Abstract
Background and Aim
After clinical screening and the serological test, many patients still require a duodenal biopsy for celiac disease diagnosis. Mild histological lesions, unspecific findings and patchiness are frequent outcomes of this mandatory diagnostic tool, thus complicating clinical decisions.
Methods
We analyzed the lymphoid components [number of total intraepithelial lymphocytes (IELs), TcR-γδ and CD3−IELs] of the duodenal epithelium by flow cytometry in samples obtained from bulb and distal duodenum during upper gastrointestinal endoscopies performed for diagnostic purposes.
Results
IEL counts and IEL subset distribution (IEL lymphogram) remain invariant along duodenal mucosa revealing a specific profile (immunophenotype) that characterizes either a healthy mucosa or a celiac mucosa. The celiac immunophenotype persists regardless of the biopsy’s anatomical location or the corresponding histological findings.
Conclusions
We propose the IEL lymphogram by flow cytometry as an immunological parameter to discern celiac condition from healthy mucosa. This obviates not only misinterpretation of minor histological changes, but also patchiness and the concerns about the location and number of biopsies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Celiac disease (CD) is an immunologically mediated intolerance to dietary prolamins that evolves into a chronic inflammatory enteropathy with frequent extraintestinal complications. It is a common condition that can be diagnosed at any age. CD occurs in genetically susceptible patients that express HLA-DQ2 or HLA-DQ8 histocompatibility antigens leading the autoimmune response. As the consequence of gluten exposure, a specific T-cell lymphocyte immune response occurs in the lamina propria, while an innate immune reaction is also sustained in the intestinal epithelia [1, 2]. Activation of lymphoid components of both compartments contributes to the resultant histopathological lesions, characterized by an increased in the number of intraepithelial lymphocytes (IELs), crypt hyperplasia and variable degrees of villous atrophy [3–6], generally accompanied by an increase of specific antibodies [7].
The improvement of the diagnostic antibody tests have contributed to a review of the requirement of the histological assessment of the lesion [3–5, 8, 9] for a definitive CD diagnosis. These better antibody tests have also helped to update ESPGHAN guidelines [10] with novel recommendations to skip the diagnostic biopsy in specific situations. However, in the daily clinical routine clinicians have to deal with a heterogeneous group of atypical or non-classical CD forms, complicating the diagnosis. In this scenario, biopsy still emerges as a pivotal diagnostic tool. Milder or less precise histological lesions are frequent in this at-risk group, and additional supportive evidence is often required to ensure diagnosis, such as IEL TcR-γδ counts [11–13], IgA anti-transglutaminase-2 autoantibodies (tTG) intestinal deposits [14] or the analysis by flow cytometry of IEL subsets [15].
Additionally, CD lesions are neither pathognomonic nor specific (less than 10 % of low-grade enteropathy is CD) [1–5, 16, 17] and can even be patchy along duodenal mucosa [1, 4, 16, 18–20]. Despite controversy [4, 5, 9, 18–21], biopsies ought to be taken from the second/third portion of the duodenum (at least four) and at least one from the duodenal bulb to improve the diagnostic yield, thereby adding complexity to the histological interpretation [4, 9, 18].
At a cellular level, the lymphoid-mediated immune response in celiac mucosa can be tested in the laboratory by flow cytometric analysis of small intestinal immune cells, in particular IEL subsets (αβ, γδ and CD3−IELs, what we have termed the “IEL lymphogram”) [15, 22]. IELs represent 5–15 % of the cells isolated from the epithelium [23]. The majority of IELs are CD3+ T cells (70 %) (80 % TcR-αβ (αβ IELs) and 5–15 % TcR-γδ (γδ IELs)). The second subset in size is the CD3−CD103+ lymphoid IEL population (10–20 % in adults, 20–40 % in children [13] (reviewed in [23, 24] ). Intraepithelial lymphocytosis is a key feature in the evaluation of celiac enteropathy [11, 25]. The detectable immune abnormalities in this intraepithelial compartment are an increase in the absolute and relative numbers of TcR-αβ and TcR-γδ IELs [6, 15, 26] and a decrease of CD3−CD103+IELs [13], even previous to the histological alterations [6]. In previous reports, we quantified the normal ranges of these IEL subsets [23] and demonstrated the specificity and sensitivity of the combined study of total IEL numbers and percentage of TcR-γδ and CD3−CD103+IEL subsets in CD diagnosis [15].
