Abstract
During the last decade biomaterial sciences and tissue engineering have become new scientific fields supplying rising demand of regenerative therapy. Tissue engineering requires consolidation of a broad knowledge of cell biology and modern biotechnology investigating biocompatibility of materials and their application for the reconstruction of damaged organs and tissues. Stem cell-based tissue regeneration started from the direct cell transplantation into damaged tissues or blood vessels. However, it is difficult to track transplanted cells and keep them in one particular place of diseased organ. Recently, new technologies such as cultivation of stem cell on the scaffolds and subsequently their implantation into injured tissue have been extensively developed. Successful tissue regeneration requires scaffolds with particular mechanical stability or biodegradability, appropriate size, surface roughness and porosity to provide a suitable microenvironment for the sufficient cell–cell interaction, cell migration, proliferation and differentiation. Further functioning of implanted cells highly depends on the scaffold pore sizes that play an essential role in nutrient and oxygen diffusion and waste removal. In addition, pore sizes strongly influence cell adhesion, cell–cell interaction and cell transmigration across the membrane depending on the various purposes of tissue regeneration. Therefore, this review will highlight contemporary tendencies in application of non-degradable scaffolds and stem cells in regenerative medicine with a particular focus on the pore sizes significantly affecting final recover of diseased organs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The successful application of scaffold in tissue engineering depends on many features such as biocompatibility, biodegradability or resistance, mechanical and chemical properties, scaffold architecture and manufacturing technologies (O’Brien 2011). Cell attachment and migration along and/or across the membrane is a fundamental part of tissue formation or regeneration and is influenced by many factors such as intracellular signals, intercellular and extracellular integrin–integrin and -ligand connections regulating cell–cell and cell-extracellular matrix (ECM) interactions (Palecek et al. 1997). The focal cell adhesion to the various surfaces of scaffold is important for the initiation of various signals further stimulating cell proliferation and differentiation (Lee et al. 2004). Therefore, the proper regulation of cell–cell and cell-scaffold interaction can fulfill many aims of multifunctional tissue engineering.
Generally, the main three groups of biomaterials—ceramics, synthetic polymers and natural polymers are used in the fabrication of scaffold for tissue regeneration. Ceramic scaffolds such as hydroxyapatite and tri-calcium phosphate were mainly used to regenerate impaired bones. Numerous synthetic polymers such as polystyrene, poly-lactic acid (PLLA), polyglycolic acid (PGA) and poly-dl-lactic-co-glycolic acid (PLGA), as scaffold materials for the engineering of various tissues, have also been highly investigated (O’Brien 2011). Natural biological materials such as collagen (and its combinations with other materials), proteoglycans, alginate-based substrates and chitosan are also promising in cell-based therapy due to their higher biocompatibility compared to the synthetic ones (O’Brien 2011). However, all of the previously mentioned biomaterials have some drawbacks: ceramics are too stiff, synthetic polymers—not degradable and natural polymers might possess poor mechanical properties (O’Brien 2011). Therefore, finding the best combinations of biomaterials of various origins subsequently improving scaffold biocompatibility is one of the main goals of contemporary tissue engineering.
Another very important factor of applied scaffolds influencing cell adhesion, migration, proliferation and differentiation is the pore size (Harley et al. 2008). The pore sizes can be divided into nano size (nano-roughness, <100 nm), micro pore size (micro-roughness, 100 nm–100 μm) and macro-roughness (100 μm–millimeters) (Vagaska et al. 2010). Different pore sizes might influence different cell processes: the nano pore size membranes was shown to be important for the formation of collagen fibers and ECM, whereas macropores play an important role in cell seeding, distribution, migration and further neo-vascularization in vivo (Liu and Ma 2004; Smith et al. 2009). It was also shown that cell migration linearly depended (up to certain level) on the pore size and that cell-secreted proteolytic MMPs supported cell migration (Fitton et al. 1998; Wolf et al. 2013). However, the instability of pore size is not always a desirable issue, especially for the tissue regeneration through the paracrine or anchorage-dependent cell–cell stimulation. Considering the impact of the ECM on cell behavior, tissue engineering has recently shifted towards the development of biomimetic 3D cell culture systems that more naturally accorded the native environment (Owen and Shoichet 2010). In this review we will overlook the processes that can be regulated just by varying the pore sizes of scaffold and how their purposeful application might promote tissue engineering.
Requirements for an ideal scaffold
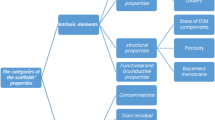
After the injection of suspended stem cells into human tissue or blood stream, it is almost impossible to control further cell location in the body and their functioning. An ideal scaffold should provide a frame that helps injected stem cells to attach, proliferate and differentiate into required tissue cells leading to a full recovery of injured organ (Fig. 1). First of all, the scaffold biomaterials have to be non-toxic to human, resistant to the quick degradation and with the corresponding pore size or porosity. Scaffolds should also allow formation of functional gap junctions and appropriate interaction with other cells and/or ECM. For the indirect cell–cell interaction across scaffold, pore size should be large enough to ensure cellular nutrition but not too large to prevent cell migration. On the other hand, for the transmigration of transplanted cells out of the scaffold to damaged tissue, the ratio between the cell and pore size is also an important factor. All together, the combination of scaffold features such as surface topography and chemistry (wettability, softness, stiffness and roughness), microstructure (porosity, pore size, pore shape and interconnectivity) together with mechanical properties might have a significant influence to the total cell bioactivity and effective organ regeneration in vivo.
The application of scaffolds and stem cells in regeneration medicine. Tissue regeneration by autologous stem cells includes: isolation of adult stem cells, characterization and selection of required stem cell population, multiplication of stem cells in vitro, seeding on selected scaffold and transplantation into damaged tissue. Tissue regeneration by iPS requires: isolation and characterization of adult somatic cells and subsequent induction of pluripotent cells (iPS). Further procedures are the same as with the stem cells. The pore size regulates the final functioning and adaptation of implanted stem cells in injured tissue leading to its successful regeneration
Scaffolds and nanotechnologies
The investigation and application of new types of nanofibers in scaffold-based tissue regeneration is rapidly expanding. Fiber scaffolds with inner pore diameters <1 μm are termed as nanofibers. They are produced using a variety of nano-techniques such as drawing (Nain et al. 2006), template synthesis (Tao and Desai 2007) temperature-induced phase separation (Liu et al. 2005), molecular self-assembly (Paramonov et al. 2006) and electrospinning (Lim and Mao 2009). Among these, electrospinning seems to be the most popular technique generating polymeric nanofibers from a variety of polymer solutions and melts. Electrospinning technique is simple, elegant, reproducible, continuous and scalable (McHugh et al. 2013). By this technique, it is possible to make fibers of various diameters ranging from 3 nm to 6 μm and up to several meters in length (Kumbar et al. 2008; McHugh et al. 2013). Although the electrospun scaffolds were able to prevent stem cells migration and are the most popular porous scaffolds known today, their pore size varies.
