are shown as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. VD3 1,25-Dihydroxy vitamin D3, PT–G pepsin–trypsin-resistant gliadin, TJ tight junction
Suppressive Effect of VD3 Treatment on Zonulin Release
As reported previously, PT–G-induced TJ injury is caused by zonulin release [9,10,11]. Therefore, to determine how VD3 attenuates the decrease in TJ protein expression induced by PT–G, we investigated whether VD3 treatment can influence zonulin release in the Caco-2 monolayer. The results showed that zonulin release began to increase significantly after the monolayers were treated with PT–G for 2 h and reached a peak at 10 h (Fig. 3). These results were consistent with those reported in previous studies [6, 23]. However, zonulin release levels decreased significantly when the monolayers were treated with PT–G and different concentrations of VD3. Moreover, zonulin release levels were the lowest in the group treated with 10−8 M VD3, as shown in Fig. 3.
Suppressive effect of VD3 treatment on zonulin release. Zonulin release levels in the culture medium of the monolayers were detected at different intervention times. As shown in the figure, zonulin level began to increase significant in the PT–G group after being treated for 4 h and reached to a peak at the time point of 10 h. However, the increased zonulin levels could be significantly attenuated in the PT–G + VD3 groups, especially in the group with a concentration of 10−8 M VD3. All data present the results of three independent experiments and are shown as mean ± SD. VD3 1,25-Dihydroxy vitamin D3, PT–G pepsin–trypsin-resistant gliadin
Inhibitory Effects of VD3 Treatment on MyD88 Expression, an Upstream Effector of the Zonulin Release Signaling Pathway
Previous studies have shown that PT–G-induced zonulin release was dependent on the recruitment and activation of MyD88 [12], an upstream intracellular signal factor that is also associated with activation of the innate immune system [28]. In addition, VD3 was previously reported to regulate innate immunity in the intestine by inhibiting MyD88 expression [20,21,22]. Thus, we hypothesized that the suppressive effects of vitamin D3 on zonulin release may also be related to inhibition of MyD88. Therefore, we measured MyD88 mRNA and protein levels in the Caco-2 monolayer by qPCR and western blot analysis, respectively, after the monolayer was treated as described above. The results showed that both mRNA and protein expression levels were significantly increased in the PT–G-treated group compared with the control group, and these increases were attenuated by dual treatment with PT–G and different concentrations of VD3, especially for 24 h. Moreover, treatment with VD3 at a concentration of 10−8 M was more effective than the other two concentrations, as shown in Fig. 4.
Inhibitory effect of VD3 treatment on MyD88 expression. a qPCR analysis of MyD88 mRNA expression level after being treated with different reagents for 24 h. b, c Western bolt analysis of MyD88 protein expression level after being treated with different reagents for both 4 and 24 h. All data present the results of more than three independent experiments and are expressed as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. VD3 1,25-Dihydroxy vitamin D3, PT–G pepsin–trypsin-resistant gliadin, MyD88 myeloid differentiation factor 88
VD3 Reduced Intestinal Mucosal Barrier Permeability in Gluten-Sensitized Mice
To confirm the effects of VD3 on PT–G-induced TJ injuries in vivo, we established a gluten-sensitized mouse model according to the previous studies [25,26,27]. First, we assessed intestinal mucosal barrier function by measuring serum FD-4 concentrations. The results showed that FD-4 concentrations were significantly higher in the PT–G group than in the control group (0.63 ± 0.16 vs. 0.39 ± 0.04 μg/mL, P = 0.0016). However, serum FD4 concentrations in PT–G + VD3 group were much lower than those in the PT–G group (0.44 ± 0.06 vs. 0.63 ± 0.16 μg/mL, P = 0.0095) and had no significant difference compared with the control group (P = 0.10), as shown in Fig. 5.
