Abstract
Spermatogonial stem cells (SSCs) are the only type of cells that transmit genes to the subsequent generations. The proliferation, cultivation and identification of SSCs in vitro are critical to understanding of male infertility, genetic resources and conservation of endangered species. To investigate the effects of glial cell-derived neurotrophic factor (GDNF) and leukemia inhibitory factor (LIF) on the proliferation of mouse SSCs in vitro, supplement of GDNF and/or LIF were designed to culture SSCs. The testes of 6–8 d mouse were harvested and digested by two-step enzyme digestion method. The SSCs and Sertoli cells were separated by differential plating. Then the SSCs were identified by alkaline phosphatase staining, RT-PCR and indirect immunofluorescence cell analysis. The cellular proliferation capacity was measured by methyl thiazolyl tetrazolium assay. The results showed that addition of 20 and 40 ng/ml of GDNF could strongly promote growth of mouse SSCs (p < 0.05). There was no significant difference between LIF treatment groups and the control group in promoting proliferation of the mouse SSCs (p > 0.05). However, the combination of 20 ng/ml GDNF and 1,000 U/ml LIF could significantly enhance the invitro proliferation of mouse SSCs (p < 0.05), and the OD490 value was 0.696 at day 5 of culture when the density of SSCs was 5–10 × 104 cells/ml.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spermatogenesis is a process that generates numerous spermatozoa for a lifetime. The spermatogonial stem cells (SSCs) support the continual spermatogenesis for their capacity of self-renewal and differentiation (Aponte et al. 2005). Long-term culture of SSCs provides a theoretical basis for researches about SSCs. The established SSCs strains provided not only a novel tool to analyze spermatogenesis or stem cell self-renewal but also opened up new areas for practical application (Kanatsu-Shinohara et al. 2003). This concerned widely the supplement of growth factors for regulation of in vitro and in vivo cell growth and proliferation. Recently, some growth factors were identified which have great importance for the proliferation and differentiation of SSCs, such as glial cell-derived neurotrophic factor (GDNF), leukemia inhibitory factor (LIF), and so on (Kanatsu-Shinohara et al. 2007; Oatley et al. 2007).
Glial cell-derived neurotrophic factor (GDNF) is produced by Sertoli cells and regulates cell fate decisions of undifferentiated spermatogonial cells. Over-expression of GDNF resulted in accumulation of SSCs (Meng et al. 2000). GDNF has a positive effect on stem cells maintenance, and was also involved in spermatogonial proliferation and differentiation (Hofmann et al. 2005). LIF has an important role in the regulation of spermatogonial cells compartment (Dorval-Coiffec et al. 2005). It was reported that LIF enhanced the formation of germ cell colonies in neonatal mouse testis culture (Kanatsu-Shinohara et al. 2007). Under these conditions, SSCs from mammalian testicular tissue can differentiate into ES-like cells in vitro (Kanatsu-Shinohara et al. 2004; Guan et al. 2006; Conrad et al. 2008). These researches suggest that LIF is involved in the maintenance of the pluripotency of cells.
Despite these valuable insights, the effects of GDNF and/or LIF on the mouse SSCs proliferation were rarely reported. In this study, mouse SSCs were obtained from the testes of 6–8 d mice, and the effects of GDNF and/or LIF on the mouse SSCs proliferation were investigated.
Materials and methods
Animals
Donor testis cells were obtained from ICR (Institute for Cancer Research) mice (purchased from the Experimental Animal Center of the Medical college of the Xi’an Jiaotong University). Male ICR mice at 6–8 d of age were killed by cervical vertebra dislocation and the testes were collected. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Northwest A&F University.
Isolation and purification of mouse SSCs
The mice testes (6-day-old) were excised and the albuginea was carefully removed to fully expose seminiferous tubules. Single cell suspension of donor testes was collected by a two-step enzymatic digestion with 1 mg/ml collagenase (Sigma Chemical Co., St. Louis, MO, USA) and 0.25 % trypsin (Sigma Chemical Co.). The dispersed cells were filtrated with a 400-eyes mesh and washed twice with DMEM (Gibco, Beijing, China) by 500×g centrifugation. The pellet was suspended in DMEM, which was placed into 25-cm2 cells culture flask and maintained at 37 °C in a humidified atmosphere with 5 % CO2. The cells were cultured by a two-step differential plating process to separate SSCs and Sertoli cells. Briefly, 106 cells per millilitre were cultured in 60-mm dishes for 12 h at 37 °C and 5 % CO2. Then the non-adhering cells (majority of them were SSCs) were transferred into a new plate to culture in the same condition, respectively, and were observed every 4 h. The medium was changed every 2–3 d.
