Abstract
Background and Aims
Differentiation of gallbladder (GB) carcinoma from benign GB wall thickening is challenging. The recent introduction of second-generation ultrasonic contrast agents has made contrast harmonic imaging with EUS possible. The aim of our study was to evaluate the utility of contrast-enhanced harmonic EUS (CH-EUS) for the differential diagnosis of GB wall thickening.
Methods
Thirty-six consecutive patients with GB wall thickening imaged by CH-EUS and then underwent surgery were enrolled in this study. After the lesions were observed with conventional harmonic EUS (H-EUS), CH-EUS was performed with intravenous injection of 0.015 ml/kg of Sonazoid. Three reviewers with various levels of experience of EUS (Reviewer A: experienced endosonographer, B: EUS trainee, C: experienced gastroenterologist with expertise in transabdominal ultrasound but no EUS experience) were blinded to findings of recorded video of H-EUS and CH-EUS. The diagnostic accuracy of H-EUS and CH-EUS for malignant GB wall thickening was compared.
Results
Final diagnoses based on surgical histology were GB carcinoma in 16, cholecystitis in 11, adenomyomatosis in 6 and cholesterolosis in 3. Overall sensitivity, specificity and accuracy for diagnosing malignant GB wall thickening of H-EUS and CH-EUS were 83.3 versus 89.6, 65 versus 98 % (p < 0.001) and 73.1 versus 94.4 % (p < 0.001). The inter-observer agreement for H-EUS was moderate (κ = 0.51), whereas that for CH-EUS was substantial (κ = 0.77). The inhomogeneous enhanced pattern on CH-EUS was a strong predictive factor of malignant GB wall thickening.
Conclusion
CH-EUS has the potential to improve the preoperative diagnostic accuracy and inter-observer agreement in the differential diagnosis of GB wall thickening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A thickened gallbladder (GB) wall is frequently detected on computed tomography (CT) and ultrasonography (US) performed for either diagnostic evaluation or health screening. The findings of GB wall thickening may be due to a broad spectrum of pathologies including GB carcinoma, chronic cholecystitis and adenomyomatosis. Differentiation between GB carcinoma, adenomyomatosis and GB inflammatory diseases presenting as wall thickening is an important clinical issue, because misinterpretation of GB wall thickening might lead to unnecessary extended surgery in patients with GB inflammatory diseases or delayed an appropriate treatment in patients with GB carcinoma. A lot of studies using various imaging modalities have been conducted to provide a precise preoperative diagnosis for GB wall thickening [1–5]. At the present, multidetector CT (MDCT) is considered to be ideally suited for the assessment of GB wall thickening because this modality allows for enhanced visualization of GB wall thickening in arterial and venous phase [6–8]. However, there are few prospective studies with large series, and the correlation between enhancement pattern on MDCT and the nature of GB wall thickening is unknown [6–8]. Therefore, differential diagnosis of GB pathologies presenting GB wall thickening such as GB carcinoma, adenomyomatosis and cholecystitis with disruptive layered structure is still challenging.
Although endoscopic ultrasonography (EUS) can demonstrate the layered structures of GB wall and is widely used to diagnose GB diseases [3, 4], EUS has been assumed to show no advantage over MDCT in the differential diagnosis of GB wall thickening. However, the recent development of second-generation ultrasonographic contrast agents, including Sonazoid and Sonovue, has permitted contrast-enhanced harmonic imaging of digestive organs with EUS [9–11]. Contrast-enhanced harmonic EUS (CH-EUS) is gaining popularity among endosonographers, and it was reported that CH-EUS was useful especially in the diagnosis of pancreatic pathology [12]. The key advantage of CH-EUS is that the influx and washout of contrast in the target lesion can be observed in real time, and the microvasculature can be imaged in real time. Regarding GB lesions, a few studies showed that CH-EUS might be useful for the diagnosis of malignant GB polyps [13, 14]. However, there are no reports on the utility of CH-EUS for the differential diagnosis of malignant and non-malignant GB wall thickening. The aim of this study was to evaluate if standard harmonic EUS (H-EUS) followed by CH-EUS could improve the diagnostic accuracy for GB wall thickening.
