Abstract
Background and Aims
The anti-inflammatory and reparative properties of mesenchymal stem cells (MSCs) make them a promising tool for treating immune-mediated and inflammatory disorders. However, whether MSCs can be used for treatment of inflammatory bowel disease (IBD) still remains unclear. In this study, a dextran sulfate sodium (DSS)-induced mouse colitis model was used to test the hypothesis that infused bone marrow-derived MSCs could exert anti-inflammatory effects against experimental colitis.
Methods
DSS-induced colitis mice were injected with 1 × 106 MSCs [in phosphate-buffered saline (PBS)] via the tail vein. Control colitis mice received PBS alone. To trace the injected cells in vivo, MSCs were labeled with chloromethyl-benzamidodialkylcarbocyanine (CM-DiI). On day 15 of the experiment, the colon was sectioned and examined for histopathological changes. Pro-inflammatory cytokines [tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β] in the inflamed colon were analyzed by real-time reverse-transcription polymerase chain reaction (RT-PCR). Serum values of TNF-α in mice were evaluated quantitatively by enzyme-linked immunosorbent assay (ELISA) analysis.
Results
DSS-induced colitis showed symptoms similar to ulcerative colitis in humans, including body weight loss, bloody diarrhea, mucosal ulceration, and shortening of the colon. Bone marrow-derived MSCs significantly ameliorated the clinical and histopathologic severity of DSS colitis compared with non-MSC control. Pro-inflammatory cytokines in both the inflamed colon (TNF-α, IL-1β) and serum (TNF-α) were downregulated in MSC-treated mice in contrast to control. CM-DiI-labeled MSCs accumulated in inflamed regions of the colon, mainly in the submucosa.
Conclusions
Systemic infusion of bone marrow-derived MSCs may exert therapeutic efficacy on acute DSS-induced colitis in mice through their anti-inflammatory effects, which demonstrates the feasibility of using bone marrow-derived MSCs to treat IBD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic relapsing disorder associated with uncontrolled inflammation within the gastrointestinal tract. Currently, the widely accepted hypothesis on the cause of IBD is a disturbed interaction of the host immune system with the commensal microflora and other luminal antigens, which leads to ongoing mucosal inflammation [1]. In addition, there is mounting evidence that impaired epithelial barrier function is critical in IBD pathophysiology [2, 3]. Despite the significant advances in clinical management for IBD, none of the existing therapeutic agents have a curative effect and few “nontoxic” therapeutic options are available to modulate intestinal inflammation. It is still challenging to develop new therapeutic approaches that are more effective and have fewer side-effects for IBD.
Bone marrow contains pluripotent mesenchymal stem cells (MSCs) that can be reproducibly and safely isolated and purified. MSCs, lacking major histocompatibility II and several costimulatory molecules, are relatively immunoprivileged and have the ability to escape immune recognition, or at least are hypoimmunogenic [4, 5]. These stem cells are capable of proliferating quickly in vitro, and have the potential to differentiate along the osteogenic, chondrogenic, and adipogenic lineages both in vitro and in vivo [6–8]. In the last several years, it has been proposed that MSCs could serve as an effective therapeutic agent for tissue repair [9, 10]. More recently, MSCs were shown to modulate innate and adaptive immunity [11–14]. These cells may inhibit the function of the major immune cell populations, including dendritic cells, T cells, B cells, and natural killer cells. The immunomodulatory and anti-inflammatory properties of MSCs have been tested in a variety of animal models and have been applied in specific clinical settings. The most exciting data are from in vivo studies conducted in humans demonstrating successful treatment of life-threatening graft-versus-host disease (GVHD) with MSCs [15–17].
In view of their anti-inflammatory properties, as well as of their role in tissue repair, MSCs represent a promising tool for treating immune-mediated and inflammatory disorders. However, whether MSCs can be used for treatment of IBD still remains unclear. In this study, we investigated the role of infused mouse bone marrow-derived MSCs (BM-MSCs) in a dextran sulfate sodium (DSS)-induced mouse colitis model and explored the possible mechanisms by which MSCs exerted anti-inflammatory effects against experimental colitis.