The electrospun scaffolds are generally comprised of hundreds of nanometers in diameter nonwoven fibers that can further interconnect and make pores larger than 10 μm (Liang et al. 2007). The silk fibroin scaffolds with pore sizes ranging from 50 to 300 μm were made using a freezing-drying technique (Zhang et al. 2001). The high ratio of fibrous surface area versus its volume ensures abundant area for the cell attachment, which permits cell cultivation at high density similar to that of a two-dimensional surface. Additionally, the high morphological resemblance of electrospun nanofibers to the native ECM suggests their broad application in the scaffold construction as a supportive matrix for the proper stem cell functioning. Many studies investigating the electrospun fibrous scaffolds were focused on the biocompatibility of scaffolds which is especially actual for the generation of tissue-like constructs in vitro (Lim and Mao 2009). However, regardless of all technical problems of scaffold manufacturing, the stem cells and their functioning on the scaffold surface is another critical step for the development of bioactive scaffold (Atala 2012; Chang and Wangs 2011).
Stem cells for tissue regeneration
As aforementioned, the most important strategies for successful tissue engineering are: finding of an appropriate biomaterial and pore sizes for the manufacturing of scaffold and choosing the cell type properly functioning on the scaffold and successfully regenerating injured tissue. Cell-based regenerative technologies are mainly related to the direct cell injection into damaged tissue or blood vessel and paracrine cell–cell and cell-ECM-based stimulation. However, terminally differentiated cell types of native organ have limited regenerative application due to their limited growth in vitro. Therefore, the application of various types of stem cells for tissue engineering is a big step forward improving cell-based therapy.
Starting from the discovery of hematopoietic stem cells (Becker et al. 1963), a particular attention in the field of cell-based therapy has been paid to the adult human mesenchymal stem cells (MSCs) due to their pluripotency (Pittenger et al. 1999). Later studies showed that the MSCs can be isolated from almost every tissue in the body, such as amniotic and synovial fluids, adipose, dental tissues, umbilical cord, peripheral bloods, dermis, brain, muscle and even tumors (Reynolds and Weiss 1992; Erices et al. 2000; Seale and Rudnicki 2000; Zuk et al. 2001; Roufosse et al. 2004; De Coppi et al. 2007; Haniffa et al. 2007; Huang et al. 2009; Yan et al. 2012; de Sousa et al. 2014). Although the MSCs have a big therapeutic potential, there are still many problems related to the sudden death of transplanted cells, migration out of transplanted organ, sedimentation into other organs causing unwanted differentiation, inflammation or even secondary cancers. Not less important requirement for the transplanted MSCs is their full adaptation and purposeful functioning in transplanted organ in order to restore injured organ. The embryonic cells (ESc) are also very promising due to their totipotency, however, they cause many ethical problems, form teratomas and might be rejected and destroyed by the host immune system (Boyd et al. 2012).
These problems could be avoided by reprogramming/de-differentiation of somatic cells into pluripotent state whereby cells adopt features of ESc. Since new stem cell technologies such as cloning or use of viral vectors have been shown to have some limitations, production of induced pluripotent stem cells can be done through the various reprogramming techniques such as somatic nuclear transfer, somatic cell hybrids and production of induced pluripotent cells (iPS) (Wenceslau et al. 2013). The activation of essential stemness genes by combination of different reprogramming factors trough a cascade of transcriptional activity has been suggested (Takahashi and Yamanaka 2006). Recently many other reprogramming strategies based on genes, proteins, iRNR and different chemicals are also available for the reprogramming of somatic cells (Yu et al. 2007; Nakagawa et al. 2008). The de-differentiation method induce the expression of genes that are not normally expressed in fibroblasts or other adult cells, change their morphology, mode of growth and differentiation potential, and is a very attractive tool for tissue regeneration. However, the search for new somatic cell reprogramming strategies omitting harmful viral technologies of the gene delivery is still of future challenge.
Nanometric pore sizes for cell attachment and functioning
Cell proliferation, differentiation and migration are mainly cell-anchorage-dependent processes that inhibit cell apoptosis and activate cytoskeleton reorganization (Wozniak et al. 2004). Therefore, the initial cell adhesion is important for the further cell functioning. The dependence of cell attachment and differentiation on the pore size of polycarbonate (PC) surfaces was nicely shown by Lee et al. (2004). The authors investigated attachment of MG63 human osteoblasts on the membrane with 0.2–8 μm pores and showed that the cells were fully adhered and spread on the surface with 0.2–1 μm pores, whereas cells became spherical with few fillopodia and lamellapodia on the membrane with the larger micropores (3.0–8.0 μm in diameter). Additionally, the cells growing on the 5.0–8.0 μm pore size membrane showed increasing osteogenic differentiation with the highest differentiation reached on 8 μm pores (Lee et al. 2004). It was also shown that nano-fibrous (50–500 nm) PLLA scaffolds enhance protein adsorption contributing to cell attachment (Woo et al. 2003). It was also shown that the osteoblasts attached and proliferated more effectively on the rough surface (0.81 μm pore size) in comparison with the smooth one (Hatano et al. 1999). Additionally, the proliferation of osteoblast-like MG63 cells decreased with increasing pore size from 200 nm to 8 μm, whereas their osteogenic differentiation improved (Lee et al. 2004). Other studies also showed that nano-fibrous scaffolds stimulated neurogenic, osteogenic, chondrogenic and other types of cell differentiation (Li et al. 2003; Yang et al. 2004; Woo et al. 2007). Additionally, the main ECM component collagen I was shown to be important for the cell attachment: it can be enhanced by 1.7-fold mimicking collagen on nano-fibers (Grinnell 1982; Woo et al. 2003). All together, nano pore sizes improve cell attachment and further cell functioning. Though, the nano pores should be of proper nanometer size: cells on too smooth scaffold surface start to make clamps around the scaffold edges disturbing diffusion of nutrients, removal of cell waste and impairing further cell functioning, whereas too big pores disorganize cell attachment (Yannas 1992).