The analysis by flow cytometry of these IEL subsets (TcR-αβ, TcR-γδ and CD3−CD103+IEL) is a highly specific and sensitive immuno-parameter (IEL lymphogram) in celiac disease diagnosis. This celiac immunophenotype is particularly helpful in potential, latent and atypical presentations or when there are diagnostic doubts [27].
Materials and Methods
Patients
Biopsies (4 samples from the second/third portion of the duodenum and 4 samples from the duodenal bulb) were obtained from 159 pediatric patients undergoing upper gastrointestinal endoscopy on the Pediatric Gastroenterological Unit of Hospital Universitario Ramón y Cajal (Madrid, Spain), over a period of 8 years (2005–2013).
Patients were defined as having celiac disease if they had the combination of positive antibodies (endomysial antibodies (EMA) or IgA anti-transglutaminase-2 antibodies (tTG), a compatible HLA genotype and histological enteropathy Marsh grade 3a–3c and IEL lymphogram of CD.
The groups were as follows:
-
Patients with CD: 109 at the time of diagnosis while the patients were on a gluten-containing diet. The diagnosis of CD was ascertained subsequently by a favorable clinical response to a GFD and the disappearance of anti-endomysium and anti-transglutaminase-2 antibodies from the sera.
Ten patients on a gluten-free diet (GFD).
-
Control group: normal intestinal biopsy specimens from 40 children were included in this group. The final diagnoses of these patients were as follows: food allergy (8 patients), toddler’s diarrhea and growth failure of extra intestinal origin (4 patients), eosinophilic esophagitis (6 patients), functional dyspepsia (9 patients), gastroesophageal reflux (5 patients), H. pylori associated gastritis with histologically normal intestinal mucosa (8 patients). All patient included in this group had histologically normal bulb and distal duodenal biopsies and IEL lymphograms were not characteristic of celiac enteropathy. Isolated changes of one IEL subset (TcR-γδ or CD3− subset) could be occasionally observed, but never in the characteristic combination of the celiac IEL profile (increase of TcR-γδ together with decrease of CD3− subset) as can be seen in Fig. 1 in the NC columns. In these cases, the isolated IEL changes also remained permanent along the duodenal mucosa.
Fig. 1 Distribution of the IEL subsets: IELs, CD3+TcR-γδ and CD3−CD103+ analyzed for the diagnostic of celiac disease, comparing the values between distal duodenum and duodenal bulb in patients with active celiac disease (CD), controls (NC) and celiac in a gluten-free diet (GFD) and statistical significance (p) of IELs changes. % = percentage: of IELs relative to total cellularity of epithelium, of each IEL subset relative to total IELs. Normal values for IEL subsets: IELs <12 % relative to total cellularity of epithelium, CD3+γδ-T lymphocytes <10 % relative to IELs, CD3−CD103+ >10 % relative to IELs [15, 24]
Histology Assessment
The histological lesions of the intestinal mucosa were evaluated according to the Marsh classification, modified by Oberhuber et al. [28]: 0 = normal, 1 = raised IELs, 2 = raised IELs with crypt hyperplasia, 3a = raised IELs, crypt hyperplasia and partial villous, 3b = raised IELs, crypt hyperplasia and subtotal villous atrophy, 3c = raised IELs, crypt hyperplasia and total villous atrophy.
Methods
Isolation and Flow Cytometry Analysis of IELs Subsets
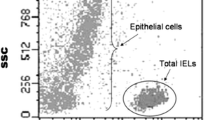
Single-cell suspensions were prepared from the epithelial layer of small intestinal biopsies as stated in [15, 24]. IELs are identified on the basis of low side scatter and CD45 expression. The intraepithelial nature of the gated IELs was assessed by CD103 expression.
Analysis by FCM focuses on three different cellular subsets: percentage of gated CD45+ relative to total cellularity of epithelium (IELs) and percentages of CD3+ γδ-T cells and CD103+CD3− cells relative to total IELs.
Statistical Analysis
The results are expressed as percentages with mean and standard error. Significance of the differences in IEL subset densities between patient groups was performed using the Mann–Whitney test, which considered statistical significance a value of p < .05.
The study was approved by the hospital’s ethical committee.
Results
Duodenal bulb and distal duodenum have identical IEL subset distribution in healthy mucosa (Table 1; Fig. 1).
IEL counts and subset distribution were quantified in biopsy samples obtained from bulb and distal duodenum from controls. Values are expressed in Table 1 (mean ± standard error) as the percentages of the three IEL subsets defined for analysis, both in bulb and distal duodenum.
Statistical values of p > .05.
Therefore, IEL lymphogram remains stable at any location of healthy duodenal mucosa.
