Abstract
This study aims to investigate the expression and significance of EphA2 and EphrinA-1 in human gastric adenocarcinoma progression and prognosis. The expression of EphA2 and EphrinA-1 was detected in the cell lines and tissues of gastric adenocarcinoma. Different expression levels of EphA2 and EphrinA-1 were found in two cell lines. The expression of EphA2 and EphrinA-1 was significantly higher in gastric adenocarcinoma tissues than in normal tissues. Statistical analysis showed a significant correlation of EphA2 expression with the depth of tumor invasion, tumor-node-metastasis (TNM) stages, and lymph node metastasis. EphrinA-1 over-expression was significantly correlated with TNM stages and lymph node metastasis, while EphA2 expression was found to be an independent prognostic factor of postoperative gastric adenocarcinoma. In conclusion, the increased expression of EphA2 and EphrinA-1 plays an important role in the progression of human gastric adenocarcinoma, in which elevated EphA2 expression is an independent factor that indicates poor prognosis in postoperative gastric adenocarcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer remains the fourth most common malignancy and the second leading cause of cancer-related death in the world in 2005, accounting for an estimated 930,000 new cases and 700,000 deaths per year [1]. The current best approach for treating gastric cancer is the complete surgical removal of the tumor with the adjacent lymph nodes. However, the efficacy of this therapeutic approach as well as chemotherapy, hormone, and radiotherapy is very limited. The prognosis of gastric cancer is poor, with an estimated relative 5-year survival rate of <25% worldwide [2, 3]. Invasion, metastasis, and recurrence are the major causes of death in gastric cancer patients and the key factors affecting clinical treatment and prognosis. However, the mechanisms of gastric cancer progression, invasion, and metastasis remain unclear and, therefore, need further investigation.
Receptor tyrosine kinases (RTKs), the common products of transforming oncogenes, have been widely used as indicators in the genesis and progression of human tumors [4]. Until now, the erythropoietin-producing hepatocellular (Eph) receptors have been recognized as the largest family of RTKs, which contains 14 distinct receptors, including eight identified ligands. Eph receptors are involved in many cellular processes through combining with their ephrin ligands, including neural development, angiogenesis, and vascular network assembly [5]. In the Eph family, EphA2 that locates on human chromosome 1p36.1 is expressed at a lowest level in epithelial cells [6]. However, such expression increased in carcinoma cells, and the functional alterations of Eph receptor kinases are prevalent in many tumors, including breast, lung, colorectal, and esophageal cancers [7–10]. It is reported that high EphA2 expression level was correlated with the metastasis and poor prognosis in both lung cancer and esophageal cancer [8, 10]. More and more evidence has suggested that the over-expression of EphA2 plays an important role in the process of many human cancers [11, 12]. However, the expression and clinical significance of EphA2 and its ligand EphrinA-1 in human gastric adenocarcinoma progression and prognosis remains unclear.
In the present study, we investigated the expression and clinical significance of EphA2 and EphrinA-1 in two different cell lines and postoperative gastric adenocarcinoma tissues.
Materials and Methods
Cell Culture
Two human gastric adenocarcinoma cell lines AGS and SGC-7901 were purchased from the Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences (Shanghai, China). The cancer cell lines were derived from gastric adenocarcinoma with different aggressive behaviors, such as highly aggressive cancer cell line AGS and moderately aggressive cancer cell line SGC-7901 [13, 14]. The cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA), 100 IU/ml penicillin and 100 μg/ml streptomycin at 37°C, and 5% CO2 in a humidified atmosphere.
