Abstract
Background and Aims
Extra-intestinal manifestations are well recognized in inflammatory bowel disease (IBD). To what extent the commonly recognized extra-intestinal manifestations seen in IBD patients are attributable to IBD is, however, not clear due to the limited number of controlled studies published.
Methods
We have conducted a study of these manifestations using electronic primary care records. We have identified extra-intestinal manifestations in IBD and non-IBD patients and derived odds ratios (ORs) using conditional logistic regression.
Results
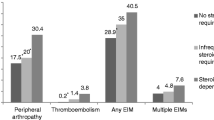
A total of 56,097 IBD patients (32.5 % Crohn’s disease, 48.3 % ulcerative colitis (UC) and 19.2 % not classified) were matched to 280,382 non-IBD controls. We found records of pyoderma gangrenosum (OR = 29.24), erythema nodosum (OR = 5.95), primary sclerosing cholangitis (OR = 188.25), uveitis (OR = 2.81), ankylosing spondylitis (OR = 7.07), sacroiliitis (OR = 2.79) and non-rheumatoid inflammatory arthritides (OR = 2.66) to be associated with IBD. One or more of these was recorded in 8.1 % of IBD patients and 2.3 % of controls. Non-specific arthritides were present in many more patients, affecting 30 % of IBD patients and 23.8 % of controls overall. We also found weaker associations with a number of conditions not generally considered to be extra-intestinal manifestations including psoriasis, ischemic heart disease, multiple sclerosis and hay fever.
Conclusion
Although “classical” extra-intestinal manifestations are strongly associated with IBD, most IBD patients remain unaffected. Arthropathies, perceived to be the commonest extra-intestinal manifestation, are not strongly associated with IBD, and the proportion of arthropathies attributable to IBD is likely to be small.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well recognized that inflammatory bowel disease (IBD) is associated with an increased risk of a number of extra-intestinal diseases. Some of these, such as venous thromboembolism (VTE), are regarded as complications of the disease process, whereas others are considered to be extra-intestinal features of the underlying disease process. These extra-intestinal manifestations (EIMs) usually include skin manifestations (pyoderma gangrenosum and erythema nodosum), joint manifestations (ankylosing spondylitis and seronegative arthritis), eye manifestations (iritis), and aphthous ulceration of the mouth. Primary sclerosing cholangitis (PSC) is sometimes considered as part of this group. Though there is an extensive literature describing the association of complications with IBD, demonstrating for example that VTE is 3–4 times more common in patients with IBD than in the general population [1, 2], the number of controlled studies of extra-intestinal manifestations available is remarkably few [3, 4]. Most of the literature constitutes case series or cohort studies of IBD patients describing the frequency of these manifestations, which does not allow us to examine variations in the frequency of these problems between patients with and without IBD [5, 6]. It is believed from these studies that extra-intestinal manifestations affect about 40 % of IBD patients [5, 7]. To our knowledge only one other recent high-quality epidemiological study has compared extra-intestinal manifestations (EIMs) in IBD to general population controls, but that study did not report on peripheral arthritis which is the most commonly reported EIM in many series [8].
Since examination of the associations of IBD with EIMs has led to further insights into their etiology [9, 10], a clear understanding of the strengths of these associations is of importance. Equally, knowledge of their prevalence in the population is of value to allow provision of services since affected patients will have greater need of health services and in some instances may be at an increased risk of death [11]. To determine the strength of association between IBD and EIMs including arthritis and how commonly they occur, we have carried out a cross-sectional study examining the comparative risks of a series of EIMs in both IBD and non-IBD patients.
Materials and Methods
Data Source
The Clinical Practice Research Datalink (CPRD, formerly General Practice Research Database) is a prospectively gathered database of longitudinal records of consultations, diagnoses, investigations and referrals from UK primary care. Clinical data have been submitted by participating primary care practices since 1987. They are coded using the Read code system which is the standard coding system in UK general practice and provides hierarchical coding not only of diagnoses, but also signs, symptoms, interventions and a number of biographical and administrative items. CPRD has been widely used for epidemiological research, and the validity of diagnosis of IBD in these data has been specifically assessed [12], with 92 % of diagnoses found highly probable and 95 % at least probable.
Study Cohort
We used CPRD records between 1987 and January 2011 to identify a cohort of patients with inflammatory bowel disease (IBD), based upon the presence of Read codes within their records indicating the diagnosis. These were matched on sex, date of birth (within 366 days) and GP practice with up to five patients without any records of an IBD diagnosis. Where more than five suitable controls were available, we selected randomly within the available controls.