Micrometric pores for cell–cell interaction
Heterotypic cell–cell interactions might control the development of various tissues. However, the direct cell–cell contacts are not always desirable in tissue engineering. For example, it was shown that co-culturing of endothelial cells together with smooth muscle cells led to the inhibition of endothelial cell growth (Saunders and D’Amore 1992). This problem can be solved by co-culturing of heterogeneous cell populations across the porous membrane. The authors showed that pore size of 0.02, 0.4, 0.6 and 0.8 microns was optimal for the cultivation of endothelial and smooth muscle cells as homogenous populations, whereas smooth muscle cells started to migrate across the membrane with pores size of 2.0 microns (Saunders and D’Amore 1992). Moreover, fibroblasts grown on one side of a membrane with 1.2 μm pore size were capable of reaching and contacting other cells grown on the opposite side of the same membrane (Kim et al. 2014). The human embryonic stem cells (hESCs) (CHA3 and H9) and feeder (STO and MEF) cells were grown on opposite sides of a membrane with 1, 3 and 8 μm pore size for 5 days (Kim et al. 2007). Data showed that attachment of hESCs on a membrane with 3 and 8 μm pore size was better compared to that of 1 μm pores. Moreover, the scaffold membrane with >3 μm pore size allowed feeder cells to migrate upwards, whereas 1 μm pore size was optimal for the growth of hESCs without contacting feeder cells. Even if hESC and feeder cells were not cross-contaminated during co-culture on the opposite sides of membrane with 3 μm pore size, they were able to have an anchorage-dependent contact with the feeder cells. Even 1 μm pores showed some protrusion of cellular bodies such as lamellipodium and filopodia permitting slight cell–cell contact. Additionally, the 3 μm pores, but not the 1 μm pores, were able to prolong growth of hESC in vitro from 15 to 25 passages (Kim et al. 2007).
Similarly to the above findings, it was shown that scaffolds with 0.45 μm pores were able to separate hematopoietic progenitors from stromal cells, which was essential for the production of mature blood elements, prevention of differentiation and preservation of hematopoietic progenitors (Verfaillie 1992). The initiation of differentiation of bladder smooth muscle cell by epithelium cell was also changed from indirect to direct by changing the range of pore size from 1 to 10 μm, respectively (Liu et al. 2000). In summary, data of various studies demonstrate that smaller cells require smaller pore size to prevent cell migration across the 2D scaffold. However, it should be taken into account that the other factors such as level of membrane porosity and pore inter-connectivity are also important factors for the sufficient cell supply by nutrition and oxygen improving further cell functioning (Chang and Wangs 2011).
Threshold of pore size for cell migration across the membrane
Cell migration across the membrane requires balance between cell size, attachment, scaffold pore size and surface topography. It was shown that the migration of human umbilical cord-derived mesenchymal stem cells (hUCMSCs) through the polycarbonate (PC) membrane with 0.4, 3.0 and 8.0 μm pores was 0, 1.8 and 8.0 %, respectively (Li et al. 2011). Migration of NIH 3T3 fibroblasts and human embryonic cells was also blocked if the membrane pore diameter was <1 μm (Kim et al. 2014). Migration of MSCs across scaffold membranes made from poly(ethylene glycol) (PEG) with pore sizes of 7, 12 and 17 μm, surprisingly, was the best through the intermediate (12 μm) pores (Peyton et al. 2011). On the other hand, the glioma cell migration through the collagen gel was hindered by the small pores (5–12 μm), whereas cell invasion distance was not a pore size-depended process (Yang et al. 2010). Additionally, it was shown that migration of polymorphonuclear cells (PMN) was reduced by 90 and 99 % through the polycarbonate membrane pores of 1.49–1.78 and 1.26–1.38 μm in diameter, respectively (Wolf et al. 2013).
Migration of stem cells through the 2D scaffold can be artificially stimulated independently of the pore size. It was shown that hMSCs migration through the membrane pores of 8 μm in diameter was more efficient when the normal dermal fibroblasts were replaced by the keloid-derived ones on the bottom of a dual-modified Boyden chamber. The hMSCs were able to migrate across the membrane with 3 μm pores only when the same keloid-derived fibroblasts were placed on the bottom of the plates (Akino et al. 2008). Similarly, the migration of hMSCs trough the 3 μm transwell membrane made from polyethylene terephthalate was effective and used for the wound healing test of human type II alveolar epithelial cell line on the opposite side of the same membrane (Akram et al. 2013). Noteworthy, cell migration through the matrix metalloproteinase (MMP)-degradable scaffolds such as collagen strongly depends upon MMP-depended ECM cleavage (Wolf et al. 2013). In summary, data mentioned in this paragraph show that cell migration across the membrane is optimal when the pore size is higher than 3 μm. It should be taken into account that cell migration trough the critical pore size might change depending on the cell type and growth conditions. The impact of pore size on the regulation of cell transmigration is shown in (Table 1).
Macroporous 3D scaffolds for cell functioning
As highlighted in the previous section, scaffold membranes with pore sizes ranging approximately from 50 nm to 12 μm regulate cellular attachment, cell–cell interaction and migration across the membrane. However, the 3D scaffolds with large pore size (around 100 μm or more) have higher amount of functional units necessary for the regeneration of various tissues. It was shown that attachment of MSCs to the island-patterned PLLA scaffold was better if pore diameter was 100 μm instead of 60 μm (Lee et al. 2009). In addition, the attachment and growth of MSC on PLLA was improved after the precoating of island-patterned scaffold with collagen and fibronectin (Lee et al. 2009). The collagen-glucosaminoglycan scaffolds with 85, 120, and 325 μm pore sizes were also investigated for the adhesion and differentiation of osteoblasts (Murphy et al. 2010). Surprisingly, the cell adhesion and proliferation during 48 h of culturing was better on the scaffold with 120 μm pores, whereas in 7 days the number of osteoblasts was higher on the scaffold with 325 μm pore sizes. The same study showed that pore size around 100 μm was important for the cell adhesion and proliferation, whereas cells migration was faster trough the scaffolds with 325 μm pore size. The membranes with smallest pore size (85 μm) showed lowest intensity of cell adhesion and migration (Murphy et al. 2010). In agreement with these results, it was shown that cell adhesion surface on scaffold was decreasing with increased pore size and had inverse linear dependence in the range of 90–151 μm (O’Brien et al. 2007). However, when the pore size increased from 85 to 325 μm the inverse linear relationship between cell adhesion and pore size was disrupted. Additionally, the poly(lactic co-glycolic acid) (PLGA) electrospun scaffold with the pore size around 100 μm also showed better cell–matrix and cell–cell interaction compared to the other pore sizes (Li et al. 2002).
Summarized impact of pore size on cell functioning on 2D and 3D scaffolds is presented in Fig. 2. However, individual goals of regenerative therapy require individual experimental conditions and best cell-scaffold interaction model. Some cell-scaffold interaction-based tissue regeneration models with particular role of pore size in it will be discussed below.