Patients and Tissue Preparation
During the period from January 2000 to March 2003, 176 patients with gastric adenocarcinoma, including 111 males and 65 females, aged between 34–78 years (59.8 ± 12.1 years), in the Department of General Surgery of Xiangya Hospital in Central South University were selected (Table 1). Radical resection was performed on these patients. Their cancer tissues were excised and fixed in 10% neutral buffered formalin and then embedded in paraffin blocks. Twenty-two cases of gastric adenocarcinoma and paired normal tissues (6-cm distances from the cancer) were randomly collected from each patient during operation from November 2007 to March 2008, of which gastric adenocarcinoma and paired normal tissues were validated by a pathologist. One half of the specimen was snap-frozen immediately and stored at −80°C for RNA extraction. The other half was fixed in formalin and embedded in paraffin for immunohistochemical staining. All of the patients had not received either chemotherapy or radiotherapy before surgery. The study was approved by the Research Ethics Committee of Central South University, China. Informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
Real-Time Reverse Transcriptase Polymerase Chain Reaction
The EphA2 and EphrinA-1 mRNA levels in two gastric adenocarcinoma cell lines and 22 paired case tissues were measured by real-time reverse transcriptase polymerase chain reaction (RT-PCR). The total RNA of cell lines and tissues were extracted using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The concentration of RNA was measured by a spectrophotometer. The cDNA pool was synthesized using 1 μg of total RNA and TaqMan® Reverse Transcription Reagents (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. According to Herath et al. [16], primers were synthesized by the TaKaRa Biotechnology (Dalian, China). The primers for EphA2, EphrinA-1, and 18S rRNA (internal control) were designed as follows: EphA2 primer, forward 5′-GGGACCTGATGCAGAACATC-3′, reverse 5′-AGTTGGTGCGGAGCCAGT-3′; EphrinA-1 primer, forward 5′-CCGGAGAAGCTGTCTGAGAA-3′, reverse 5′- GGTTTGGAGATGTAGTAGTAGCTGTG-3′; 18S rRNA forward 5′-GACTCAACACGGGAAACCTC-3′, reverse 5′-AGCATGCCAGAGTCTCGTTC-3′. EphA2, EphrinA-1, and 18S rRNA genes were amplified from the cDNA pool using gene-specific primers and Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) in an ABI PRISM® 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The PCR cycling conditions in this study were as follows: 50°C/10 min, and 95°C/30 s, 60°C/1 min, 72°C/1 min for 50 cycles. Quantitative RT-PCR was done at least thrice, including a no-template control as the negative control. The relative levels of EphA2 and EphrinA-1 mRNA expression were represented as the ratio of EphA2 or EphrinA-1–18S rRNA and were calculated from the standard curve.
Western Blot
The cells were lyzed in extraction solution as previously described [17]. Each 50 μg of SDS sample was loaded onto 12% SDS-PAGE gels and blotted onto an Optitran BA-S85 membrane (Schlischer & Schell, Dassel, Germany). The transferred membranes were subsequently incubated with rabbit antibody against EphA2 (sc-924, dilution 1:200) or EphrinA-1 (sc-911, dilution 1:200) (Santa Cruz, CA, USA) at 4°C overnight. After washing, they were incubated with ECL HRP-linked anti-rabbit IgG (Amersham Biosciences, Piscataway, NJ, USA) for 1 h at room temperature. Bands were visualized by employing the ECL Advance™ Detection System (Amersham Biosciences, Piscataway, NJ, USA). EphA2 or EphrinA-1 protein expression levels were represented as the densitometric ratio of the targeted protein to the housekeeping protein of β-actin. All reported results were the average ratio obtained from three different independent experiments for each cell line.
Immunohistochemistry
The tumor samples and the normal tissue as the control were prepared by pathologists. The paraffin-embedded sections were cut 4 μm thick and then deparaffinized and rehydrated. Immunohistochemical staining was performed to detect the expression of EphA2 or EphrinA-1 using the DAKO EnVision™ System (Dako, Glostrup, Denmark). After proteolytic digestion, the slides were blocked for peroxidase with 2.5% hydrogen peroxide in methanol for 30 min at room temperature and then incubated with primary antibody against EphA2 (sc-924, dilution 1:100) or EphrinA-1 (sc-911, dilution 1:100) (Santa Cruz, CA, USA) overnight at 4°C. After washing, the slices were incubated with peroxidase labeled polymer and substrate chromogen. Finally, the sections were incubated in phosphate buffered solution (PBS) containing diaminobenzidine (DAB) for 5 min at room temperature. The staining results of the targeted proteins were observed under an Olympus microscope. Negative controls were prepared by substituting the primary antibody with non-immune rabbit serum (sc-2338, Santa Cruz, CA, USA). Moreover, EphA2- and EphrinA-1-positive breast cancer tissues were prepared as positive controls for immunohistochemistry. Two independent investigators evaluated and scored the sections in ten random visual fields for each section (double-blinded). The evaluation of the staining results for EphA2 and EphrinA-1 was graded semiquantitatively as previously [18, 19], and the expression intensity scores (0 points = 0–5%; 2 points = 6–50%; 3 points = more than 50%) and positive staining cell scores (1 point = weak intensity; 2 points = moderate intensity; 3 points = strong intensity) were summed. Sum of scores ≥3 points were considered as significant over-expression and noted as positive to simplify the data analysis.