Materials and Methods
Mice
Specific pathogen-free male BALB/c (H-2d) mice (aged 6–7 weeks, weighing between 19 and 21 g) were purchased from the Experiment Animal Center of Sun Yat-sen University in Guangzhou, China. The experimental protocol was approved by the Sun Yat-sen University Animal Care and Research Committee. All animals received care in accordance with National Institutes of Health guidelines for the use of experimental animals.
Isolation and Expansion of BM-MSCs
Three-week-old male BALB/c (H-2d) mice were killed by cervical dislocation and immersed in 75 % ethanol for 5 min. The bilateral femurs and tibias were aseptically excised, stripped of connective tissues, and then stored in PBS supplemented with 1× penicillin/streptomycin on ice. The ends of the bones were trimmed, and the bone marrow was flushed out of the marrow shafts by using complete cell culture medium consisting of α-minimum essential medium (α-MEM; Gibco, Invitrogen Corp., Grand Island, NY, USA) supplemented with 10 % fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT, USA). The bone marrow cells were dispersed by gently drawing medium and cells up and down, filtered through a 70-μm cell strainer (BD Biosciences, Mississauga, ON, Canada), and then centrifuged for 5 min at 352 × g. After removal of the supernatant, cells were plated in plastic tissue culture flask (Corning, NY, USA) at concentration of 106 cells/cm2 using mouse Complete Mesencult as medium (Stem Cell Technologies, Vancouver, BC, Canada). Nonadherent cells were removed after 48 h, and fresh medium was added. Thereafter, the medium was refreshed every 3–4 days, for about 1–2 weeks; then, only adherent cells were collected following 5-min incubation at 37 °C with 0.25 % trypsin solution (Gibco, Invitrogen Corp., Grand Island, NY, USA). Cells were replated at density of 3 × 103 cells/cm2 and passaged at approximately 80 % confluency. Third- and fourth-passage cells were used for all experiments.
Cell-Surface Marker Analysis of BM-MSCs
Cells were trypsinized, collected, and incubated for 30 min in the dark at 4 °C with fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD29, CD45, major histocompatibility complex (MHC) class I, MHC class II or phycoerythrin (PE)-conjugated anti-mouse CD34, CD44. Afterward, they were washed twice with PBS, resuspended in 1 mL PBS, and immediately analyzed. Detection of PE and FITC labeling was accomplished by flow cytometry (FACSCalibur flow cytometer; Becton, Dickinson and Company, Franklin Lakes, NJ, USA). At least 10,000 events were collected. WinMDI 2.9 software was used to create the histograms.
Animal Studies
To induce colitis, the mice received 4 % (w/v) dextran sulfate sodium (DSS; molecular weight, 36,000–50,000; International Laboratory, USA) in their drinking water from day 1 to day 7, according to the published protocol [18]. On day 8, all animals returned to drinking plain water. Syngeneic BM-MSCs (1 × 106 cells in 0.3 ml PBS) were injected via the tail vein in the DSS-treated mice, respectively, on day 2, day 5, and day 8. In control, DSS-treated mice received 0.3 ml PBS without BM-MSCs per time. Weight measurements, evaluation of stool consistency, and fecal occult blood test were performed daily. A validated clinical disease activity index (DAI) ranging from 0 to 4 was calculated using the parameters described previously [19]. The mice were sacrificed on day 15, and blood was collected by cardiac puncture for analysis of serum inflammatory mediators. The entire colon was removed from the cecum to the anus. Colon length was measured before dividing the colon for histology and evaluation of expression of inflammatory cytokines.
Histology
Histopathological analysis was performed on three samples of distal colon for each animal. Colon samples were fixed in 10 % formalin solution, embedded in paraffin, and sliced into sections of 4 μm thickness before staining with hematoxylin and eosin. Histological evaluation was completed in a double-blind fashion by two investigators using a scoring system [20]. Briefly, histology was scored as follows: Epithelium (E): 0, normal morphology; 1, loss of goblet cells; 2, loss of goblet cells in large areas; 3, loss of crypts; 4, loss of crypts in large areas. Infiltration (I): 0, no infiltrate; 1, infiltrate around crypt basis; 2, infiltrate reaching to L. muscularis mucosa; 3, extensive infiltration reaching the L. muscularis mucosa and thickening of the mucosa with abundant edema; 4, infiltration of the L. submucosa. The histological score was defined as the sum of the two parameters (total score = E + I).