Schematic presentation how pore sizes regulate cell attachment, interaction and migration. a 2D scaffold membrane with pore size <1 μm for the better cell attachment. b 2D scaffold membrane with the pore size ranging from 1 to 3 μm for the anchorage-dependent cell–cell interaction. c 2D scaffold membrane with the pore sizes of 3–12 μm for the direct cell–cell contacts, migration and/or invasion. d 3D scaffold with the surface pore sizes of 1–3 μm and porous internal structure for the indirect cell–cell or cell-ECM interaction. e Cell migration in and out of 3D scaffold through the pore size ranging from 100 to 800 μm which depends on the aim of tissue regeneration
Impact of pore sizes in tissue engineering
Pore sizes regulating bone regeneration
The application of scaffolds, especially biodegradable, for the musculoskeletal regeneration has been intensively investigated (Agrawal and Ray 2001). Based on various studies, the minimum requirement for pore size in 3D bone regeneration is considered to range from 100 μm to more than 300 μm (Karageorgiou and Kaplan 2005). Moreover, the pore size around 100 μm favored hypoxic conditions inducing osteochondral formation before osteogenesis, whereas larger pore size (>300 μm) directly initiated osteogenesis (Karageorgiou and Kaplan 2005). It was also shown that bone regeneration in ceramic scaffolds with pore sizes smaller than 350 μm varied: pore sizes between 100 and 300 μm initiated bone formation, while pore sizes between 10 and 100 μm conditioned fibrous tissue or unmineralized osteoids (Hulbert et al. 1970). Similar data were observed in osteogenic differentiation of BMP7 gene-transfected MSCs: the differentiation was better on the silk fibroin protein scaffold with pore size between 100 and 300 μm compared to 50–100 μm (Zhang et al. 2010). Another study showed that the bone ingrowths predominated in porous poly(methyl methacrylate) (PMMA) scaffold with pore size around 450 μm (Ashman and Moss 1977). Similarly, the osteoblast migration was faster inside microcellular porous polymers derived from the high internal phase emulsions (polyHIPEs) scaffolds with 100 μm pore size compared to 40 and 60 μm ones (Akay et al. 2004). The same study showed that modification of polymer with hydroxyapatite increased osteogenesis; however, the larger pores increased rate of osteoblasts migration into the scaffold but not the depth—maximum was 1.4 mm for all pore sizes (Akay et al. 2004).
It should be noted that the level of porosity is not less important than the pore size for the bone regeneration (Zhang et al. 2001). The osteogenesis of skeletal stem cells was especially effective on the new type of biphasic hydroxyapatite/tricalcium (HA/TCP) scaffolds with high porosity (45 pores per inch) (Aarvold et al. 2013). Microporous polycaprolactone (PCL) matrice with various pore sizes might be used to stimulate osteogenesis by delivery of various protein such as lysozyme (100–200 μm), collagenases (80–200 μm), catalase (100–250 μm), lactose (45–90, 90–125 μm) and gelatin (125–250 μm) into damaged tissue (Wang et al. 2007, 2009). Correspondingly, osteoblasts proliferation and bone formation on the PLGA scaffold was the best with the pore-size ranging from 150 to 300 μm (Peter et al. 1998). Parallelly, it was shown that PLGA scaffolds with pore size ranging from 150 to 710 μm had no impact on the osteoblasts attachment and proliferation, whereas tissue mineralization was the best on the scaffolds with 300–500 μm pores (Ishaug et al. 1997).
Pore sizes for the regeneration of other connective tissues
Cartilage loss is the most prominent feature of arthritis, whereas cartilage regeneration is a difficult and time-consuming process. Recent biotechnological achievements in tissue engineering were able to suggest broad variety of scaffolds and diverse MSC sources for the regeneration of connective tissue, particularly cartilage. The scaffolds with pore sizes ranging from 200 to 400 μm and with an oval to round pore shape was shown to be important not only for the functioning of osteoblasts but also for the differentiation of chondrocytes (Boyan et al. 1996). Implants consisting of biodegradable Estane polymers comprising macropores of 150–355 μm that are highly interconnected with micropores (<50 μm) have been found to be conducive to ingrowth into fibrocartilaginous tissue preventing degeneration of the articular cartilage (Tienen et al. 2006). Recent investigation of MSC chondrogenesis on collagen-hyaluronic acid (CHyA) scaffolds with pore-sizes of 94, 130, and 300 μm in diameter showed that scaffolds with the largest (300 μm) pores significantly stimulated expression of chondrogenic genes (Matsiko et al. 2015). Additional precoating of PLGA scaffolds (90–180 μm of pore size) with fibrin increased chondrogenesis and deposition of cartilage-specific ECM (Sha’ban et al. 2008). The successful application of iPS for the regeneration and restoration of cartilage defects on 3D nanofibrous scaffolds with similar pore size has been also recently shown (Liu et al. 2014).
The ECM is a heterogeneous complex providing structural support for cell growth, migration and signaling. During cell migration and spreading, focal adhesion is basically an integrin-mediated process, whereas adhesion of cells to fibrillar ECM generates extracellular fibrils and fibronectin (Pankov et al. 2000). Fibroblasts, as main ECM generators, have slightly different adhesion properties to the 3D structures compared to the 2D and require high cell density for the ECM generation and better attachment (Cukierman et al. 2001). It was shown that the poly(lactic acid) (Karageorgiou and Kaplan 2005) and PLGA scaffolds produced by salt leaching with pores smaller than 160 μm were optimal for attachment and growth of human skin fibroblasts (Yang et al. 2002). The invasion of human endothelial cells into PLGA scaffolds was even better under the sheared stress conditions (Koo et al. 2014). The generation of connective tissue by fibroblasts seeded on the molded/salt leached poly(lactic-co-glycolic acid)/polybutylene terephthalate (PEGT/PBT) copolymer scaffolds was efficient with an average of interconnecting pores of 160 ± 56 μm (Wang et al. 2005). On the other hand, it was shown that the canine dermal fibroblasts were the least selective for pore size and showed similar cell proliferation and ECM formation on the poly(L-lactic acid) (L-PLA) scaffold with pores ranging from 38 to 150 μm (Zeltinger et al. 2001). Additionally, it was shown that the sphere-template polymers (poly(2-hydroxyethylmethacrylate); poly(pHEMA)) with 40 μm pores were able to recapitulate key elements of both dermal and epidermal layers of skin, whereas migration of keratinocytes into pores was limited (Fukano et al. 2010). Data presented in this section show that the impact of pore size in successful regeneration of various connecting tissues depends not only on the origin of scaffold’s biomaterial and fabrication techniques but also on the geometry of pores.
Pore sizes for the regeneration of nerve system
The brain and nervous systems have limited capacity to regenerate, which is very often a reason of neurodegenerative diseases. The golden standard for the nerve reconstruction is autologous nerve grafting but with limited natural sources. Therefore, the successful application of apropos cells and scaffolds could extend endogenous regeneration and/or replacement of defective neural cells. It was shown that Schwann cells seeded on the fibrin precoated polyurethane scaffolds with uniaxially-oriented pore structure in the range of 2 μm (the pore wall) and 75 × 750 μm (elongated pores) could significantly regenerate peripheral axons (Hausner et al. 2007). In parallel, the axon regeneration on poly(dimethyl siloxane) scaffold precoated with poly-l-lysine and laminin required the neurite bridging and was augmented with increasing groove width from 50 to 200 μm (Goldner et al. 2006). It was also shown that the axon outgrowth along the longitudinal direction of 3D hydrogel alginate increased with increasing capillary diameter displaying the highest axon density within the scaffold capillary diameter of 71–86 μm (Pawar et al. 2011). Similarly, neurites of PC12 cells also displayed an increasing parallel orientation on the surface with groove width ranging from 20 to 60 μm (Mahoney et al. 2005). Pore width of collagen I scaffold ranging from 20 to 50 μm was also better compared to the 50–100 μm for the glial and axonal growth (Bozkurt et al. 2009). The laminin-coated cryogel scaffolds with pore sizes of 80–100 microns were successfully used for the regeneration of brain neurons (Jurga et al. 2011). Similarly to the previous results, it was shown that collagen membrane with 100-μm-diameter pores were proper for the transplantation and further differentiation of neural stem cells (Yuan et al. 2014).