Patients and Methods
Study Design
The study was approved with the institutional review board. This was a retrospective review of consecutive patients who were referred for CH-EUS assessment of GB wall thickening suspicious for malignancy from the results of preceding multimodal imaging tests, including transabdominal US, MRI and MDCT, and in whom definitive diagnosis for GB wall thickening was obtained with surgical resection from May 2008 to April 2011. A thickened GB wall was defined as GB wall thickness more than 3 mm based on any imaging modality [4, 8]. Inclusion criteria were (1) patients who underwent CH-EUS assessment of focal or diffuse GB wall thickening detected with transabdominal US, MRI or MDCT; (2) histological diagnosis for GB wall thickening was available after surgical resection; (3) at least 1-year follow-up after definitive diagnosis; and (4) Video sequences adequate for evaluation. Any patients with predominantly GB polyps and acute phase of cholecystitis were excluded.
Standard H-EUS screening of the pancreaticobiliary system without the use of contrast agent was performed first, with special attention to the GB wall thickening [15]. Tissue harmonic imaging exploits the effect of nonlinear propagation on the acoustic signal as it travels through the human tissue. It produces ultrasound images by using second harmonic signals, generated by the tissue itself during this nonlinear propagation of acoustic energy. The resultant advantages are improved lateral resolution, reduced side lobe artifacts, and an increased signal-to-noise ratio [16]. Therefore, H-EUS can provide better quality images, compared with fundamental EUS [17]. Subsequent to standard H-EUS screening, intravenous administration of 0.015 ml/kg/body of contrast agent, Sonazoid (Daiichi-Sankyo Co., Ltd., Tokyo, Japan), was performed. Then, the GB wall thickening and surrounding organs were observed for at least 3 min after injection of the contrast agent until the echogenicity of contrast decreased after the early vascular phase and became difficult to analyze. All procedures were performed by a single endosonographer, and all ultrasonographic data were recorded on digital videos.
Three reviewers who were blinded to the results of previous investigations for GB wall thickening reviewed the recorded ultrasonographic video clips of H-EUS followed by CH-EUS (H-EUS/CH-EUS) for GB wall thickening. These three reviewers consisted of reviewer A who was a proficient of EUS with experience of more than 300 cases of CH-EUS, reviewer B who was a trainee of EUS, and reviewer C who was a gastroenterologist without any experience of EUS, but knowledgeable about contrast-enhanced harmonic transabdominal ultrasound for hepatocellular carcinoma. All video clips were recorded at least one year before reviewing, because only patients who received at least one-year follow-up after definitive diagnosis were enrolled in this study to avoid the reviewer’s recognition of cases from the endosonographic appearance. Before reviewing video clips of H-EUS/CH-EUS for GB wall thickening in this study, reviewer B and C with limited knowledge of CH-EUS for GB pathologies underwent a training session during which they were given video clips of H-EUS/CH-EUS of six typical cases with GB wall thickening (2 cases with GB carcinoma, 2 cases with chronic cholecystitis, and 2 cases with GB adenomyomatosis) and practiced the interpretation of finding of GB carcinoma, chronic cholecystis and adenomyomatosis. After this training session, a blind review of video clips of H-EUS/CH-EUS for GB wall thickening by three reviewers (A, B and C) was performed, and reviewers were asked to document the following endosonographic finding: (1) magnitude of the greatest GB wall thickness (mm) on H-EUS, (2) internal echogenicity of the area of GB wall thickening on H-EUS, compared with the surrounding normal liver parenchyma, (hypoechoic or iso-/hyperechoic) (3) internal echo pattern of the area of GB wall thickening on H-EUS (inhomogeneous or homogenous), (4) the disruption of GB wall layer structure on H-EUS (presence or absence), (5) the diagnosis of GB wall thickening on H-EUS (GB carcinoma or non-malignant lesion), (6) the overall degree of enhancement on CH-EUS (hypoenhancement or iso-/hyperenhancement), compared with the surrounding normal liver parenchyma, (7) the distribution pattern of contrast agent on CH-EUS (inhomogeneous or homogenous), and (8) the diagnosis of GB wall thickening on CH-EUS (GB carcinoma or non-malignant lesion). During the process of reviewing, H-EUS findings were documented first, followed by findings of CH-EUS. The reviewer’s evaluation of the degree of enhancement and the distribution of contrast on CH-EUS was performed in the arterial and early venous phase until around 90 s from the first arrival of contrast in accordance with recent European guideline [18]. The reference standard was the histological finding after surgery, and the sensitivity and specificity to detect malignant GB wall thickening between H-EUS and CH-EUS were compared, and inter-observer agreement among reviewers with various levels of experience of EUS was assessed.