Covariates
The associations between IBD and extra-intestinal manifestations are estimated with adjustment for body mass index (BMI) and tobacco use status so that these risk factors for numerous diseases (including ischemic heart disease, atrial fibrillation and hypertension) should not confound our estimates.
We calculated a trimmed mean of BMI for each patient after removing values that fell outside the 1st- and 99th-centile. For patients without any records of BMI, we calculated BMI from their trimmed mean heights and weights. We categorized BMI as underweight (BMI under 18.5), normal (18.5–25.0), overweight (25.0–30.0), or obese (over 30.0). We classified tobacco use for each patient as the highest recorded of the following status (from highest to lowest): “current smoker,” “ex-smoker,” or “not current.” We used an “unknown” category for missing values in body mass index or tobacco use, assuming that missingness did not occur at random.
Extra-Gastrointestinal Manifestations
Outcomes were defined in a similar manner to the diagnosis of IBD, by the presence of diagnostic codes for “red eyes” (divided into uveitis and non-uveitis causes), sclerosing cholangitis, psoriasis, erythema nodosum (both with and without sulfasalazine users to avoid conflating drug side effects with disease effects), pyoderma gangrenosum, arthropathies (divided into osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, sacroiliitis and other specified inflammatory arthritis, arthritis not otherwise specified). Where previously validated code lists were not available, we derived them by manually searching the list of Read codes. All lists were compiled by at least two of the authors.
Patients with IBD are likely to consult their general practitioners more often than non-IBD patients, which may lead to bias in the ascertainment of extra-gastrointestinal manifestations since these conditions may also occur in the general population. Hence, we have also examined the following conditions, which are not known to be strongly associated with inflammatory bowel disease: atrial fibrillation, primary hypertension, ischemic heart disease, hay fever, sinusitis, migraine and multiple sclerosis to ensure any study findings are not due to ascertainment bias.
Statistical Analysis
We examined the association between the risk of developing any extra-gastrointestinal manifestations and inflammatory bowel disease by estimating the odds ratio for each of the outcomes. Odds ratios (ORs) were estimated after adjusting for age, BMI and tobacco use in a multivariate conditional logistic regression model and are presented with 95 % confidence intervals (CIs).
Regulatory and Ethical Approval
As this work utilized existing anonymized data, no separate ethical approval was required. The work was approved by the Independent Scientific Advisory Committee for MHRA database research; protocol number 10_147.
Results
We identified a cohort of 56,097 IBD patients who had valid sex and date of birth recorded, and who contributed prospective data to CPRD. To these cases we matched 280,382 non-IBD patients.
Half of the patients in our IBD cohort were diagnosed with ulcerative colitis (UC) and about one-third had Crohn’s disease (Table 1) (the remaining IBD patients had either codes for both UC and Crohn’s or only codes for indeterminate or non-specific IBD). IBD patients were less likely to be current smokers (28.6 vs. 30.5 %) and more likely to be ex-smokers (19.8 vs. 14.5 %) than non-IBD patients. Non-IBD patients were more likely to be obese (14.6 %) than IBD patients (13.1 %). The proportion of non-IBD patients with missing BMI or tobacco use status was higher, presumably due to fewer opportunities for these to be recorded.
With the exception of hypertension, all of the extra-intestinal conditions we examined were more common in patients with IBD (Table 2). The association of IBD with extra-intestinal disease was particularly strong for uveitis (OR 2.81, 95 % CI 2.65–2.98), sclerosing cholangitis (OR 188.25, 95 % CI 90.97–389.55), erythema nodosum (OR 5.95, 95 % CI 5.38–6.59, and after excluding sulfasalazine users OR 4.84, 95 % CI 4.35–5.39), pyoderma gangrenosum (OR 29.24, 95 % CI 20.99–40.75), ankylosing spondylitis (OR 7.07, 95 % CI 6.24–8.01), sacroiliitis (OR 2.79, 95 % CI 2.45–3.17), and other inflammatory arthritis (OR 2.66, 95 % CI 2.44–2.91) (Table 3). The association was moderate between IBD and multiple sclerosis (OR 1.45, 95 % CI 1.26–1.67), all arthropathies combined (OR 1.47, 95 % CI 1.43–1.50), and psoriasis (OR 1.36, 95 % CI 1.30–1.42). Although the associations were substantial, the proportion of IBD patients affected by each of those conditions was small: from 0.5 % with pyoderma gangrenosum to 3.4 % with uveitis. Overall 8.1 % of IBD patients and 2.3 % of controls were affected by at least one of these well recognized associations, i.e., an excess of 5.8 % among IBD patients. Many IBD patients had presented to their GP with arthropathy (n = 16,854, 30.0 %) or a red eye (n = 12,102, 21.6 %), but most of those were attributed to non-specific arthritis (n = 11,585) or non-uveitis causes of red eye (n = 11,073).