In Vivo Tracing of BM-MSCs by CM-DiI Label
To clarify the in vivo localization of infused BM-MSCs in the inflamed colonic tissues, we used chloromethyl-benzamidodialkylcarbocyanine (CM-DiI, MW 1,051.50; Molecular Probes, Eugene, OR, USA) as labeling agent. Briefly, BM-MSCs were incubated with 2 μg/ml CM-DiI (C7000) for 5 min at 37 °C, and then for an additional 15 min at 4 °C. Labeled BM-MSCs were administered via the tail vein using the same protocol described above. Frozen tissue sections obtained from the colon of the inflamed region and the proximal colon of the noninflamed region were observed with a fluorescence microscope (E600; Nikon, Tokyo, Japan), using the tetramethylrhodamine isothiocyanate filter set (excitation, 540 nm; dichroic mirror, 565 nm). Micrographs were taken with a digital camera system.
Detection of Serum Pro-inflammatory Cytokine
Blood samples were centrifuged at 1,000 × g for 15 min, and the sera were stored at −80 °C until cytokine determination. The TNF-α concentrations in the sera were determined by mouse TNF-alpha ELISA kit (BioSource, Inc., Camarillo, CA, USA) according to the manufacturer’s protocol.
Determination of mRNA Expression of Inflammatory Cytokines in the Colon
Colon segments were frozen in liquid nitrogen until use at −80 °C. Total RNA from individual colons was isolated by using TRI reagent (Invitrogen) according to the manufacturer’s protocol. Reverse transcription of total RNA was performed with ReverTra Ace®qPCR RT kit (FSQ-101, Toyobo). Then, a real-time PCR reaction with final volume of 50 ml was completed using the SYBR® Green Realtime PCR Master Mix (QPK-201, Toyobo). All reactions were performed in triplicate, and the thermal cycling conditions were 1 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 15 s at 58 °C, and 45 s at 72 °C using the Applied Biosystems 7500 real-time PCR system.
Primer pairs were designed according to data from GenBank and evaluated by nucleotide BLAST standard search to avoid cross-reactivity with other known sequences. The GenBank accession numbers chosen to design primers were CCDS28691 for TNF-α, CCDS16726 for IL-1β, and CCDS19833 for β-actin. The designed sequences were as follows: 5′-GAC AAG CCT GTA GCC CAC GT-3′ (TNF-α sense primer), 5′-ACA AGG TAC AAC CCA TCG GC-3′ (TNF-α antisense primer); 5′-TGA CGG ACC CCA AAA GAT GA-3′ (IL-1β sense primer), 5′-ACA GCT TCT CCA CAG CCA CA-3′ (IL-1β antisense primer); 5′-CTT CAA CAC CCC AGC CAT GT-3′ (β-actin sense primer), 5′-TGG CGT GAG GGA GAG CAT AG-3′ (β-actin antisense primer). The amplified products are all 146 bp in length for TNF-α, IL-1β, and β-actin. For relative quantification, we compared the amount of target normalized to the β-actin amplification.
Statistical Analysis
All numerical data are expressed as mean ± standard error of mean (SEM); n refers to the number of animals. Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 15 and Microsoft EXCEL version 2003. To compare two groups, parametric and nonparametric analyses were performed using an unpaired t test and the Mann–Whitney U test, respectively. p values <0.05 were considered statistically significant.
Results
Characterization of Isolated BM-MSCs
After plating of whole marrow cells and removal of nonadherent cells, cells with a spindle-shape characteristic of MSCs appeared to gradually predominate in the cultures. At passage 3 or 4, cultures formed homogeneous spindle-shaped cell colonies. Flow cytometric analysis confirmed that BM-MSCs do not express hematopoietic surface markers CD34 and CD45 but stain positive for CD29, CD44, and MHC class I (Fig. 1).