Equipment and Technique of CH-EUS
Sonazoid
All patients received intravenous administration of Sonazoid in this study. Sonazoid was a second-generation microbubble ultrasonographic agent consisting of perflubutane microbubbles with a median diameter of 2–3 μm [19]. It was reconstituted with 2 ml sterile water for injection. The dose of 0.015 ml of Sonazoid per kilogram body weight was recommended for injection. It was injected as a bolus at a rate of 1 ml/s with a 22-gauge cannula placed in the antecubital vein and flushed with 10 ml normal saline.
EUS Imaging Technique
An electronic radial echoendoscope (GF-UE260, Olympus Medical Systems, Tokyo, Japan) or a curved linear array echoendoscope (GF-UCT260, Olympus Medical Systems, Tokyo, Japan) and the Prosound alpha-10 processor (Hitachi Aloka Medical Co., Ltd., Tokyo, Japan) were used. Prosound alpha-10 had two kinds of mode for EUS imaging, which consisted of conventional tissue harmonic echo (THE) and extended pure harmonic detection (ExPHD) mode. ExPHD mode was specific to contrast-enhanced harmonic ultrasonography, which combined receiving frequencies of filtered fundamental and second harmonic components with transmitting frequency of 5 or 6 MHz. In this specific mode for contrast enhancement, tissue structures became dark and obscure, although contrast microbubble was clearly imaged as high echoic dot. Therefore, THE mode was applied for standard H-EUS screening and ExPHD mode for CH-EUS examination. The acoustic power of CH-EUS was set with a mechanical index of 0.25–0.3, and a single focus point was set at the most distant margin of GB wall thickening from the transducer.
Statistical Analysis
Stata version 10 software (StataCorp, TX, USA) was used for all statistical comparison, and p < 0.05 was considered to be statistically significant. MacNemer test was used for the pair-wise comparison of accuracy of H-EUS and CH-EUS. Univariate logistic regression analysis was performed to calculate odds ratio for each of the four variables of endosonographic findings on H-EUS which consisted of the size of GB wall thickness, the internal echogenicity, the internal echo pattern and the presence of disruption of layer structure, and the 2 variables of endosonographic findings on CH-EUS which consisted of the degree of enhancement and the distribution pattern of contrast. Then, we ran 2 sets of multivariate logistic regression models to constructed diagnostic models to predict factors of malignant GB wall thickening. For the first model (EUS model), the full model included the 4 variables exclusively on H-EUS. For the second model (CH-EUS model), the full model included the 2 variables on CH-EUS in addition to the variables selected on H-EUS model. To construct the final model, a backward stepwise method was used to remove the variables with p value less than 0.10. AUC was compared between the final model of H-EUS and the CH-EUS to determine whether the addition of the CH-EUS findings could improve the diagnostic accuracy.
Results
Patient Characteristics
During the study period, H-EUS/CH-EUS was performed in forty-one patients with GB wall thickening suspicious for malignancy from the results of preceding multimodal imaging tests, including transabdominal US, MRI and MDCT. Among 41 patients, 2 patients had ascites and were diagnosed with advanced gallbladder carcinoma and therefore underwent chemotherapy without surgical resection. In addition, because multimodal imaging tests consisted of H-EUS/CH-EUS, MRI and MDCT showed typical findings of adenomyomatosis of gallbladder in 3 patients, they did not undergo surgical resection. Finally, thirty-six patients with GB wall thickening met inclusion criteria and were analyzed: all cases underwent H-EUS/CH-EUS, and surgical histopathological diagnoses were available. Clinical characteristics of patients were shown in Table 1. The final diagnosis based on surgical resection was GB carcinoma in 16 cases, chronic cholecystitis in 11, adenomyomatosis in 6 and cholesterolosis of gallbladder in 3.