Statistical Analysis
Continuous variables were expressed as χ ± SD. Student’s two-tailed t-test or the χ 2 test was used to compare the expression between the cancer and paired normal tissues. The protein expression and clinicopathologic parameters were compared by the Mann–Whitney U test or χ 2 test. The overall survival duration for 176 cases of postoperative gastric adenocarcinoma was calculated from the date of operation to that of death or 31st March 2008 using the method of Kaplan–Meier. Those patients who died of accidents or other diseases except gastric cancer were excluded. The log-rank with trend test was used for the ordinal datum of univariate analysis and a Cox proportional hazards regression model was used for the multivariate analysis of survival duration. Statistical analyses were performed with SPSS software (version 13.0, SPSS, Inc., Chicago, USA). A P-value < 0.05 was considered to be statistically significant.
Results
Expression of EphA2 and EphrinA-1 in Human Gastric Adenocarcinoma Cell Lines
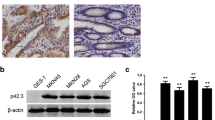
The expression levels of EphA2 and EphrinA-1 mRNA in human gastric adenocarcinoma cell lines AGS and SGC-7901 were detected by real-time RT-PCR (Fig. 1a). The relative expression levels of EphA2 and EphrinA-1 mRNA were higher in AGS (highly aggressive) than that in SGC-7901 (moderately aggressive). Western blot analysis showed that EphA2 and EphrinA-1 protein were highly expressed in the two gastric adenocarcinoma cell lines. However, compared with the SGC-7901 cell line, both EphA2 and EphrinA-1 protein expressed slightly higher in the AGS cell line (Fig. 1b), which is consistent with the result of real-time RT-PCR.
Expression of EphA2 and EphrinA-1 in human gastric adenocarcinoma cell lines AGS and SGC-7901. a The mRNA expression of EphA2 and EphrinA-1 normalized with 18sRNA mRNA quantified by real-time reverse transcriptase polymerase chain reaction (RT-PCR). Columns: three parallel experiments; bars: standard deviation. b The protein expression of EphA2 and EphrinA-1 detected by Western blot, with β-actin as the internal marker
Expression of EphA2 and EphrinA-1 in Human Gastric Adenocarcinoma Tissues
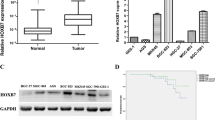
The expression levels of EphA2 and EphrinA-1 mRNA in 22 cases of gastric adenocarcinoma and paired normal tissues were compared by real-time RT-PCR. The relative expression levels of EphA2 mRNA were significantly higher in gastric adenocarcinoma tissues than that in normal tissues (2.092 ± 0.701 vs. 0.781 ± 0.335, t = 22.573, P < 0.001). Similarly, the relative expression levels of EphrinA-1 mRNA were significantly higher in gastric adenocarcinoma tissues than that in normal tissues (1.805 ± 0.620 vs. 0.865 ± 0.369, t = 20.117, P < 0.001). Immunohistochemistry was used to analyze the protein expression of EphA2 and EphrinA-1 in 22 paired cases. Positive EphA2 and EphrinA-1 immunostaining was diffusely distributed throughout the cytoplasm. The positive rate of EphA2 expression was 77.3% (17/22) in gastric adenocarcinoma and 31.8% (7/22) in normal tissues. Positive expression of EphrinA-1 was found in 16 cases of gastric adenocarcinoma tissues (72.7%) and eight cases of normal tissues (36.4%). The χ 2 test indicated that the expression of both EphA2 and EphrinA-1 protein in gastric adenocarcinoma tissues was significantly higher than that in normal tissues (χ 2 = 9.167, P = 0.002 for EphA2; χ 2 = 5.867, P = 0.015 for EphrinA-1). The immunohistochemistry staining result of the EphA2 and EphrinA-1 proteins is shown (Fig. 2).
Immunohistochemistry of EphA2 and EphrinA-1 in human gastric adenocarcinoma and normal tissues. The arrows indicate the positive staining in gastric glandular epithelium (brown–yellow color). a Negative expression of EphA2 in normal tissues (EnVision method, ×400). b Positive expression of EphA2 in gastric adenocarcinoma tissues (EnVision method, ×400). c Negative expression of EphrinA-1 in normal tissues (EnVision method, ×400). d Positive expression of EphrinA-1 in gastric adenocarcinoma tissues (EnVision method, ×400)
Kaplan–Meier survival curves of patients with gastric adenocarcinoma according to the EphA2 and EphrinA-1 immunostaining results. a Patients with EphrinA-1-positive immunostaining had less survival duration than patients with EphrinA-1-negative immunostaining (P = 0.029). b No significant difference in survival duration between patients with EphrinA-1-negative and -positive immunostaining (P = 0.141)
Correlation between EphA2 and EphrinA-1 Expression and Clinicopathologic Features
The correlation between the clinicopathologic features of those patients with gastric adenocarcinoma and expressions of EphA2 and EphrinA-1 protein in 176 cases of gastric adenocarcinoma were shown in Table 1.