As highlighted in this paragraph, the regeneration of long-size peripheral axons requires long pores ranging from 200 up to 750 μm and even millimeters, whereas their ingrowth and/or outgrowth needs much smaller pores (20–70 μm) depending on the origin of biomaterial. Scaffolds with pore sizes around 100 μm seem to be more suitable for the regeneration of neurons.
Pore sizes for the regeneration of cardiovascular system
Cardiovascular diseases remain the leading cause of mortality in the world very often requiring vascular replacement as one of the ways to treat ischemic heart and peripheral vascular diseases (Isenberg et al. 2006). Limited source of autologous vessels, similarly to autologous nerves, encouraged looking for new ways to vascular regeneration including application of biodegradable scaffolds (Niklason et al. 1999). It was shown that vascular smooth muscle cells cultivated on 38–150 μm pore size L-PLA scaffold displayed equivalent cell proliferation and matrix deposition (Zeltinger et al. 2001). Similarly, other authors also found that growth of smooth muscle cells on PLGA scaffold with pore size ranging from 50 to 200 μm was not significantly affected during 14 days of cell culturing (Lee et al. 2008). Additionally, similar pore size (60–150 μm) of PLLA scaffold was successfully applied to generate smooth muscle cells (SMCs) from human iPS cells (Wang et al. 2014). Subcutaneous implantation of the last-mentioned SMC-scaffold construct in nude mice demonstrated formation of vascular tissue. However, the microvascular epithelial cells showed sparse extracellular matrix on the L-PLA scaffolds with pore size from 38 to 150 μm, while multilayered lining was formed on the scaffolds with pore size <38 μm (Zeltinger et al. 2001). It was also shown that the depth of invasion (160 μm after 4 h) of endothelial progenitor cells into electrospun fibrous scaffold and their further colonization was increased with increasing pore size (>45 μm) (Hong et al. 2015). Another study showed only 100 μm infiltration of the endothelial progenitors into electrospun fibrous scaffolds with pore size <20 μm after 7 days of culturing (Blakeney et al. 2011). It showed that higher ratio between pore and an endothelial cell size (endothelial cell diameter is around 20 μm) leads to more successful cell migration and invasion. On the other hand, the decellularized vascular matrices could be also a perfect scaffold supporting growth and invasion of endothelial cells (Lu et al. 2004).
Pore sizes for the regeneration of heart tissue
Heart failure is the leading cause of death in the world and occurs due to the loss of cardiomyocytes (Braunwald and Pfeffer 1991). To date, most of stem-cell-based strategies for cardiac repair were related to the injection of cell suspension directly into injured myocardium. Various types of stem cells and their ability to differentiate into cardiomyocytes have been intensively investigated. Recently, iPS cells have been also considered as therapeutic tool for heart regeneration (Takahashi and Yamanaka 2006). However, it was shown that iPS or ES cells-derived cardiomyocytes had different contractile properties compared to native cardiac tissue (Xi et al. 2010). Additionally, it was shown that around 90 % of transplanted cells die within 1 week and 50–90 % are extruded out of the myocardium (Zhang et al. 2001; Muller-Ehmsen et al. 2002). Therefore, the new direction in heart regeneration is related to the application of scaffold delivering stem cells into heart and/or scaffold-regulated direct cardiomyocyte stimulation. It was shown that bimodal poly(2-hydroxyethylmethacrylate-co-methacrylic acid) (pHEMA-co-MAA) hydrogel scaffolds with parallel channels of 60 μm in diameter were required to seed and promote aggregation of cardiomyocytes (Madden et al. 2010). Additionally, sphere-template material (pHEMA-co-MAA) with 40 and 80 μm pores showed formation of functional vessels within implants without complication of tricell (cardiomyocyte, endothelial and fibroblast) functioning. It was also shown that the same type of sphere-template material with larger pores (~90–160 μm) led to more fibrosis and less vascularity (Marshall et al. 2004). On the other hand, the enhanced survival of cardiomyocytes after the paracrine stimulation trough 0.22 μm pores was also shown (Kawaguchi et al. 2010).
Scientists now are developing biological pacemakers as an alternative to the electrical pacemakers with the hope to mimic the natural pacemaker and to overcome some limitations of the electronic ones. Using MSCs, as the best suited stem cell type for making a biological pacemaker, it is expected to create an appropriate cardiac response to exercise and emotions. MSCs from the biological pacemaker should respond to the physiological changes of the body and influence functioning of cardiomyocytes (Rosen et al. 2004). However, how to make the best cell-scaffold model for the biological pacemaker efficiently stimulating heart contraction is still an open issue. The pore sizes for the biological pacemaker should allow some contact of implanted MSC with cardiomyocytes but prevent their transmigration. Recently, our group is investigating non-degradable scaffolds made from various materials and with different pore sizes and their biocompatibility with bone marrow-derived MSC in order to generate the functional biological pacemaker for heart stimulation. In light of data presented in this review, the pore size of biopacemaker membrane should be around 3 μm preventing cell transmigration but providing slight MSC-cardiomyocyte interaction. Additionally, the biopacemaker should be of apropos mechanical stability and biocompatibility. So far this field is full of unsolved questions. Hopefully, in the nearest future we will be able to answer at least part of the questions concerning the application of biopacemakers/bioscaffolds in heart stimulation.
Conclusions
All aforementioned studies and data have verified that regulation of pore size is one of the most critical issues for the successful scaffold application in regenerative therapy. Summarizing data of various studies we can conclude that nano-pored scaffolds with pores <1 μm can be applied to improve cell-surface interaction, whereas the anchorage-dependent cell–cell communication requires larger pore size (around 1–3 μm). Cell migration through the scaffold surface pores needs larger pores ranging from 3 to 12 μm. Additionally, cell transfer through the pore size below 3 μm may need an additional stimulation. Finally, cell attachment was the best on 3D scaffolds with 100 μm pores, whereas pore sizes required for the regeneration of various tissues varied depending on the size and the origin of transplanted cell and regenerating tissue: the regeneration of long peripheral axons required longitudinally-oriented pores (200–750 μm); chondrogenic and osteogenic MSC differentiation demanded scaffolds with 200–400 μm pores; growth of smooth muscle endothelial, nerve cells and fibroblasts was the best on 50–160 μm pores, while microvascular epithelial cells required small pores (<38 μm).