Diagnostic Accuracy for Detection of Malignant GB Wall Thickening of GB Carcinoma with H-EUS and CH-EUS
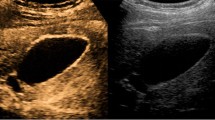
Three blind reviewers (A, B and C) with various levels of EUS experience reviewed video clips of 36 patients enrolled in this study and documented the ultrasonographic findings and the diagnosis for GB wall thickening for H-EUS and CH-EUS. For reviewer A, who was a well-experienced endosonographer at CH-EUS, sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV) and AUC for diagnosing malignant GB wall thickening with H-EUS and CH-EUS were 87.5/80/83.3/77.7/88.9 %/0.82 and 100/100/100/100/100 %/1.0, respectively. For reviewer B, who was a EUS fellow, sensitivity, specificity, accuracy, PPV, NPV and AUC for diagnosing malignant GB wall thickening with H-EUS and CH-EUS were 75/50/61.1/54.5/71.4 %/0.62 and 81.3/95/88.9/92.9/86.4 %/0.88, respectively. For reviewer C, who was a well-experienced gastroenterologist at transabdominal ultrasound without EUS experience, sensitivity, specificity and AUC for diagnosing malignant GB wall thickening with H-EUS and CH-EUS were 87.5/65/75/66.7/86.6 %/0.76 and 87.5/100/94.4/100/90.9 %/0.93, respectively. Finally, overall sensitivity, specificity, accuracy, PPV, NPV and AUC for diagnosing malignant GB wall thickening with H-EUS and CH-EUS were 83.3/65/73.1/65.6/82 %/0.74 and 89.6/98/94.4/97.7/92.2 %/0.94, respectively. Thus, overall specificity (p < 0.001), overall accuracy (p < 0.001), overall AUC (p = 0.004), accuracy (p = 0.03) and AUC (p = 0.01) of reviewer A, specificity (p = 0.0039), accuracy (p = 0.002), and AUC (p < 0.001) of reviewer B, and specificity (p = 0.01), accuracy (p = 0.04) and AUC (p = 0.01) of reviewer C with CH-EUS were significantly higher than those with H-EUS (Table 2). Representative cases with cholecystitis and GB carcinoma were shown in Figs. 1 and 2.
A representative case of localized cholecystitis that was initially suspected to be a gallbladder carcinoma with multi-imaging tests. a Conventional harmonic EUS showed a low-echoic and homogeneous gallbladder wall thickening. b Contrast-enhanced harmonic EUS showed homogenous enhancement within gallbladder wall thickening. Surgical resection was performed and histopathological findings revealed a lymphocyte infiltration, granulomatous change and fibrosis without malignant cells, which was compatible with cholecystitis
A representative case of gallbladder carcinoma that was suspected to be gallbladder carcinoma with multi-imaging tests. a Conventional harmonic EUS showed a low-echoic and homogeneous wall thickening, and the layered structure was relatively distinguishable. b Contrast-enhanced harmonic EUS showed an area of inhomogeneous enhancement within gallbladder wall thickening. Surgical histopathology revealed moderate differentiated adenocarcinoma
Inter-Observer Agreement for Providing the Differential Diagnosis of Malignant or Non-Malignant GB Wall Thickening
The level of agreement to provide the differential diagnosis of malignant or non-malignant GB wall thickening between three reviewers was moderate on H-EUS (κ = 0.51, 95 % CI 0.36–0.59), whereas that of CH-EUS was substantial (κ = 0.77, 95 % CI 0.7–0.85).
Association Between the Endosonographic Findings and Malignant GB Wall Thickening on H-EUS and CH-EUS
As to the endosonographic findings on H-EUS and CH-EUS that were documented with 3 reviewers, univariate logistic regression analysis was performed to calculate odds ratio for each of four variables of endosonographic findings on H-EUS and 2 variables of endosonographic findings on CH-EUS. GB wall thickening more than 12 mm and the presence of disruption of GB wall layer structure on H-EUS and inhomogeneous distribution pattern of contrast agent on CH-EUS were significantly linked to malignant GB wall thickening of GB carcinoma at a high odds ratio (Table 3).