There was a significant correlation between EphA2 expression and the depth of tumor invasion (χ 2 = 10.972, P = 0.012). Considering TNM staging, the tumor stage and EphA2 expression were significantly correlated (χ 2 = 12.069, P = 0.007). EphA2 was expressed significantly higher in cancer tissues from those cases with lymph node metastasis than those without lymph node metastasis (87/105 = 82.9% vs. 48/71 = 67.6%, χ 2 = 5.514, P = 0.019). However, EphA2 expression was not associated with the patient’s age, sex distribution, tumor size, and differentiation status (all P > 0.05).
EphrinA-1 expression was found to be correlated with TNM stages (χ 2 = 9.545, P = 0.023). The gastric adenocarcinoma with lymph node metastasis had a higher EphrinA-1 expression rate than that without lymph node metastasis (82/105 = 78.1% vs. 44/71 = 62.0%, χ 2 = 5.414, P = 0.020). However, the EphrinA-1 expression rate was not associated with the patient’s age, sex distribution, tumor size, depth of tumor invasion, or differentiation status (all P > 0.05).
Survival Analysis
The median survival duration in the patients with negative and positive EphA2 expression was 39 and 16 months, respectively. The patients with increased EphA2 expression had shorter survival duration (P = 0.029, Fig. 3a). The median survival duration in those patients with negative and positive EphrinA-1 expression in tumor tissues was 35 and 26 months, respectively. However, the impact of EphrinA-1 expression on patients’ survival duration was not statistically significant (P = 0.141, Fig. 3b).
In the Cox multivariate analysis, there were six important clinical parameters, including the depth of tumor invasion, TNM stages, lymph node metastasis, Helicobacter pylori infection, EphA2 expression, and EphrinA-1 expression. In these parameters, tumor TNM stages, lymph node metastasis, and EphA2 expression were found to be independent prognostic factors of human gastric adenocarcinoma after resection (Table 2, P < 0.05). The prognosis of the patients with EphA2-positive expression was significantly worse than those patients with EphA2-negative expression (hazard ratio = 2.197, 95% CI = 1.521–4.819, P = 0.022).
Discussion
In the present study, we investigated the expression of EphA2 and EphrinA-1 in two different human gastric adenocarcinoma cell lines and clinical specimens of gastric adenocarcinoma after resection. The expression levels of EphA2 and EphrinA-1 were significantly higher in gastric adenocarcinoma tissues compared with the normal tissues. Our result indicated that EphA2 and EphrinA-1 played an important role in the progression of human gastric adenocarcinoma at the cellular and tissue levels. Furthermore, this large clinical sample study proved for the first time that EphA2 was an independent predictive factor in the prognosis of postoperative gastric adenocarcinoma.
In our study, the mRNA and protein expression levels of EphA2 and EphrinA-1 were higher in gastric adenocarcinoma tissues than normal tissues, which was consistent with other reports [20]. Meanwhile, EphA2 and EphrinA-1 were obviously detected in both of the gastric adenocarcinoma cell lines (AGS, SGC-7901). Abraham et al. reported a carcinoma of the urinary bladder in which EphA2 and EphrinA-1 were highly expressed [21]. However, the underlying mechanisms for EphA2 and EphrinA-1 high expression are still unclear. Some studies hypothesized that the high EphA2 expression in tumor cells was due to increased protein stability [22–24]. Unstable cell-to-cell contacts could functionally decrease EphA2 binding possibility with its membrane-anchored ligands, further decreasing ligand-mediated degradation, which contributes to the high expression of EphA2 and EphrinA-1 in tumor cells. Wu et al. found that the mRNA expressions of EphA2 and EphrinA-1 not to be completely correlated with their protein expressions in 20 cases of cervical carcinoma, suggesting that the discordance between mRNA and protein expression levels may be due to post-transcriptional level regulation in cervical carcinoma [25]. However, both mRNA and protein of EphA2 and EphrinA-1 were over-expressed in gastric adenocarcinoma, indicating the pre-transcriptional regulatory mechanism in gastric adenocarcinoma.