VD3 reduced intestinal mucosal barrier permeability in gluten-sensitized mice. Serum FD-4 concentrations of gluten-sensitized mice were measured after being treated with different reagents for 7 days by oral administration (n = 8 in each group). All data are expressed as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. VD3 1,25-Dihydroxy vitamin D3, PT–G pepsin–trypsin-resistant gliadin, FITC-dextran 4000
VD3 Up-Regulated TJ Protein Expression in Gluten-Sensitized Mice
As stated above, VD3 regulates TJ protein expression in the Caco-2 monolayer model; thus, we assessed whether treatment with 100 ng/20 g VD3 for 7 consecutive days can influence TJ protein expression in vivo. We found that the expression levels of TJ proteins (occludin, claudin-1, and ZO-1) were significantly up-regulated in the VD3-treated group compared with the PT–G-treated group, a finding consistent with the results of experiments involving the Caco-2 monolayer model (Fig. 6).
VD3 up-regulated TJ protein expression in gluten-sensitized mice. a, b Western blot analysis of the expression levels of TJ protein (occludin, claudin-1, and ZO-1) in small intestinal epithelium of gluten-sensitized mice after being treated with different reagents for 7 days. All data are pooled from the results of more than three independent experiments and are expressed as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. VD3 1,25-Dihydroxy vitamin D3, PT–G pepsin–trypsin-resistant gliadin, TJ tight junction
VD3 Blocked the MyD88-Dependent Zonulin Release Signaling Pathway In Vivo
We also evaluated the influence of VD3 treatment on the MyD88-dependent zonulin release signaling pathway in vivo. The results showed that MyD88 expression levels increased significantly in the PT–G-treated group compared with the control group; however, this increase could be attenuated by VD3 treatment (Fig. 7a, b). Moreover, the change in zonulin release levels elicited by VD3 treatment was similar to the change in MyD88 levels, as shown in Fig. 7c.
VD3 blocked the MyD88-dependent zonulin release signaling pathway in vivo. a qPCR analysis of MyD88 mRNA expression level in small intestinal epithelium of gluten-sensitized mice after being treated with different reagents for 7 days. b Results of immunohistochemistry shown that MyD88 expression level increased significantly in the PT–G group, while significant expression of MyD88 was not observed in control group and PT–G + VD3 group. c Serum zonulin levels of gluten-sensitized mice were detected after being treated with different reagents for 7 days (control group and PT–G group: n = 10; PT–G + VD3 group: n = 8). All data are expressed as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. VD3 1,25-Dihydroxy vitamin D3, PT–G pepsin–trypsin-resistant gliadin, MyD88 myeloid differentiation factor 88
Discussion
CD is a chronic autoimmune disease that is predominantly triggered by gluten. Previous studies have shown that gluten and its components, especially PT–G, can induce CD by injuring the small intestinal mucosal barrier through the MyD88-dependent zonulin release signaling pathway. Although larazotide acetate, a new synthetic peptide that is used to block the zonulin receptor, has shown potential as an adjuvant therapy for CD [29, 30], it remains unavailable for all patients because of questions regarding its efficacy and safety. Moreover, it must also be evaluated in additional well-designed randomized clinical trials [31, 32]. In this study, we reported that VD3, a reagent that is widely used in the clinic, may be useful as an alternative to larazotide acetate.
VD3 is primarily used for the treatment of osteoporosis. Alternatively, VD3 is also used as a nutritional supplement. However, VD3 has been reported to have many additional functions in recent years. Specifically, it has anti-tumor effects [33] and can suppress the immune response [34]. We were interested in VD3 because it is also a TJ regulator that can be used to ameliorate the intestinal mucosal barrier dysfunction induced by DSS [17], LPS [18], and alcohol [19]. However, whether VD3 can protect the small intestinal mucosal barrier from PT–G remains unclear. Here, we observed that VD3 protects against PT–G-induced TJ injuries both in a Caco-2 monolayer model and in a gluten-sensitized mouse model.