Comparison of Diagnostic Models for the Prediction of Malignant GB Wall Thickening of H-EUS and CH-EUS
Stepwise selection of multivariate logistic regression model of 4 variables on H-EUS (wall thickness more than 12 mm, hypoechoic internal echogenicity, inhomogeneous internal echo pattern and disrupt layer structure) identified that 2 variables of GB wall thickness more than 12 mm and the disruption of GB wall layer structure were independently associated with malignant GB wall thickening, and the 2 variables were included in the final model of H-EUS (Table 4). Moreover, stepwise selection of multivariate logistic regression model of 2 variables on CH-EUS (hypovascular enhancement and inhomogeneous distribution pattern of contrast) in addition to 2 variables on H-EUS model identified that 2 variables of inhomogeneous enhancement pattern on CH-EUS and GB wall thickness more than 12 mm were independently associated with malignant GB wall thickening, and then, the 2 variables were included in the final model of CH-EUS (Table 5). AUC of CH-EUS model for prediction of malignant GB wall thickening was significantly higher than H-EUS model (0.94 vs. 0.84, p = 0.004) (Fig. 3).
Discussion
Ultrasonographic contrast agents were used initially as Doppler signal enhancers because they increased sensitivity in low-velocity, low-volume blood flow. However, in this setting of ultrasonographic examination with contrast agents, the main disadvantage is the presence of the inevitable artifacts such as tissue motion (flash) and blooming (overpainting) [20]. To overcome these initial limitations, contrast-enhanced harmonic technology with second-generation contrast agents and new ultrasound system such as Aloka Prosound α10 equipped with new contrast specific software mode has been developed. This technology made contrast-enhanced harmonic imaging with EUS based on low mechanical index technique to be possible [9, 10]. Second-generation contrast agents include a gas surrounded by a shell (microbubbles) that increases stability, and contrast-enhanced harmonic technology is able to visualize the microcirculation and parenchymal perfusion by selectively depicting the signals derived from the contrast agents while simultaneously filtering the signals originating from tissue. In addition, this technology can detect signals from microbubbles in vessels with very slow flow without Doppler-related artifacts. Moreover, as microbubble destruction is minimized with the use of low mechanical index, continuous real-time assessment of the microvascularization during the uptake of contrast agent can be obtained [10].
After the introduction of contrast-enhanced harmonic technology, a lot of studies of CH-EUS with second-generation contrast agents have been conducted to improve the diagnostic accuracy especially for pancreatic pathologies. Napoleon et al. [21] reported that hypointensity on CH-EUS using Sonovue could diagnose pancreatic carcinoma with a sensitivity and specificity of 89 and 88 %, respectively. A prospective study of CH-EUS using Sonazoid with large samples by Kitano et al. [22] showed hypoenhanced masses depicted with CH-EUS were diagnosed pancreatic carcinoma with high sensitivity and specificity of 95.1 and 89 %, respectively. In addition, a recent meta-analysis [12] of study for the differential diagnosis of pancreatic carcinoma with both contrast-enhanced Doppler and contrast-enhanced harmonic EUS showed the finding of hypoenhanced mass on CH-EUS provided the diagnosis of pancreatic carcinoma with the pooled sensitivity and specificity of 94 and 89 %, respectively. Moreover, CH-EUS using Sonazoid was shown to improve the accuracy in preoperative T-staging of pancreaticobiliary malignancies, compared with conventional H-EUS [11]. As another unique approach with CH-EUS, the quantitative perfusion analysis of signal echointensity within region of interest using time intensity curve was attempted to diagnosis pancreatic pseudotumor, autoimmune pancreatitis, pancreatic carcinoma and neuroendocrine tumor differentially [23–25]. Thus, hemodynamic analysis with CH-EUS can improve diagnostic performance in the differential diagnosis of various pancreatic pathologies, and T-staging of pancreatic carcinoma as well.