Compared to moderately aggressive cancer cell lines, SGC-7901, EphA2, and EphrinA-1 were highly expressed in highly aggressive cancer cell lines AGS, which was consistent with the results observed in ovarian carcinoma cell lines. It has been reported that the EphA2 expression was higher in moderately and highly aggressive ovarian cancer cells than that in poorly invasive ovarian cancer cells [18]. In this study, 176 cases of human gastric adenocarcinoma were investigated and it was found that higher EphA2 and EphrinA-1 protein expression levels correlated with more aggressive behavior, indicating the important roles of EphA2 and EphrinA-1 in the progression and metastasis of human gastric adenocarcinoma after resection, which were consistent with the results observed in ovarian carcinoma [26]. According to Ruoslahti [27], over-expression of the receptor not only affected cell proliferation but also changed their invasive behavior, that is, the receptors were mislocalized in malignant cells with high levels of EphA2, being not able to bind their ephrin ligands, and, therefore, not phosphorylated, resulting in increased extracellular matrix adhesions and higher metastatic potential. Hence, the EphrinA-1-induced down-regulation of EphA2 may provide a new strategy for tumor therapy. Recent studies suggested that the over-expression of EphA2 promoted adherent junction destabilization through a signaling pathway of recruitment of Src kinase, activating LMW-PTP and RhoA GTPase, inhibiting p190 RhoGAP [28]. EphA2 physically and functionally interacted with ErbB2 to amplify Ras/mitogen-activated protein kinase (MAPK) and RhoA signaling in tumor cells, indicating that EphA2 cooperated with ErbB2/Neu to promote mammary adenocarcinoma tumorigenesis and metastatic progression [29]. EphrinA-1 stimulated the growth of EphA2-expressing melanoma cell lines, indicating that EphrinA-1 was an autocrine growth factor for melanomas [30]. However, the possible underlying mechanism needs further investigation to prove its truth.
As revealed by univariate analysis, the patients with positive EphA2 staining had significantly poorer survival compared with those patients with negative EphA2 expression. More importantly, the Cox multivariate analysis indicated that the EphA2 protein expression was the independent prognostic factor in human gastric adenocarcinoma after resection. High EphA2 expression has been previously reported to be predictive for a shorter overall survival in human ovarian carcinoma [26]. However, our study was the first large clinical sample study regarding the predictive factor of EphA2 in gastric adenocarcinoma. As a result, a preoperative determination of EphA2 expression may be useful in predicting the therapeutic effect and postoperative survival of human gastric adenocarcinoma.
Conclusion
The increased expression of EphA2 and EphrinA-1 contributes to gastric adenocarcinoma progression, in which EphA2-elevated expression is a key indicator for poor prognosis. Since EphA2 has been considered to be a target for gene therapy, like RNA interference against pancreatic adenocarcinoma and advanced ovarian cancer [31, 32], it is plausible that EphA2 may also be beneficial for those patients with gastric adenocarcinoma.
References
American Cancer Society. Cancer facts and figures 2005. Available online at: http://www.cancer.org/downloads/STT/CAFF2005f4PWSecured.pdf.
Catalano V, Labianca R, Beretta GD, et al. Gastric cancer. Crit Rev Oncol Hematol, 2005;54:209–241. doi:10.1016/j.critrevonc.2005.01.002.
American Cancer Society. Cancer facts and figures 2008. Available online at: http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf.
van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol, 1994;10:251–337. doi:10.1146/annurev.cb.10.110194.001343.
Surawska H, Ma PC, Salgia R. The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev, 2004;15:419–433. doi:10.1016/j.cytogfr.2004.09.002.
Sulman EP, Tang XX, Allen C, et al. ECK, a human EPH-related gene, maps to 1p36.1, a common region of alteration in human cancers. Genomics, 1997;40:371–374. doi:10.1006/geno.1996.4569.
Zelinski DP, Zantek ND, Stewart JC, et al. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res, 2001;61:2301–2306.
Kinch MS, Moore MB, Harpole DH Jr. Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res, 2003;9:613–618.
Kataoka H, Igarashi H, Kanamori M, et al. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci, 2004;95:136–141. doi:10.1111/j.1349-7006.2004.tb03194.x.