Overall, successful application of scaffolds in regenerative medicine is a complex of pore-size and cell-type-dependent processes and needs an individual experimental optimization considering regenerative and therapeutic aims. However, this review shows that pore size and geometry plays a significant and very often limiting role in tissue regeneration. The large quantity of investigations performed up to these days will shorten ways for the future discoveries in the field of tissue engineering.
References
Aarvold A, Smith JO, Tayton ER, Lanham SA, Chaudhuri JB, Turner IG, Oreffo RO (2013) The effect of porosity of a biphasic ceramic scaffold on human skeletal stem cell growth and differentiation in vivo. J Biomed Mater Res A 101:3431–3437. doi:10.1002/jbm.a.34646
Agrawal CM, Ray RB (2001) Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res 55:141–150
Akay G, Birch MA, Bokhari MA (2004) Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials 25:3991–4000. doi:10.1016/j.biomaterials.2003.10.086
Akino K, Akita S, Yakabe A, Mineda T, Hayashi T, Hirano A (2008) Human mesenchymal stem cells may be involved in keloid pathogenesis. Int J Dermatol 47:1112–1117. doi:10.1111/j.1365-4632.2008.03380.x
Akram KM, Samad S, Spiteri MA, Forsyth NR (2013) Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respir Res 14:9
Ashman A, Moss ML (1977) Implantation of porous polymethylmethacrylate resin for tooth and bone replacement. J Prosthet Dent 37:657–665
Atala A (2012) Regenerative medicine strategies. J Pediatr Surg 47:17–28. doi:10.1016/j.jpedsurg.2011.10.013
Becker AJ, Mc CE, Till JE (1963) Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197:452–454
Blakeney BA, Tambralli A, Anderson JM, Andukuri A, Lim DJ, Dean DR, Jun HW (2011) Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 32:1583–1590. doi:10.1016/j.biomaterials.2010.10.056
Boyan BD, Hummert TW, Dean DD, Schwartz Z (1996) Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 17:137–146
Boyd AS, Rodrigues NP, Lui KO, Fu X, Xu Y (2012) Concise review: immune recognition of induced pluripotent stem cells. Stem Cells 30:797–803. doi:10.1002/stem.1066
Bozkurt A, Deumens R, Beckmann C, OldeDamink L, Schugner F, Heschel I, Sellhaus B, Weis J, Jahnen-Dechent W, Brook GA, Pallua N (2009) In vitro cell alignment obtained with a Schwann cell enriched microstructured nerve guide with longitudinal guidance channels. Biomaterials 30:169–179. doi:10.1016/j.biomaterials.2008.09.017
Braunwald E, Pfeffer MA (1991) Ventricular enlargement and remodeling following acute myocardial infarction: mechanisms and management. Am J Cardiol 68:1D–6D
Chang HI, Wangs Y (2011) Cell responses to surface and architecture of tissue engineering scaffolds. In: Eberli D (ed) Regenerative medicine and tissue engineering - cells and biomaterials, chapter 27. InTech, Rijeka, Croatia, pp 569– 588. doi:10.5772/21983
Cukierman E, Pankov R, Stevens DR, Yamada KM (2001) Taking cell-matrix adhesions to the third dimension. Science 294:1708–1712. doi:10.1126/science.1064829
De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25:100–106. doi:10.1038/nbt1274
de Sousa EB, Casado PL, Moura Neto V, Duarte ME, Aguiar DP (2014) Synovial fluid and synovial membrane mesenchymal stem cells: latest discoveries and therapeutic perspectives. Stem Cell Res Ther 5:112. doi:10.1186/scrt501
Erices A, Conget P, Minguell JJ (2000) Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 109:235–242
Fitton JH, Dalton BA, Beumer G, Johnson G, Griesser HJ, Steele JG (1998) Surface topography can interfere with epithelial tissue migration. J Biomed Mater Res 42:245–257
Fukano Y, Usui ML, Underwood RA, Isenhath S, Marshall AJ, Hauch KD, Ratner BD, Olerud JE, Fleckman P (2010) Epidermal and dermal integration into sphere-templated porous poly(2-hydroxyethyl methacrylate) implants in mice. J Biomed Mater Res A 94:1172–1186. doi:10.1002/jbm.a.32798
Goldner JS, Bruder JM, Li G, Gazzola D, Hoffman-Kim D (2006) Neurite bridging across micropatterned grooves. Biomaterials 27:460–472. doi:10.1016/j.biomaterials.2005.06.035
Grinnell F (1982) Cell-collagen interactions: overview. Methods Enzymol 82 Pt A:499–503
Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP (2007) Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol 179:1595–1604
Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ (2008) Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J 95:4013–4024. doi:10.1529/biophysj.107.122598
Hatano K, Inoue H, Kojo T, Matsunaga T, Tsujisawa T, Uchiyama C, Uchida Y (1999) Effect of surface roughness on proliferation and alkaline phosphatase expression of rat calvarial cells cultured on polystyrene. Bone 25:439–445
Hausner T, Schmidhammer R, Zandieh S, Hopf R, Schultz A, Gogolewski S, Hertz H, Redl H (2007) Nerve regeneration using tubular scaffolds from biodegradable polyurethane. Acta Neurochir Suppl 100:69–72
Hong JK, Bang JY, Xu G, Lee JH, Kim YJ, Lee HJ, Kim HS, Kwon SM (2015) Thickness-controllable electrospun fibers promote tubular structure formation by endothelial progenitor cells. Int J Nanomedicine 10:1189–1200. doi:10.2147/IJN.S73096
Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792–806. doi:10.1177/0022034509340867
Hulbert SF, Young FA, Mathews RS, Klawitter JJ, Talbert CD, Stelling FH (1970) Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res 4:433–456. doi:10.1002/jbm.820040309
Isenberg BC, Williams C, Tranquillo RT (2006) Small-diameter artificial arteries engineered in vitro. Circ Res 98:25–35. doi:10.1161/01.RES.0000196867.12470.84
Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG (1997) Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res 36:17–28
Jurga M, Dainiak MB, Sarnowska A, Jablonska A, Tripathi A, Plieva FM, Savina IN, Strojek L, Jungvid H, Kumar A, Lukomska B, Domanska-Janik K, Forraz N, McGuckin CP (2011) The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials 32:3423–3434. doi:10.1016/j.biomaterials.2011.01.049
Karageorgiou V, Kaplan D (2005) Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26:5474–5491. doi:10.1016/j.biomaterials.2005.02.002
Kawaguchi N, Smith AJ, Waring CD, Hasan MK, Miyamoto S, Matsuoka R, Ellison GM (2010) c-kitpos GATA-4 high rat cardiac stem cells foster adult cardiomyocyte survival through IGF-1 paracrine signalling. PLoS One 5:e14297. doi:10.1371/journal.pone.0014297