Crohn’s disease was more strongly associated with uveitis (OR 3.20, 95 % CI 2.89–3.55), ankylosing spondylitis (OR 8.98, 95 % CI 7.18–11.23), or cutaneous manifestations such as erythema nodosum (OR 9.08, 95 % CI 7.70–10.71 and after excluding sulfasalazine users: OR 7.74, 95 % CI 6.52–9.17) and pyoderma gangrenosum (OR 47.36, 95 % CI 21.94–102.26) (Table 3). UC was more strongly associated with sclerosing cholangitis (OR 313.71, 95 % CI 93.96–1047.36) than Crohn’s disease, although the number of patients affected was small (37 Crohn’s disease and 227 UC patients).
Patients with IBD were also more likely to have atrial fibrillation (OR 1.11, 95 % CI 1.05–1.16), ischemic heart disease (OR 1.21, 95 % CI 1.17–1.26), migraine (OR 1.13, 95 % CI 1.09–1.17), psoriasis (OR 1.36, 95 % CI 1.30–1.42), or multiple sclerosis (OR 1.45, 95 % CI 1.26–1.67) (Table 3).
It was notable that with the exceptions of psoriasis and multiple sclerosis all extra-intestinal conditions studied were more commonly diagnosed after than before IBD. This was particularly marked for sclerosing cholangitis which was 5 times as commonly diagnosed after IBD (Tables 4, 5).
Discussion
We have shown that the commonly recognized extra-intestinal manifestations of IBD are indeed more commonly diagnosed in IBD patients than in the general population. The degree to which they are associated with IBD varies, with the strongest associations being for sclerosing cholangitis, pyoderma gangrenosum and erythema nodosum, with uveitis and sero-negative arthropathy (except ankylosing spondylitis, which is strongly associated with IBD) being less closely associated. We have also shown that these associations are reasonably specific to the classically recognized extra-intestinal manifestations and that the proportion of IBD cases affected with sufficient severity to be reported to a general practitioner is in absolute terms only greater than that in controls by about 6 %.
Strengths and Weaknesses
With 56,097 IBD cases and 280,382 general population controls, ours is the largest study of EIMs yet conducted. This provides us with power to subdivide the manifestations studied and to examine the associations with rare diagnoses such as pyoderma gangrenosum and PSC. We are enabled to have this power by our use of routinely collected clinical data from general practitioners, which also allows us to ensure that our populations of cases and controls have been selected with minimal bias. This ensures that, unlike previous studies from tertiary referral centers, our results should be generalizable to the whole UK population. Using this data source does, however, have an appreciable scientific cost: we do not have the ability to validate the diagnoses we are describing for individual patients, or to subdivide them into more specific categories. This is not to say that the diagnostic information is unreliable. A number of the diagnoses we studied have been specifically validated in this data set [11, 12] by reference to paper records, and the plethora of validated diagnoses [13, 14] suggest that GP recorded diagnoses are generally accurate in about 90 % of patients. We cannot, however, divide arthropathy into large and small joint diseases, determine the extent of skin involvement in PG or psoriasis, or define a detailed phenotype of IBD. Perhaps most importantly when considering the differences between our findings and those of previous studies, we cannot closely examine patients for evidence of subclinical presentations of the diagnoses we are studying. Therefore, any such asymptomatic or subclinical presentations may be unrecorded when compared to previous studies. As this will affect both IBD cases and the control population equally, it will not, however, have biased our comparison between them.
Compared to Past Literature
Occurrence of EIMs
As for so many features of IBD, the earliest publications about the associations of IBD with its extra-intestinal manifestations were case reports and case series in the first half of the twentieth century [15–17]. The currently recognized associations were described very rapidly. Subsequently many groups have sought out extra-intestinal manifestations in the IBD patients under their care, or IBD in those with arthritis. Few studies, however, have included control groups and only one to our knowledge included general population controls in whom extra-intestinal manifestations were sought in the same manner as in IBD patients [8].