Characteristic IEL subset changes in CD enteropathy are present both in bulb and distal duodenum (Table 2; Fig. 1).
In Table 2, we expressed the percentages (mean ± standard error) of the three diagnostic IEL subsets analyzed in the distal duodenum (D) and duodenal bulb (B) on active CD patients and patients on GFD.
Statistical values of p > .05.
Therefore, a characteristic celiac IEL lymphogram is present either in bulb or distal duodenum.
Celiac patients with patchy lesion maintain a similar IEL lymphogram in bulb and distal duodenum (Table 3).
Patients with discrepancies among histological results showed similar IEL lymphograms in distal duodenum (D) and bulb (B); value of p > .05.
All patients were HLA DQ2 and had positive serological markers.
Therefore, differences in Marsh range at different biopsy locations are minimized by a permanent and characteristic celiac IEL lymphogram.
Discussion
Current EPSGHAN guidelines for CD diagnosis recommend that the intestinal biopsy may be omitted in some patients under certain circumstances: symptomatic cases with high anti-tTG levels and positive EMA plus DQ2 and/or DQ8 positivity. The remaining suspicious cases that do not fulfill the above safety criteria will still require a diagnostic biopsy that should be contemplating specimens from bulb and distal duodenum [10]. Relative to clinical manifestation and symptoms, it is known that clinical presentation of CD is highly heterogeneous with a wide range of variants such as silent, potential, latent or refractory forms. Complicating the scenario, histological lesions are not pathognomonic [1–5, 16, 17], while anti-tTG antibodies are not constantly present in atypical or low-grade presentations [1, 3–5, 18, 20, 29]. In addition, cell-mediated inflammation and antibody production can be influenced by different external factors, such as the amount of gluten ingested or undercurrent infections or toxins. This, together with genetic background or immunological conditions, would make possible a broad spectrum in the clinical, tissular and serological expression of this disease [3, 17, 30].
After clinical screening and the serological test, many patients require a diagnostic biopsy in order to search for characteristic lesions. At this point, information obtained from the biopsy is mandatory and has to be efficient enough to avoid unspecificities, misinterpretation and patchy lesions [1, 4, 5, 19, 30]. This is the controversial part concerning the proper location and number of duodenal biopsies for the best diagnostic efficiency [9, 16, 18]. Rather than looking for sites or thinking about necessary quantities of biopsies, it would be better to look for additional new tools to support the diagnosis. In doing so, this would avoid the traditional problems of histological interpretation or patchiness of the lesion [4, 5, 9, 19].
In previous reports, we established the normal range of IEL subsets by flow cytometry in distal duodenal mucosa and the abnormal range that occurs in celiac condition [15, 23]. Now, we show (Fig. 1) that this characteristic celiac immunophenotype is present in either bulb or distal duodenum in all celiac patients. Flow cytometry analysis of IELs allows an easy check of the immunological status of epithelium looking for cell subset changes and is particularly helpful for those patients with atypical forms of the disease or who present diagnostic doubts.
The histological changes observed in celiac enteropathy are characteristic but not exclusive to CD; however, when incorporating celiac immunophenotyping to the evaluation of a biopsy, it results in a disease-specific enteropathy. Additionally, patchy lesions represent an obstacle for proper histological interpretation. In this paper, we have shown that celiac immunophenotype is not submitted to these variations and exhibits the same profile regardless of the mucosal lesion’s grade. An IEL lymphogram on the bulb or distal duodenum provides greater diagnostic accuracy for celiac enteropathy.
Intraepithelial lymphocytes maintain the same IEL subset distribution all along duodenal mucosa, with no significant size variation between bulb and distal duodenum. Secondly, IEL subset changes, characteristic of CD, are present in both bulb and distal duodenum with a similar significance, even in patients with patchy enteropathy.
We can conclude that diagnostic biopsy, when required, combined with IEL lymphogram analysis provides specificity to the histological findings and avoids possible misinterpretation of the histological lesions.
Abbreviations
- CD:
-
Celiac disease
- EMA:
-
Endomysial antibodies
- GFD:
-
Gluten-free diet
- tTG:
-
IgA anti-transglutaminase-2 auto-antibodies
References
Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743.
Jabri B, Kasarda DD, Green PH. Innate and adaptive immunity: the yin and yang of celiac disease. Immunol Rev. 2005;206:219–231.
Dewar DH, Ciclitira P. Clinical features and diagnosis of celiac disease. Gastroenterology. 2005;128:S19–S24.
Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19.
Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002.
Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–242.