Regarding the diagnosis of GB pathologies with CH-EUS, Park et al. [14] reported sensitivity and specificity of CH-EUS to differentiate neoplastic GB polyp from cholesterol polyp based on enhancement pattern were 75 and 66.6 %, respectively. Choi et al. [13] also conducted a comparison study of conventional EUS and CH-EUS in the diagnosis of malignant GB polyp. In the article, an irregular vessel pattern and the presence of perfusion defect determined by CH-EUS were sensitive for malignant GB polyp, and sensitivity and specificity for CH-EUS and conventional EUS to diagnose malignant GB polyp were 93.5 and 93.2 % versus 90.0 and 91.1 %, respectively. Thus, CH-EUS might improve the diagnostic performance in the diagnosis of neoplastic GB polyp. However, only a few reports demonstrated the utility of CH-EUS in the differential diagnosis of gallbladder polyps. Hence, the ability of CH-EUS in the diagnosis for GB pathologies is well unknown. Furthermore, there is no report of the utility of CH-EUS in the differential diagnosis of GB wall thickening that has an expansive differential diagnosis, including cholecystitis, adenomyomatosis and GB carcinoma. Therefore, we conducted this first study to examine the role of CH-EUS in the differential diagnosis for GB wall thickening. Our study showed CH-EUS could significantly improve the specificity, accuracy and AUC for diagnosing malignant GB wall thickening, compared with H-EUS alone; CH-EUS could diagnose correctly benign GB wall thickening such as cholecystitis and adenomyomatosis that H-EUS misinterpreted as GB carcinoma. In univariate analysis, GB wall thickening more than 12 mm and the presence of disruption of GB wall layer structure on H-EUS and inhomogeneous distribution pattern of contrast agent on CH-EUS were significantly linked to malignant GB wall thickening at a high odds ratio. In the comparison of diagnostic model of H-EUS and CH-EUS, CH-EUS model, which was constructed with the endosonographic findings of CH-EUS in addition to H-EUS finding, was significantly accurate for malignant GB wall thickening, compared with H-EUS model. That is, the addition of contrast enhancement to conventional H-EUS was very useful for the differential diagnosis of GB wall thickening. In addition, inter-observer agreement for CH-EUS was substantial, whereas H-EUS was moderate. A specific contrast enhancement mode was used to highlight contrast microbubbles on CH-EUS. This was called ExPHD mode in the Aloka system used in our study. In this specific mode for contrast enhancement, tissue structures became dark and obscure, although contrast microbubbles were clearly imaged as high echoic dots, compared with conventional B-mode (THE mode). Therefore, we consider the reason for the improvement of inter-observer agreement on CH-EUS to be due to the distribution pattern of contrast (inhomogeneous or homogenous) on CH-EUS. This was a strong predictive factor of malignant GB wall thickening and was simple to interpret, compared with the interpretation of disrupt GB wall layer on H-EUS. Thus, H-EUS followed by CH-EUS might offer examiners with various levels of experience in EUS an opportunity to improve the diagnostic accuracy for GB wall thickening.
This study has several limitations. This was a non-randomized retrospective cohort study at single center. There is a possibility of patient’s selection bias, and the sample size was small. To clarify the utility of CH-EUS in the diagnosis of GB pathologies, prospective comparative trials of CH-EUS with MDCT or MRI were warranted.
In conclusion, our study examined the role of CH-EUS in the differential diagnosis for GB wall thickening. H-EUS followed by CH-EUS is a promising, reliable modality for the differential diagnosis of GB wall thickening. In particular, the inhomogeneous enhanced pattern on CH-EUS was a strong predictor of malignant GB wall thickening. Diagnostic accuracy could be improved by the addition of contrast enhancement to conventional H-EUS. Further larger prospective studies are warranted.
Abbreviations
- GB:
-
Gallbladder
- CH-EUS:
-
Contrast-enhanced harmonic EUS
- H-EUS:
-
Harmonic EUS
- US:
-
Ultrasonography
- THE:
-
Tissue harmonic echo
- CT:
-
Computed tomography
- ExPHD:
-
Extended pure harmonic detection
References
Joo I, Lee JY, Kim JH, et al. Differentiation of adenomyomatosis of the gallbladder from early-stage, wall-thickening-type gallbladder cancer using high resolution ultrasound. Eur Radiol. 2013;23:730–738.
Jan JY, Kim SW, Lee SE, et al. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann Surg. 2009;250:943–949.
Muguruma N, Okamura S, Ichikawa S, et al. Endoscopic ultrasonography in the diagnosis of gallbladder wall lesions in patients with gallstones. J Clin Ultrasound. 2001;29:395–400.