Kim S, Ahn SE, Lee JH, Lim DS, Kim KS, Chung HM, Lee SH (2007) A novel culture technique for human embryonic stem cells using porous membranes. Stem Cells 25:2601–2609. doi:10.1634/stemcells.2006-0814
Kim MY, Li DJ, Pham LK, Wong BG, Hui EE (2014) Microfabrication of high-resolution porous membranes for cell culture. J Memb Sci 452:460–469. doi:10.1016/j.memsci.2013.11.034
Koo MA, Kang JK, Lee MH, Seo HJ, Kwon BJ, You KE, Kim MS, Kim D, Park JC (2014) Stimulated migration and penetration of vascular endothelial cells into poly(l-lactic acid) scaffolds under flow conditions. Biomater Res 18:7. doi:10.1186/2055-7124-18-7
Kumbar SG, James R, Nukavarapu SP, Laurencin CT (2008) Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater 3:034002. doi:10.1088/1748-6041/3/3/034002
Lee SJ, Choi JS, Park KS, Khang G, Lee YM, Lee HB (2004) Response of MG63 osteoblast-like cells onto polycarbonate membrane surfaces with different micropore sizes. Biomaterials 25:4699–4707. doi:10.1016/j.biomaterials.2003.11.034
Lee M, Wu BM, Dunn JC (2008) Effect of scaffold architecture and pore size on smooth muscle cell growth. J Biomed Mater Res A 87:1010–1016. doi:10.1002/jbm.a.31816
Lee IC, Lee YT, Yu BY, Lai JY, Young TH (2009) The behavior of mesenchymal stem cells on micropatterned PLLA membranes. J Biomed Mater Res A 91:929–938. doi:10.1002/jbm.a.32309
Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK (2002) Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res 60:613–621
Li WJ, Danielson KG, Alexander PG, Tuan RS (2003) Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A 67:1105–1114. doi:10.1002/jbm.a.10101
Li DJ, Chai JK, Han YF, Sun TJ, Deng HP, Zhao JY, Liu LY (2011) Growth and migration of umbilical cord mesenchymal stem cells on polycarbonate membrane with different pore sizes. Zhonghua Yi Xue Za Zhi 91:699–702
Liang D, Hsiao BS, Chu B (2007) Functional electrospun nanofibrous scaffolds for biomedical applications. Adv Drug Deliv Rev 59:1392–1412. doi:10.1016/j.addr.2007.04.021
Lim SH, Mao HQ (2009) Electrospun scaffolds for stem cell engineering. Adv Drug Deliv Rev 61:1084–1096. doi:10.1016/j.addr.2009.07.011
Liu X, Ma PX (2004) Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng 32:477–486
Liu W, Li Y, Cunha S, Hayward G, Baskin L (2000) Diffusable growth factors induce bladder smooth muscle differentiation. In Vitro Cell Dev Biol Anim 36:476–484
Liu XH, Smith L, Wei G, Won YJ, Ma PX (2005) Surface engineering of nano-fibrous poly(l-lactic acid) scaffolds via self-assembly technique for bone tissue engineering. J Biomed Nanotechnol 1:54–60
Liu J, Nie H, Xu Z, Niu X, Guo S, Yin J, Guo F, Li G, Wang Y, Zhang C (2014) The effect of 3D nanofibrous scaffolds on the chondrogenesis of induced pluripotent stem cells and their application in restoration of cartilage defects. PLoS One 9:e111566. doi:10.1371/journal.pone.0111566
Lu Q, Ganesan K, Simionescu DT, Vyavahare NR (2004) Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials 25:5227–5237. doi:10.1016/j.biomaterials.2003.12.019
Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD (2010) Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci USA 107:15211–15216. doi:10.1073/pnas.1006442107
Mahoney MJ, Chen RR, Tan J, Saltzman WM (2005) The influence of microchannels on neurite growth and architecture. Biomaterials 26:771–778. doi:10.1016/j.biomaterials.2004.03.015
Marshall AJ, Irvin CA, Barker T, Sage EH, Hauch KD, Ratner BD (2004) Biomaterials with tightly controlled pore size that promote vascular ingrowth ACS. Polymer Prepr 45:100–101
Matsiko A, Gleeson JP, O’Brien FJ (2015) Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng Part A 21:486–497. doi:10.1089/ten.TEA.2013.0545
McHugh KJ, Tao SL, Saint-Geniez M (2013) A novel porous scaffold fabrication technique for epithelial and endothelial tissue engineering. J Mater Sci Mater Med 24:1659–1670. doi:10.1007/s10856-013-4934-1
Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L (2002) Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol 34:107–116. doi:10.1006/jmcc.2001.1491
Murphy CM, Haugh MG, O’Brien FJ (2010) The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31:461–466. doi:10.1016/j.biomaterials.2009.09.063
Nain AS, Wong JC, Amon CH, Sitti M (2006) Drawing suspended polymer micro-/nanofibers using glass micropipettes. Appl Phys Lett 89:183105–183107. doi:10.1063/1.2372694
Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26:101–106. doi:10.1038/nbt1374
Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R (1999) Functional arteries grown in vitro. Science 284:489–493
O’Brien FJ (2011) Biomaterials & scaffolds for tissue engineering. Mater Today 14:88–95. doi:10.1016/S1369-7021(11)70058-X
O’Brien FJ, Harley BA, Waller MA, Yannas IV, Gibson LJ, Prendergast PJ (2007) The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering. Technol Health Care 15:3–17
Owen SC, Shoichet MS (2010) Design of three-dimensional biomimetic scaffolds. J Biomed Mater Res A 94:1321–1331. doi:10.1002/jbm.a.32834
Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF (1997) Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385:537–540. doi:10.1038/385537a0
Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM (2000) Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol 148:1075–1090
Paramonov SE, Jun HW, Hartgerink JD (2006) Self-assembly of peptide-amphiphile nanofibers: the roles of hydrogen bonding and amphiphilic packing. J Am Chem Soc 128:7291–7298. doi:10.1021/ja060573x
Pawar K, Mueller R, Caioni M, Prang P, Bogdahn U, Kunz W, Weidner N (2011) Increasing capillary diameter and the incorporation of gelatin enhance axon outgrowth in alginate-based anisotropic hydrogels. Acta Biomater 7:2826–2834. doi:10.1016/j.actbio.2011.04.006
Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG (1998) Polymer concepts in tissue engineering. J Biomed Mater Res 43:422–427
Peyton SR, Kalcioglu ZI, Cohen JC, Runkle AP, Van Vliet KJ, Lauffenburger DA, Griffith LG (2011) Marrow-derived stem cell motility in 3D synthetic scaffold is governed by geometry along with adhesivity and stiffness. Biotechnol Bioeng 108:1181–1193. doi:10.1002/bit.23027
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710