Xu F, Zhong W, Li J, et al. Predictive value of EphA2 and EphrinA-1 expression in oesophageal squamous cell carcinoma. Anticancer Res, 2005;25:2943–2950.
Ogawa K, Pasqualini R, Lindberg RA, et al. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene, 2000;19:6043–6052. doi:10.1038/sj.onc.1204004.
Fang WB, Brantley-Sieders DM, Parker MA, et al. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene, 2005;24:7859–7868. doi:10.1038/sj.onc.1208937.
Barranco SC, Townsend CM Jr, Casartelli C, et al. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res, 1983;43:1703–1709.
Lin CH, Fu ZM, Liu YL, et al. Investigation of SGC-7901 cell line established from human gastric carcinoma cells. Chin Med J, 1984;97:831–834.
Sobin LH, Wittekind Ch. International Union Against Cancer. TNM Classification of Malignant Tumors, 5th edn. New York: Wiley; 1997.
Herath NI, Spanevello MD, Sabesan S, et al. Over-expression of Eph and ephrin genes in advanced ovarian cancer: ephrin gene expression correlates with shortened survival. BMC Cancer, 2006;6:144. doi:10.1186/1471-2407-6-144.
Shen H, Zhang M, Minuk GY, et al. Different effects of rat interferon alpha, beta and gamma on rat hepatic stellate cell proliferation and activation. BMC Cell Biol, 2002;3:9. doi:10.1186/1471-2121-3-9.
Thaker PH, Deavers M, Celestino J, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res, 2004;10:5145–5150. doi:10.1158/1078-0432.CCR-03-0589.
Lin YG, Han LY, Kamat AA, et al. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer, 2007;109:332–340. doi:10.1002/cncr.22415.
Nakamura R, Kataoka H, Sato N, et al. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci, 2005;96:42–47.
Abraham S, Knapp DW, Cheng L, et al. Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary bladder. Clin Cancer Res, 2006;12:353–360. doi:10.1158/1078-0432.CCR-05-1505.
Zantek ND, Walker-Daniels J, Stewart J, et al. MCF-10A-NeoST: a new cell system for studying cell-ECM and cell-cell interactions in breast cancer. Clin Cancer Res, 2001;7:3640–3648.
Walker-Daniels J, Riese DJ 2nd, Kinch MS. c-Cbl-dependent EphA2 protein degradation is induced by ligand binding. Mol Cancer Res, 2002;1:79–87.
Kinch MS, Carles-Kinch K. Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin Exp Metastasis, 2003;20:59–68. doi:10.1023/A:1022546620495.
Wu D, Suo Z, Kristensen GB, et al. Prognostic value of EphA2 and EphrinA-1 in squamous cell cervical carcinoma. Gynecol Oncol, 2004;94:312–319. doi:10.1016/j.ygyno.2004.05.019.
Han L, Dong Z, Qiao Y, et al. The clinical significance of EphA2 and Ephrin A-1 in epithelial ovarian carcinomas. Gynecol Oncol, 2005;99:278–286. doi:10.1016/j.ygyno.2005.06.036.
Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res, 1999;76:1–20. doi:10.1016/S0065-230X(08)60772-1.
Fang WB, Ireton RC, Zhuang G, et al. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci, 2008;121:358–368. doi:10.1242/jcs.017145.
Brantley-Sieders DM, Zhuang G, Hicks D, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest, 2008;118:64–78. doi:10.1172/JCI33154.
Easty DJ, Guthrie BA, Maung K, et al. Protein B61 as a new growth factor: expression of B61 and up-regulation of its receptor epithelial cell kinase during melanoma progression. Cancer Res, 1995;55:2528–2532.
Duxbury MS, Ito H, Zinner MJ, et al. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene, 2004;23:1448–1456. doi:10.1038/sj.onc.1207247.
Landen CN Jr, Chavez-Reyes A, Bucana C, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res, 2005;65:6910–6918. doi:10.1158/0008-5472.CAN-05-0530.
Acknowledgments
This work is supported by a grant from the Natural Science Foundation, Health Department of Hunan Province, China (no. C2006-008), and a grant from the Provincial Administration of Traditional Chinese Medicine, Health Department of Hunan Province, China (no. 06103). The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, WJ., Ge, J., Chen, ZK. et al. Over-Expression of EphA2 and EphrinA-1 in Human Gastric Adenocarcinoma and Its Prognostic Value for Postoperative Patients. Dig Dis Sci 54, 2410–2417 (2009). https://doi.org/10.1007/s10620-008-0649-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-008-0649-4