Flow cytometric analysis of cell-surface antigens of BALB/c mouse bone marrow-derived mesenchymal stem cells. Cells were stained with monoclonal antibodies conjugated to FITC or PE and thereafter analyzed with a FACSCalibur cytometer and WinMDI 2.9 software. These data are representative of four experiments
Therapeutic Efficacy of Infused MSCs for DSS-Induced Colitis
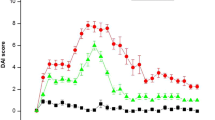
In BALB/c mice, administration of 4 % DSS for 7 days resulted in reproducible histologic inflammation mainly in the distal colon. All DSS-treated mice developed clinical signs similar to ulcerative colitis in humans, including body weight loss, bloody diarrhea, mucosal ulceration, and shortening of the colon. Mice receiving no DSS in drinking water did not show any signs of colitis and gained weight over time. In DSS-induced colitis mice, BM-MSCs were transfused on day 2, day 5, and day 8. The individual scores of weight loss (DSS 4 % + PBS, 2.6 ± 0.5 versus DSS 4 % + MSCs, 1.4 ± 0.4, p = 0.03), stool consistency (DSS 4 % + PBS, 1.6 ± 0.3 versus DSS 4 % + MSCs, 0.8 ± 0.15, p = 0.04), and rectal bleeding (DSS 4 % + PBS, 1.6 ± 0.4 versus DSS 4 % + MSCs, 0.8 ± 0.19, p = 0.04) after 15 days were significantly improved in the MSC-treated group compared with PBS-treated control (Fig. 2).
Clinical therapeutic efficacy of administration of exogenous MSCs on relative body weight, stool consistency, and rectal bleeding. BALB/c (H-2d) mice received 4 % dextran sulfate sodium (DSS) with drinking water for 7 consecutive days. Syngeneic bone marrow-derived mesenchymal stem cells (MSCs) were infused via the tail vein on days 2, 5, and 8. DSS-induced colitis mice receiving no MSCs served as control. The individual score, consisting of weight loss, stool consistency, and rectal bleeding, is broken down at day 15. Data are represented as mean ± SEM (n = 6 mice per group, results are from three separate experiments). *p < 0.05 versus PBS-treated group
Mice were killed after 15 days, and the entire colon was placed on cellulose without tension for measurement of length. DSS addition led to a reduced colon length compared with normal control group. Infusion of BM-MSCs reduced the extent of DSS-induced colon shortening (Fig. 3). A significant difference of colon length was observed between 4 % DSS + PBS and 4 % DSS + MSCs (6.62 ± 0.31 cm versus 7.24 ± 0.36 cm, p = 0.02).
Effect of MSC therapy on a macroscopic findings and b colon length. BALB/c (H-2d) mice received 4 % dextran sulfate sodium (DSS) with drinking water for 7 consecutive days. Syngeneic bone marrow-derived mesenchymal stem cells (MSCs) were infused via the tail vein on days 2, 5, and 8. DSS-induced colitis mice receiving no MSCs served as control. A representative preparation of the colon of a non-DSS-fed mouse is given in A-Normal. Treatment with MSCs significantly inhibited shortening of the colon 15 days after induction of colitis with dextran sulfate sodium (DSS). Values are mean ± SEM (n = 6 mice per group, results are from three separate experiments). *p < 0.05 versus DSS 4 % + PBS and normal control
Histological Improvement of DSS-Induced Colitis by MSC Treatment
Consistent with the previous findings, histological changes in DSS-induced colitis were mainly observed in the distal colon with severity becoming progressively less towards the proximal site. Thus, we evaluated the histological severity of colitis in the distal colon. Treatment with MSCs reduced the extent of the inflamed area in the rectum. The crypt damage and infiltration of inflammatory cells were decreased compared with the control group (Fig. 4). In the MSC-treated group, the histological colitis score was significantly reduced compared with the vehicle control group (DSS 4 % + PBS, 4.75 ± 0.48 versus DSS 4 % + MSCs, 2.5 ± 0.28, p = 0.007; Fig. 5).