In the Caco-2 monolayer model, TEER decreased and FD-4 flux increased significantly after the monolayer was treated with PT–G for 24 h, indicating that PT–G induced a significant increase in Caco-2 monolayer permeability. We subsequently measured TJ protein mRNA and protein levels by qPCR and western blot analysis, respectively. The results showed that occludin, claudin-1, and ZO-1 levels decreased slightly in the PT–G-treated group compared with the control group after the monolayers had been treated with PT–G for 4 h. These decreases became more significant when the treatment time was extended to 24 h. These findings suggest that PT–G down-regulates TJ protein levels. Moreover, we also evaluated zonulin release signaling pathway activity by measuring MyD88 expression and zonulin release levels. The results showed that both MyD88 expression and zonulin release levels increased significantly in the PT–G-treated group compared with the control group, which indicates that PT–G activates the zonulin release signaling pathway. All these results were consistent with those of previously published studies, which reported that gliadin or PT–G increased zonulin release, resulting in TJ protein disassembly and increases in Caco-2 monolayer barrier permeability [35,36,37].
However, treatment with VD3 significantly increased TEER and significantly decreased FD-4 flux. In addition, treatment with VD3 increased occludin, claudin-1, and ZO-1 both at the mRNA and at protein levels. Moreover, VD3-containing treatment also significantly decreased MyD88 expression and zonulin release levels. All these results suggest that VD3 can suppress zonulin release signaling pathway activity, up-regulate TJ protein expression, and attenuate increases in Caco-2 monolayer permeability.
The following three different concentrations of VD3 were used in this study: 10−7, 10−8, and 10−9 M. Our results showed that the protective effects of 10−8 M VD3 tended to be superior to those of 10−9 M VD3. However, when the concentration was increased to 10−7 M, this enhancement disappeared. These findings suggest that the protective effects of VD3 on PT–G-induced TJ injury may be dose dependent and that 10−8 M may be the optimum concentration for treatment. These results differ slightly from those of other studies focusing on TJ injuries induced by TNF-α [18] or alcohol [19]. The results of previous studies suggested that 10−7 M was the most effective concentration compared with 10−8 and 10−9 M. We attribute the differences between our results and those of the above-mentioned studies to the tumor cell-killing effects of high concentrations of VD3 [38,39,40], and this effect may be enhanced by PT–G through an unknown mechanism. Additionally, the source of the Caco-2 cell line, different experimental conditions, and experimental devices may also contribute to the difference.
To confirm our findings in vivo, we used a gluten-sensitized mice model. The mice were sensitized with PT–G for 30 consecutive days, which resulted in significant increases in small intestinal permeability and significant decreases in TJ protein expression, as previously reported [41, 42]. Moreover, the above treatment also resulted in MyD88-dependent zonulin release signaling pathway activation. However, these changes were attenuated by treatment with oral VD3 for 7 consecutive days, a finding that confirmed VD3 has protective effects against PT–G-induced intestinal mucosal barrier injuries in vivo.
Herein, we reported that VD3 can protect the intestinal mucosal barrier from PT–G both in vitro and in vivo. However, the study also had some limitations that must be addressed. First, the changes in distribution of the TJ proteins in the Caco-2 monolayer were not shown by immunofluorescence, as in most published studies [17,18,19], because the cells were too thick to be stained and were likely to flake off after being cultured for more than 21 days. Second, neither MyD88 nor zonulin antagonists were used to identify the specific molecule targeted by VD3 to block the zonulin release signaling pathway. Third, we did not explore the mechanism through which VD3 blocks the above signaling pathway. However, based on the results of previous studies [17,18,19], which reported that VD3 acts by binding to its nuclear ligand, i.e., the VDR, we suspect that VD3 may also inhibit the above pathway by binding to its ligand. However, further studies involving this ligand are needed to confirm this hypothesis. Finally, the models used herein were also limited. The Caco-2 monolayer model is often used to study diseases associated with the large intestinal mucosal barrier, as the characteristics of the Caco-2 monolayer are thought to be more similar to those of the colon. Thus, using this model to study small intestinal mucosal barrier function may not be appropriate. Moreover, the gluten-sensitized mouse model is also not widely used for studies of CD, and most published studies used the DQ8 or DQ2 transgenic mouse model instead, which is a better system but is difficult to obtain in China. Additionally, the side effects, such as renal stones and hypercalcemia, of VD3 were not evaluated in the animal experiments.