We find uveitis to be recorded in 3.4 % of our IBD cases which is within the range of values (1.8-6 %) found in large modern series [5, 6, 8, 18]. For arthropathy overall, we report 30 % of our IBD patients as being affected, which is among the higher figures reported. For a number of other EIMs, including EN, PG, PSC and AS, we report prevalence lower than in some previous studies. The reasons for this discrepancy may include the fact that we have included all patients with IBD regardless of the length of their history, while it is recognized that EIMs may appear more common if one waits many years after diagnosis [6, 8]. Equally it may be that as many of the previous studies have been conducted from single centers with an interest in IBD, some cases which would have been subclinical, and hence undetected in the routine practice we describe will have been detected. One observation which might lend weight to this idea is that our figures are rather more similar to more dated British figures from an era before extra-intestinal manifestations might have been expected to be sought out by the clinician [19]. It is of course also possible that these conditions are left undetected in the UK despite causing symptoms, and finally it may be that we are seeing a different spectrum of disease due to the presence in our more recent cohort of milder IBD which would in the past itself have remained undiagnosed, or even because of the success of modern IBD management in suppressing these problems.
With respect to the variation between UC and CD we find, as is commonly reported, that apart from PSC, most EIMs are commoner in CD [5, 6, 18] and they occur/emerge after the diagnosis of IBD.
Association with IBD
One great advantage of the present study is the presence of general population controls, which allows determination of the degree of association between the conditions studied and IBD. For each of the classically recognized EIMs, we have considered there is an important association with an odds ratio above 2. It is interesting to note, however, that a number of other conditions not commonly thought of as EIMs which we included primarily to consider specificity are also statistically significantly associated though with a lower OR. In some of these conditions, such as OA or RA, this may represent evidence of the misdiagnosis of a clinically similar EIM, but in others such as hay fever, sinusitis or migraine it is most reasonable to see this as evidence of ascertainment bias perhaps because increased medical contact increases the opportunity for diagnoses in those followed up with IBD. For ischemic heart diseases and multiple sclerosis, it might be argued that they are neither related to a known EIM, nor certain not to be related to IBD. Each has previously been proposed to be associated with IBD [20, 21], but neither is well recognized to be. Each condition is weakly associated with IBD, but unlike the classical EIMs, they do not appear to occur more commonly in IBD patients before their IBD is diagnosed. We believe therefore that ascertainment bias remains a possible explanation of these associations also.
One other advantage of using controls of course is that we can speculate as to how much of an associated condition in IBD patients might be attributable to the IBD. The attributable risks of the extra-intestinal conditions (calculated making the assumption that the associations are causal) will be the risk in those with IBD minus that in the general population. For example overall arthropathy of one sort or another is reported by 30 % of IBD patients, but was also reported by 24 % of general population controls. We could argue therefore that in only about 6 % at most of IBD patients is this likely to be truly an extra-intestinal manifestation of disease, or that only one-fifth of the arthritis in IBD patients is likely to be related to it. If we compare this to PSC which is very rare in the general population, though PSC occurs in only 0.6 % of our IBD cohort, for almost all of these it is likely that the two conditions are related.
Clinical Implications
For patients with IBD who present with EN, PG and PSC, it is quite likely that the etiology of these conditions may be closely linked to IBD. For patients with arthropathy, this link is far less likely and is in fact likely to be the case in no more than one-fifth of cases. Previous reports of EIMs occurring in 40 % or more of IBD cases are likely to have overestimated the prevalence of EIMs by including patients with arthropathy which was not truly related to IBD. Though IHD, MS and psoriasis which are not commonly seen as extra-intestinal manifestations have been proposed to be associated with IBD, we find their associations to be a good deal weaker than those of the classical EIMs, and with odds ratios little higher than those found for associations with hay fever or migraine, it is hard to rule out the possibilities of ascertainment bias or confounding as explanations.
Summary
We have found that commonly recognized skin, joint and eye extra-intestinal manifestations of IBD occur in only about 6 % more commonly in IBD cases than in the general population. Most arthropathy in IBD patients is unlikely to be a true extra-intestinal manifestation.
Abbreviations
- IBD:
-
Inflammatory bowel disease
- UC:
-
Ulcerative colitis
- PSC:
-
Primary sclerosing cholangitis
- VTE:
-
Venous thromboembolism
- EIMs:
-
Extra-intestinal manifestations
- CPRD:
-
Clinical Practice Research Datalink
- BMI:
-
Body mass index
- OR:
-
Odds ratio
- CI:
-
Confidence interval
References
Bernstein CN, Blanchard JF, Houston DS, et al. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population-based cohort study. Thromb Haemost. 2001;85:430–434.
Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657–663. doi:10.1016/S0140-6736(09)61963-2.