Rosen MR, Brink PR, Cohen IS, Robinson RB (2004) Genes, stem cells and biological pacemakers. Cardiovasc Res 64:12–23. doi:10.1016/j.cardiores.2004.05.012
Roufosse CA, Direkze NC, Otto WR, Wright NA (2004) Circulating mesenchymal stem cells. Int J Biochem Cell Biol 36:585–597. doi:10.1016/j.biocel.2003.10.007
Saunders KB, D’Amore PA (1992) An in vitro model for cell–cell interactions. In Vitro Cell Dev Biol 28A:521–528
Seale P, Rudnicki MA (2000) A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol 218:115–124. doi:10.1006/dbio.1999.9565
Sha’ban M, Kim SH, Idrus RB, Khang G (2008) Fibrin and poly(lactic-co-glycolic acid) hybrid scaffold promotes early chondrogenesis of articular chondrocytes: an in vitro study. J Orthop Surg Res 3:17. doi:10.1186/1749-799X-3-17
Smith IO, Liu XH, Smith LA, Ma PX (2009) Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 1:226–236. doi:10.1002/wnan.26
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. doi:10.1016/j.cell.2006.07.024
Tao SL, Desai TA (2007) Aligned arrays of biodegradable poly(epsilon-caprolactone) nanowires and nanofibers by template synthesis. Nano Lett 7:1463–1468. doi:10.1021/nl0700346
Tienen TG, Heijkants RG, de Groot JH, Pennings AJ, Schouten AJ, Veth RP, Buma P (2006) Replacement of the knee meniscus by a porous polymer implant: a study in dogs. Am J Sports Med 34:64–71. doi:10.1177/0363546505280905
Tucker SP, Melsen LR, Compans RW (1992) Migration of polarized epithelial cells through permeable membrane substrates of defined pore size. Eur J Cell Biol 58:280–290
Vagaska B, Bacakova L, Filova E, Balik K (2010) Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol Res 59:309–322
Verfaillie CM (1992) Direct contact between human primitive hematopoietic progenitors and bone marrow stroma is not required for long-term in vitro hematopoiesis. Blood 79:2821–2826
Wang H, Pieper J, Peters F, van Blitterswijk CA, Lamme EN (2005) Synthetic scaffold morphology controls human dermal connective tissue formation. J Biomed Mater Res A 74:523–532. doi:10.1002/jbm.a.30232
Wang Y, Chang HI, Wertheim DF, Jones AS, Jackson C, Coombes AG (2007) Characterisation of the macroporosity of polycaprolactone-based biocomposites and release kinetics for drug delivery. Biomaterials 28:4619–4627. doi:10.1016/j.biomaterials.2007.07.006
Wang Y, Chang HI, Li X, Alpar O, Coombes AG (2009) Delivery of bioactive macromolecules from microporous polymer matrices: release and activity profiles of lysozyme, collagenase and catalase. Eur J Pharm Sci 37:387–394. doi:10.1016/j.ejps.2009.03.010
Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao C, Chang LJ, Chen YE, Ma PX, Yang B (2014) Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials 35:8960–8969. doi:10.1016/j.biomaterials.2014.07.011
Wenceslau CV, Kerkis I, Lizier NF, Kerkis A (2013) De-Differentiation of somatic cells to a pluripotent state. In: Bhartiya D, Lenka N (eds) Pluripotent stem cells, chapter 3. InTech, Rijeka, Croatia, pp 39–63. doi:10.5772/54372
Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P (2013) Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 201:1069–1084. doi:10.1083/jcb.201210152
Woo KM, Chen VJ, Ma PX (2003) Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res A 67:531–537. doi:10.1002/jbm.a.10098
Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, Kim GS, Somerman MJ, Ma PX (2007) Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials 28:335–343. doi:10.1016/j.biomaterials.2006.06.013
Wozniak MA, Modzelewska K, Kwong L, Keely PJ (2004) Focal adhesion regulation of cell behavior. Biochim Biophys Acta 1692:103–119. doi:10.1016/j.bbamcr.2004.04.007
Xi J, Khalil M, Shishechian N, Hannes T, Pfannkuche K, Liang H, Fatima A, Haustein M, Suhr F, Bloch W, Reppel M, Saric T, Wernig M, Janisch R, Brockmeier K, Hescheler J, Pillekamp F (2010) Comparison of contractile behavior of native murine ventricular tissue and cardiomyocytes derived from embryonic or induced pluripotent stem cells. FASEB J 24:2739–2751. doi:10.1096/fj.09-145177
Yan XL, Fu CJ, Chen L, Qin JH, Zeng Q, Yuan HF, Nan X, Chen HX, Zhou JN, Lin YL, Zhang XM, Yu CZ, Yue W, Pei XT (2012) Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat 132:153–164. doi:10.1007/s10549-011-1577-0
Yang J, Shi G, Bei J, Wang S, Cao Y, Shang Q, Yang G, Wang W (2002) Fabrication and surface modification of macroporous poly(l-lactic acid) and poly(l-lactic-co-glycolic acid) (70/30) cell scaffolds for human skin fibroblast cell culture. J Biomed Mater Res 62:438–446. doi:10.1002/jbm.10318
Yang F, Xu CY, Kotaki M, Wang S, Ramakrishna S (2004) Characterization of neural stem cells on electrospun poly(l-lactic acid) nanofibrous scaffold. J Biomater Sci Polym Ed 15:1483–1497
Yang YL, Motte S, Kaufman LJ (2010) Pore size variable type I collagen gels and their interaction with glioma cells. Biomaterials 31:5678–5688. doi:10.1016/j.biomaterials.2010.03.039
Yannas IV (1992) Tissue regeneration by use of collagen–glycosaminoglycan copolymers. Clin Mater 9:179–187
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920. doi:10.1126/science.1151526
Yuan N, Tian W, Sun L, Yuan R, Tao J, Chen D (2014) Neural stem cell transplantation in a double-layer collagen membrane with unequal pore sizes for spinal cord injury repair. Neural Regen Res 9:1014–1019. doi:10.4103/1673-5374.133160
Zeltinger J, Sherwood JK, Graham DA, Mueller R, Griffith LG (2001) Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng 7:557–572. doi:10.1089/107632701753213183
Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE (2001) Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol 33:907–921. doi:10.1006/jmcc.2001.1367
Zhang Y, Fan W, Ma Z, Wu C, Fang W, Liu G, Xiao Y (2010) The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater 6:3021–3028. doi:10.1016/j.actbio.2010.02.030
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228. doi:10.1089/107632701300062859
Acknowledgments
This work was supported by EU project „Biocardiostim“ (No. VP1-3.1-ŠMM-10-V-02-029).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bružauskaitė, I., Bironaitė, D., Bagdonas, E. et al. Scaffolds and cells for tissue regeneration: different scaffold pore sizes—different cell effects. Cytotechnology 68, 355–369 (2016). https://doi.org/10.1007/s10616-015-9895-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-015-9895-4