In conclusion, our study reported that VD3 exerts a protective effect on the small intestinal mucosal barrier from PT–G by up-regulating TJ protein expression. In addition, the mechanism of this protective effect may be related to the blockage of the MyD88-dependent zonulin release signaling pathway. Our findings may facilitate the development of new treatment strategies for CD. However, additional randomized control clinical trials are still needed to confirm the efficacy and proper dose of VD3 as a treatment for CD in the future.
References
Kang JY, Kang AH, Green A, Gwee KA, Ho KY. Systematic review: Worldwide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther. 2013;38:226–245.
Caminero A, Galipeau HJ, McCarville JL, et al. Duodenal bacteria from patients with celiac disease and healthy subjects distinctly affect gluten breakdown and immunogenicity. Gastroenterology. 2016;151:670–683.
Maiuri L, Ciacci C, Ricciardelli I, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–37.
Almeida LM, Gandolfi L, Pratesi R, et al. Presence of DQ2.2 associated with DQ2.5 increases the risk for celiac disease. Autoimmune Dis. 2016;2016:5409653.
Lammers KM, Khandelwal S, Chaudhry F, et al. Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR3-dependent manner only in patients with coeliac disease. Immunology. 2011;132:432–440.
Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194.e3–204.e3.
Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4:e1251384.
Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Ann N Y Acad Sci. 2012;1258:25–33.
Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–1449.
Vanuytsel T, Vermeire S, Cleynen I. The role of Haptoglobin and its related protein, Zonulin, in inflammatory bowel disease. Tissue Barriers. 2013;1:e27321.
Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778:588–600.
Thomas KE, Sapone A, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol. 2006;176:2512–2521.
Chung BH, Kim BM, Doh KC, et al. Suppressive effect of 1α,25-dihydroxyvitamin D3 on Th17-immune responses in kidney transplant recipients with tacrolimus-based immunosuppression. Transplantation. 2016;101:1711.
Li M, Li L, Zhang L, et al. 1,25-Dihydroxyvitamin D3 suppresses gastric cancer cell growth through VDR- and mutant p53-mediated induction of p21. Life Sci. 2017;179:88.
Kim SH, Pei QM, Jiang P, Yang M, Qian XJ, Liu JB. Effect of active vitamin D3 on VEGF-induced ADAM33 expression and proliferation in human airway smooth muscle cells: implications for asthma treatment. Respir Res. 2017;18:7.
Dai ZH, Tan B, Yang H, Wang O, Qian JM, Lv H. 1,25-Hydroxyvitamin D relieves colitis in rats via down-regulation of toll-like receptor 9 expression. Croat Med J. 2015;56:515–524.
Zhao H, Zhang H, Wu H, et al. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;30:57.
Chen S, Zhu J, Chen G, et al. 1,25-Dihydroxyvitamin D3 preserves intestinal epithelial barrier function from TNF-α induced injury via suppression of NF-kB p65 mediated MLCK-P-MLC signaling pathway. Biochem Biophys Res Commun. 2015;460:873–878.
Chen SW, Ma YY, Zhu J, et al. Protective effect of 1,25-dihydroxyvitamin D3 on ethanol-induced intestinal barrier injury both in vitro and in vivo. Toxicol Lett. 2015;237:79–88.
Jiang J, Shi D, Zhou XQ, et al. Vitamin D inhibits lipopolysaccharide-induced inflammatory response potentially through the Toll-like receptor 4 signalling pathway in the intestine and enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian). Br J Nutr. 2015;114:1560–1568.
Li B, Baylink DJ, Deb C, et al. 1,25-Dihydroxyvitamin D3 suppresses TLR8 expression and TLR8-mediated inflammatory responses in monocytes in vitro and experimental autoimmune encephalomyelitis in vivo. PLoS ONE. 2013;8:e58808.
Equils O, Naiki Y, Shapiro AM, et al. 1,25-Dihydroxyvitamin D inhibits lipopolysaccharide-induced immune activation in human endothelial cells. Clin Exp Immunol. 2006;143:58–64.
Gujral N, Suh JW, Sunwoo HH. Effect of anti-gliadin IgY antibody on epithelial intestinal integrity and inflammatory response induced by gliadin. BMC Immunol. 2015;9:41.
Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375–387.