Hammer B, Ashurst P, Naish J. Diseases associated with ulcerative colitis and Crohn’s disease. Gut. 1968;9:17–21. doi:10.1136/gut.9.1.17.
Acheson ED. An association between ulcerative colitis, regional enteritis, and ankylosing spondylitis. Q J Med. 1960;29:489–499. http://www.ncbi.nlm.nih.gov/pubmed/13681207.
Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110–119. doi:10.1038/ajg.2010.343.
Lakatos L, Pandur T, David G, et al. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300–2307. http://www.ncbi.nlm.nih.gov/pubmed/14562397. Accessed Jun 12, 2013.
Williams H, Walker D, Orchard TR. Extraintestinal manifestations of inflammatory bowel disease. Curr Gastroenterol Rep. 2008;10:597–605. http://www.ncbi.nlm.nih.gov/pubmed/19006617. Accessed Oct 9, 2013.
Bernstein CN, Blanchard JF, Rawsthorne P, et al. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116–1122. doi:10.1111/j.1572-0241.2001.03756.x.
Alonso Farto JC, Arias IA, Lopez Longo FJ, et al. Clinical significance of abdominal scintigraphy using 99mTc-HMPAO-labelled leucocytes in patients with seronegative spondyloarthropathies. Eur J Nucl Med. 2000;27:1768–1773. doi:10.1007/s002590000393.
Ciccacci C, Biancone L, Di Fusco D, et al. TRAF3IP2 gene is associated with cutaneous extraintestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2013;7:44–52. doi:10.1016/j.crohns.2012.02.020.
Langan SM, Groves RW, Card TR, et al. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol. 2012;132:2166–2170. doi:10.1038/jid.2012.130.
Lewis JD, Brensinger C, Bilker WB, et al. Validity and completeness of the General Practice Research Database for studies of inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2002;11:211–218. doi:10.1002/pds.698.
Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69:4–14. doi:10.1111/j.1365-2125.2009.03537.x.
Jick H, Terris BZ, Derby LE, et al. Further validation of information recorded on a general practitioner based computerized data resource in the united kingdom. Pharmacoepidemiol Drug Saf. 1992;1:347–349. doi:10.1002/pds.2630010607.
Cullinan E. Ulcerative colitis: clinical aspects. Br Med J. 1938;2:1351–1356. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2211132/. Accessed Dec 2, 2013.
Steinberg VL, Storey G. Ankylosing spondylitis and chronic inflammatory lesions of the intestines. Br Med J. 1957;2:1157–1159. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1963013&tool=pmcentrez&rendertype=abstract. Accessed Dec 2, 2013.
Billson FA, De Dombal FT, Watkinson G, et al. Ocular complications of ulcerative colitis. Gut. 1967;8:102–106. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1552509&tool=pmcentrez&rendertype=abstract. Accessed Oct 14, 2013.
Christodoulou DK, Katsanos KH, Kitsanou M, et al. Frequency of extraintestinal manifestations in patients with inflammatory bowel disease in Northwest Greece and review of the literature. Dig Liver Dis. 2002;34:781–786.
Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. III. Complications. Gut. 1964;5:1–22. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1552214&tool=pmcentrez&rendertype=abstract. Accessed May 30 2012.
Bernstein CN, Wajda A, Blanchard JF. The incidence of arterial thromboembolic diseases in inflammatory bowel disease: a population-based study. Clin Gastroenterol Hepatol. 2008;6:41–45. doi:10.1016/j.cgh.2007.09.016.
Gupta G, Gelfand JM, Lewis JD. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology. 2005;129:819–826.
Acknowledgments
This work was funded by a grant from Crohn’s and Colitis UK (NACC) (Grant Number M/10/01). This study was conceived and designed collaboratively by all authors. Dr. Card obtained funding. Dr. Chu carried out all analysis. All authors contributed to the interpretation of the results. Drs. Card and Chu jointly wrote the first draft after discussions among all authors, and all authors edited and amended this and all subsequent drafts. All authors approved the final draft. Dr. Langan is funded by an NIHR Clinician Scientist Fellowship (NIHR/CS/010/014). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the UK Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare they have no conflict of interest relating to the presented work.
Rights and permissions
About this article
Cite this article
Card, T.R., Langan, S.M. & Chu, T.P.C. Extra-Gastrointestinal Manifestations of Inflammatory Bowel Disease May Be Less Common Than Previously Reported. Dig Dis Sci 61, 2619–2626 (2016). https://doi.org/10.1007/s10620-016-4195-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4195-1