Giersiepen K, Lelgeman M, Stuhldreher N, et al. Accuracy of diagnostic antibody tests for coeliac disease in children: summary of an evidence report. J Pediatr Gastroenterol Nutr. 2012;54:229–241.
European Society of Paediatric Gastroenterology and Nutrition. Revised criteria for diagnosis of coeliac disease. Arch Dis Child. 1990;65:909–911.
Rashid M, MacDonald A. Importance of duodenal bulb biopsies in children for diagnosis of celiac disease in clinical practice. BMC Gastroenterol. 2009;9:78.
Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160.
Holm K, Mäki M, Savilahti E, et al. Intraepithelial gamma delta T-cell-receptor lymphocytes and genetic susceptibility to coeliac disease. Lancet. 1992;339:1500–1503.
Savilahti E, Arato A, Verkasalo M. Intestinal gamma/delta receptor-bearing T lymphocytes in celiac disease and inflammatory bowel disease in children. Constant increase in celiac disease. Pediatr Res. 1990;28:579–581.
Spencer J, MacDonald TT, Diss TC, et al. Changes in intraepithelial lymphocyte subpopulations in coeliac disease and enteropathy associated T cell lymphoma (malignant histiocytosis of the intestine). Gut. 1989;30:339–346.
Koskinen O, Collin P, Lindfors K, et al. Usefulness of small-bowel mucosal transglutaminase-2 specific autoantibody deposits in the diagnosis and follow-up of celiac disease. J Clin Gastroenterol. 2010;44:483–488.
Camarero C, Eiras P, Asensio A, et al. Intraepithelial lymphocytes and coeliac disease: permanent changes in CD3−/CD7+ and T cell receptor gd subsets studied by flow cytometry. Acta Paediatr. 2000;89:285–290.
Bonamico M, Thanasi E, Mariani P, et al. Duodenal bulb biopsies in celiac disease: a multicenter study. J Pediatr Gastroenterol Nutr. 2008;47:618–622.
Tjon JM, van Bergen J, Koning F. Celiac disease: how complicated can it get? Immunogenetics. 2010;62:641–651.
Prasad KK, Thapa BR, Nain CK, et al. Assessment of the diagnostic value of duodenal bulb histology in patients with celiac disease, using multiple biopsy sites. J Clin Gastroenterol. 2009;43:307–311.
Zawahir S, Safta A, Fasano A. Pediatric celiac disease. Curr Opin Pediatr. 2009;21:655–660.
Hopper AD, Cross SS, Sanders DS. Patchy villous atrophy in adult patients with suspected gluten-sensitive enteropathy: is a multiple duodenal biopsy strategy appropriate? Endoscopy. 2008;40:219–224.
Bonamico M, Mariani P, Thanasi E, et al. Patchy villous atrophy of the duodenum in chidhood celiac disease. J Pediatr Gastroenterol Nutr. 2004;38:204–207.
Leon F. Flow cytometry of intestinal intraepithelial lymphocytes in celiac disease. J Immunol Methods. 2011;363:177–186.
Camarero C, León F, Sánchez L, et al. Age-related variation of intraepithelial lymphocytes subsets in normal human duodenal mucosa. Dig Dis Sci. 2007;52:685–691.
Eiras P, Roldán E, Camarero C, et al. Flow cytometry description of a novel CD3−/CD7+ intraepithelial lymphocyte subset in human biopsies: potential diagnostic value in coeliac disease. Cytometry. 1998;34:95–102.
Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–391.
Halstensen TS, Scott H, Fausa O, et al. Gluten stimulation of coeliac mucosa in vitro induces activation (CD25) of lamina propria CD4+ T cells and macrophages but no crypt-cell hyperplasia. Scand J Immunol. 1993;38:581–590.
Leon F, Eiras P, Roy G, et al. Intestinal intraepithelial lymphocytes and anti-transglutaminase in a screening algorithm for coeliac disease. Gut. 2002;50:740–741.
Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194.
Arranz E, Ferguson A. Intestinal antibody pattern of celiac disease: occurrence in patients with normal jejunal biopsy histology. Gastroenterology. 1993;104:1263–1272.
Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419–2426.
Acknowledgments
Supported by grant MEC I+D SAF2006-01403 from the Spanish Ministry for Education and Science.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Andrés, A., Camarero, C. & Roy, G. Distal Duodenum Versus Duodenal Bulb: Intraepithelial Lymphocytes Have Something to Say in Celiac Disease Diagnosis. Dig Dis Sci 60, 1004–1009 (2015). https://doi.org/10.1007/s10620-014-3414-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3414-x