Histologic improvement after MSCs therapy in murine colitis. Representative H&E-stained paraffin sections of distal colon in mice 15 days after induction of colitis with dextran sulfate sodium (DSS). a (×40) and b (×100): PBS-treated group; c (×40) and d (×100): MSC-treated group. Treatment with MSCs reduced the crypt damage and infiltration of inflammatory cells within the lamina propria and the submucosa. (The photo is representative of three separate experiments)
Effect of MSC therapy on histologic colonic severity. Mice fed with 4 % dextran sulfate sodium (DSS) and receiving MSC therapy showed a significant lower histologic severity score comprising depth of inflammation infiltration and crypt damage compared with PBS-treated control. Values are mean ± SEM (n = 6 mice per group, results are from three separate experiments). *p < 0.01 versus DSS 4 % + PBS and normal control
Homing of Infused BM-MSCs to the Inflamed Colon Induced by DSS
Infused BM-MSCs were labeled with CM-DiI to detect in vivo localization of MSCs in inflamed colon. The fluorescein signal was mainly observed in the submucosa of the inflamed colon (Fig. 6). In noninflamed colonic segments or in control mice, no labeled MSCs were detected.
Effects of MSCs on Serum TNF-α Production
TNF-α is considered one of the important inflammatory mediators playing a key role in the pathogenesis of IBD. For this reason, we evaluated the effect of administration of MSCs on serum TNF-α production in the DSS-induced colitis mice. As shown in Fig. 7, the serum level of TNF-α, as assessed by ELISA assay, was markedly reduced in MSC-treated mice with DSS-induced colitis in comparison with PBS-treated control group (DSS 4 % + MSCs, 40.67 ± 4.26 pg/ml versus DSS 4 % + PBS, 85.41 ± 6.91 pg/ml, p < 0.01).
mRNA Expression of Inflammatory Mediators in Colonic Tissues
To provide further insight into the molecular mechanisms underlying the suppression of colitis by MSC administration, messenger RNA (mRNA) expression levels of TNF-α and IL-1β in the colon were measured by real-time RT-PCR. As shown in Fig. 8, a significant increase in mRNA expression of TNF-α and IL-1β was observed in PBS-treated mice with DSS-induced colitis compared with normal control group (p < 0.01). Treatment with MSCs resulted in a reduction in mRNA expression of TNF-α and IL-1β in mice with DSS-induced colitis (p < 0.01).
Colonic mRNA expression of inflammatory mediators (TNF-α, IL-1β). The distal colon tissue from MSC-treated and from PBS-treated mice was prepared on day 15 after DSS exposure, and real-time RT-PCR was performed as described in the “Materials and Methods” section. Values are mean ± SEM. *p < 0.01 versus DSS 4 % + PBS and normal control
Discussion
Although animal models of IBD do not represent the complexity of human disease, they are valuable tools for studying many important disease aspects that are difficult to address in humans, such as the effect of emerging therapeutic strategies. In our study, the colitis mouse model chemically induced by DSS has shown symptoms similar to ulcerative colitis (UC) in humans, including body weight loss, bloody diarrhea, mucosal ulceration, and shortening of the colon.
The precise mechanism of UC still remains unknown, but there is accumulating evidence that increase of pro-inflammatory cytokines such as TNF-α and IL-1β within colonic tissues plays an important role in the pathogenesis of UC [21]. MSCs have been shown to decrease expression of a wide panel of inflammatory cytokines and chemokines, decrease infiltration of inflammatory cells, and increase expression of growth factors in models of inflammation [22, 23]. In addition, MSCs have also been reported to influence the cytokine secretion profile of the different T cell subsets, as their addition to an in vitro activated T cell culture results in decreased production of the pro-inflammatory cytokines interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-17 and increased levels of anti-inflammatory cytokines such as IL-4 and IL-10 [24–26].
In the present study, we used bone marrow-derived MSCs to treat DSS-induced colitis in immunocompetent mice. The colitis mice received one exogenously infused dose of MSCs at 1 × 106 cells for three times per mouse. Body weight was improved in MSC-treated group compared with PBS control. A marked histological improvement in the inflamed area of the colon was also noted. The severity of the disease was significantly reduced following treatment with MSCs. Furthermore, serum assays showed reduced TNF-α level in blood samples following MSCs treatment of mice in which DSS was used to induce colitis. Also, information gathered from real-time RT-PCR results suggests that the stem cells downregulated inflammation markers such as TNF-α (p < 0.01) and IL-1β (p < 0.01) at mRNA level. These data suggest that MSCs may mediate some of these benefits through an anti-inflammatory mechanism.