Papista C, Gerakopoulos V, Kourelis A, et al. Gluten induces coeliac-like disease in sensitised mice involving IgA, CD71 and transglutaminase 2 interactions that are prevented by probiotics. Lab Invest. 2012;92:625–635.
Huber M, Baier W, Bessler WG, Heinevetter L. Modulation of the Th1/Th2 bias by lipopeptide and saponin adjuvants in orally immunized mice. Immunobiology. 2002;205:61–73.
Gourbeyre P, Denery-Papini S, Larré C, Gaudin JC, Brossard C, Bodinier M. Wheat gliadins modified by deamidation are more efficient than native gliadins in inducing a Th2 response in Balb/c mice experimentally sensitized to wheat allergens. Mol Nutr Food Res. 2012;56:336–344.
Everard A, Geurts L, Caesar R, et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat Commun. 2014;5:5648.
Gopalakrishnan S, Durai M, Kitchens K, et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides. 2012;35:86–94.
Khaleghi S, Ju JM, Lamba A, Murray JA. The potential utility of tight junction regulation in celiac disease: focus on larazotide acetate. Therap Adv Gastroenterol. 2016;9:37–49.
Leffler DA, Kelly CP, Green PH, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology. 2015;148:1311.e6–1319.e6.
Leffler DA, Kelly CP, Abdallah HZ, et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol. 2012;107:1554–1562.
Lange TS, Stuckey AR, Robison K, et al. Effect of a vitamin D3 derivative (B3CD) with postulated anti-cancer activity in an ovarian cancer animal model. Investig New Drugs. 2010;28:543–553.
Gui B, Chen Q, Hu C, Zhu C, He G. Effects of calcitriol (1,25-dihydroxy-vitamin D3) on the inflammatory response induced by H9N2 influenza virus infection in human lung A549 epithelial cells and in mice. Virol J. 2017;14:10.
Orlando A, Linsalata M, Notarnicola M, Tutino V, Russo F. Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: The role of cellular polyamines. BMC Microbiol. 2014;14:19.
Datta P, Weis MT. Calcium glycerophosphate preserves transepithelial integrity in the Caco-2 model of intestinal transport. WJG. 2015;21:9055–9066.
Barilli A, Rotoli BM, Visigalli R, et al. Gliadin-mediated production of polyamines by RAW264.7 macrophages modulates intestinal epithelial permeability in vitro. Biochim Biophys Acta. 2015;1852:1779–1786.
Dormoy V, Béraud C, Lindner V, et al. Vitamin D3 triggers antitumor activity through targeting hedgehog signaling in human renal cell carcinoma. Carcinogenesis. 2012;33:2084–2093.
Chen S, Zhu J, Zuo S, et al. 1,25(OH)2D3 attenuates TGF-β1/β2-induced increased migration and invasion via inhibiting epithelial-mesenchymal transition in colon cancer cells. Biochem Biophys Res Commun. 2015;468:130–135.
Aggarwal A, Kállay E. Cross talk between the calcium-sensing receptor and the vitamin D system in prevention of cancer. Front Physiol. 2016;7:451. (eCollection 2016).
Abe R, Shimizu S, Yasuda K, et al. Evaluation of reduced allergenicity of deamidated gliadin in a mouse model of wheat-gliadin allergy using an antibody prepared by a peptide containing three epitopes. J Agric Food Chem. 2014;62:2845–2852.
Silva MA, Jury J, Sanz Y, et al. Increased bacterial translocation in gluten-sensitive mice is independent of small intestinal paracellular permeability defect. Dig Dis Sci. 2012;57:38–47. doi:10.1007/s10620-011-1847-z.
Author’s contribution
SD performed the majority of the experiments. XW and HY performed the animal experiments and analyzed the data. HW and TPS designed the research. SD, TPS, and HW wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there are no conflict of interests for this article.
Rights and permissions
About this article
Cite this article
Dong, S., Singh, T.P., Wei, X. et al. Protective Effect of 1,25-Dihydroxy Vitamin D3 on Pepsin–Trypsin-Resistant Gliadin-Induced Tight Junction Injuries. Dig Dis Sci 63, 92–104 (2018). https://doi.org/10.1007/s10620-017-4738-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4738-0