Kim HJ, Park JH, Park DI, et al. Clinical usefulness of endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig Dis Sci. 2012;57:508–515.
Yoshimitsu K, Honda H, Jimi M, et al. MR diagnosis of adenomyomatosis of the gallbladder and differentiation from gallbladder carcinoma: importance of showing Rokitansky-Aschoff sinuses. AJR. 1999;172:1535–1540.
Furlan A, Ferris JV, Hosseinzadeh K, Borhani AA. Gallbladder carcinoma update: multimodality imaging evaluation, staging, and treatment option. AJR. 2008;191:1440–1447.
Piligrim CHC, Groeschl RT, Pappas SG, Gamblin TCG. An often overlooked diagnosis: imaging features of gallbladder cancer. J Am Coll Surg. 2013;216:333–339.
Kim SJ, Lee JM, Lee JY, et al. Analysis of enhancement pattern of flat gallbladder wall thickening on MDCT to differentiate gallbladder cancer from cholecystitis. AJR. 2008;191:765–771.
Dietrich CF, Ignee A, Frey H. Contrast-enhanced endoscopic ultrasound with low mechanical index: a new technique. Z Gastroenterol. 2005;43:1219–1223.
Kitano M, Sakamoto H, Matsui U, et al. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video). Gastrointest Endosc. 2008;67:141–150.
Imazu H, Uchiyama Y, Matsunaga K, et al. Contrast-enhanced harmonic EUS with novel ultrasonographic contrast (Sonazoid) in the preoperative T-staging for pancreaticobiliary malignancies. Scand J Gastroenterol. 2010;45:732–738.
Gong TT, Hu DM, Zhu Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: a meta-analysis. Gastrointest Endosc. 2012;76:301–309.
Choi JH, Seo DW, Choi JH, et al. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos). Gastrointest Endosc. 2013;78:484–493.
Park CH, Chung MJ, Oh TG, et al. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg Endosc. 2013;27:1414–1421.
Maguchi H. Education and training for endoscopic ultrasonography in Japan. Dig Endosc. 2004;16:S148–S152.
Hong HS, Han JK, Kim TK, et al. Ultrasonographic evaluation of the gallbladder: comparison of fundamental, tissue harmonic, and pulse inversion harmonic imaging. J Ultrasound Med. 2001;20:35–41.
Ishikawa H, Hirooka Y, Itoh A, et al. A comparison of imaging quality between tissue harmonic imaging and fundamental imaging with an electronic radial scanning echoendoscope in the diagnosis of pancreatic diseases. Gastrointest Endosc. 2003;57:931–936.
Piscaglia F, Nolsoe C, Dietrich CF, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59.
Sontum PC, Ostensen J, Dyrstad K, Hoff L. Acoustic properties of NC100100 and their relation with the microbubble size distribution. Invest Radiol. 1999;34:268–275.
Dietrich CF. Contrast-enhanced low mechanical index endoscopic ultrasound (CELMI-EUS). Endoscopy 2009; 41:02E43–02E44.
Napoleon B, Alvarez-Sanchez MV, Gincoul R, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564–570.
Kitano M, Kudo M, Yamao K, et al. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303–310.
Matsubara H, Itoh A, Kawashima H, et al. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas. 2011;40:1073–1079.
Imazu H, Kanazawa K, Mori N, et al. Novel quantitative perfusion analysis with contrast-enhanced harmonic EUS for differentiation of autoimmune pancreatitis from pancreatic carcinoma. Scand J Gastroenterol. 2012;47:853–860.
Gheonea DJ, Streba CT, Ciurea T, Saftoiu A. Quantitative low mechanical index contrast-enhanced endoscopic ultrasound for the differential diagnosis of chronic pseudotumoral pancreatitis and pancreatic cancer. BMC Gastronterol. 2013;13:2.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imazu, H., Mori, N., Kanazawa, K. et al. Contrast-Enhanced Harmonic Endoscopic Ultrasonography in the Differential Diagnosis of Gallbladder Wall Thickening. Dig Dis Sci 59, 1909–1916 (2014). https://doi.org/10.1007/s10620-014-3115-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3115-5