Another intriguing characteristic of MSCs is their apparent ability to home into sites of injury when delivered systemically. In the last several years, it has been proposed that MSCs could serve as a powerful “natural system for tissue repair,” and they could be effective therapeutic agents in a variety of experimental models of tissue injuries [7, 27, 28]. Previous studies using animal models of IBD have shown that transplanted bone marrow cells can contribute to tissue repair by forming epithelial cells [29] and activated myofibroblasts [30]. A recent study by Khalil et al. [31] demonstrated that exogenously administered murine CD34-negative stem cells from mouse bone marrow and peripheral blood could facilitate mucosal repair in a model of DSS-induced colitis. The authors found that the transplanted stem cells were detected predominantly in the submucosa of the damaged colon epithelium, and epithelial repair in experimental IBD was mediated either by induction of improved vasculogenesis or by differentiation of the stem cells into endothelial cells. In our study, homing of the delivered MSCs to the inflamed colon was confirmed by in vivo cell tracking. Further investigations are needed to evaluate the potential of MSCs to differentiate into various types of cells as replacement and repair parts for damaged tissues.
Although there is general agreement that MSCs can be cultured in vitro with no risk of malignant transformation [32], several studies have shown that, in some experimental models, administered animal-derived MSCs can enhance tumor growth [33]. Therefore, the safety profile of this procedure and possible long-term adverse effects, including uncontrolled proliferative processes and development of neoplasms, require further and thorough examinations.
In conclusion, systemic infusion of bone marrow-derived MSCs may exert therapeutic efficacy on acute DSS-induced colitis in mice through their anti-inflammatory effects. Our present findings demonstrate the feasibility of using bone marrow-derived MSCs to treat IBD.
References
Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640.
Xavier RJ, Podilsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434.
Kucharzik T, Maaser C, Lügering A, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1083.
Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434.
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896.
Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319.
Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347.
Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford). 2008;47:126–131.
Brooke G, Cook M, Blair C, et al. Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol. 2007;18:846–858.
Păunescu V, Deak E, Herman D, et al. In vitro differentiation of human mesenchymal stem cells to epithelial lineage. J Cell Mol Med. 2007;11:502–508.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736.
Fibbe WE, Nauta AJ, Roelofs H. Modulation of immune responses by mesenchymal stem cells. Ann N Y Acad Sci. 2007;1106:272–278.
Noël D, Djouad F, Bouffi C, et al. Multipotent mesenchymal stromal cells and immune tolerance. Leuk Lymphoma. 2007;48:1283–1289.
Zhang B, Liu R, Shi D, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113:46–57.
Rinden O, Uzunel M, Rassmuson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397.
Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441.
Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus host disease: a phase II study. Lancet. 2008;371:1579–1586.
Wirtz S, Neufert C, Weigmann B. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546.
Naito Y, Takagi T, Kuroda M, et al. An orally active matrix metalloproteinase inhibitor, ONO-4817, reduces dextran sulfate sodium induced colitis in mice. Inflamm Res. 2004;53:462–468.
Obermeier F, Kojouharoff G, Hans W, et al. Interferon-gamma (IFN-gamma) and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–245.
Ogata H, Hibi T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr Pharm Des. 2003;9:1107–1113.
Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose derived mesenchymal stem cells. Arthr Rheum. 2009;60:1006–1019.
Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946.
Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stemcells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761.
Prevosto C, Zancolli M, Canevali P, et al. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888.
Kong QF, Sun B, Bai SS, et al. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J Neuroimmunol. 2009;207:83–91.
Phinney D, Prockop D. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdiffererentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902.
Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–116.
Komori M, Tsuji S, Tsujii M, et al. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005;13:109–118.
Brittan M, Chance V, Elia G, et al. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology. 2005;128:1984–1995.
Khalil PN, Weiler V, Nelson PJ, et al. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944–954.
Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149.
Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 30872461) and Guangdong Natural Science Foundation (no. 8151008901000107).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiao-Wen He and Xiao-Sheng He contributed equally to this work.
Rights and permissions
About this article
Cite this article
He, XW., He, XS., Lian, L. et al. Systemic Infusion of Bone Marrow-Derived Mesenchymal Stem Cells for Treatment of Experimental Colitis in Mice. Dig Dis Sci 57, 3136–3144 (2012). https://doi.org/10.1007/s10620-012-2290-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2290-5