Culture medium for mouse SSCs
The primary component of culture medium was DMEM/F12 supplemented with 10 % FBS (Hyclone, Logan, UT, USA), 100 IU/ml penicillin (Gibco/Invitrogen), 100 μg/ml streptomycin (Gibco/Invitrogen), and 0.1 mM Non-Essential Amino Acids (NEAA) (Gibco/Invitrogen). The culture schemes of GDNF and LIF (both: Sigma) are shown in Table 1.
Alkaline phosphatase (AKP) assay
AKP is a pluripotency cell marker (Neri et al. 2007; Park et al. 2008). AKP positive cells have the properties of stem cells that they can be stained into blue violet or brown. Mouse SSCs were fixed with 4 % paraformaldehyde and then stained with NBT/BCIP AKP substrate (Solarbio, Beijing, China) for 10–15 min. Then the cells were observed and photographed under an inverted phase contrast microscope (Nikon Imaging Sales Co Ltd., Tokyo, Japan).
Indirect immunofluorescence cell analysis
CD9 was considered as a marker to identify for undifferentiated type A spermatogonia (Abu Elhija et al. 2012) by indirect immunofluorescence staining. Briefly, the cells were fixed in 4 % paraformaldehyde for 15 min and then rinsed triply in PBS with 0.1 % Tween-20. Subsequently, the cells were resuspended in phosphate-buffered saline with bovine serum albumin (PBS-BSA) over an hour at 37 °C. Anti-CD9 antibody (Abcam, Cambridge, UK) (final concentration 1:1,000) was added to the solution for 12 h at 37 °C and then triply washed in PBS. The Cy3 AffiniPure Goat Anti-Rabbit IgG 140 (H + L) (Invitrogen, Carlsbad, CA, USA) (final concentration 1:1,000) was added and incubated for an hour at 37 °C. Finally, the solution was incubated with Hoechst 33258 (Invitrogen) (1:800) for 8 min and then washed thrice with PBS, and the cells were resuspended in 0.5 ml PBS-BSA. The fluorescence was monitored using a 8- and a 644-nm double band-pass filter by fluorescence microscope (Nikon Imaging Sales Co Ltd., Tokyo, Japan).
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis
Total cellular RNA was isolated from mouse SSCs and Sertoli cells by RNA simple Total RNA Kit (TianGen Co. Ltd., Beijing, China) and was reversed transcribed by superscript first-strand synthesis system. The cDNA were then analyzed by polymerase chain reaction (PCR). The primer sequences, GenBank accession numbers, and sequence sizes were as follows: the Ngn3 (GeneBank No. NM_009719.6) Forward: 5′-TTG GCA CTC AGC AAA CAG C-3′; Reverse: 5′-TCC CTT TCC ACT AGC ACC C-3′ 467 bp; Oct4 (GeneBank No. NM_013633.2) Forward: 5′-CCC CAA TGC CGT GAA GTT-3′; Reverse: 5′-GAA A GG TGT CCC TGT AGC C-3′ 556 bp; β-actin (GeneBank No.NM_007393.3) Forward: 5′-GCC TTC CTT CTT GGG TAT-3′; Reverse: 5′- CCT TCA CCG TTC CAG TTT-3′ 549 bp; Integrin alpha 6 (GeneBank No.NM_008397.3) Forward: 5′-ATG ATG AAA GTC TCG TGC-3′; Reverse: 5′-CAT AGC CAA ACG AGG AAG-3′, 222 bp; Integrin beta 1 (GeneBank No. NM_010578.2) Forward: 5′ TTG ATG AAT GAA ATG AGG AG 3′, Reverse: 5′-TCC AGA TAT GCG TTG CTG-3′, 225 bp; Sycp3 (GeneBank No. NM_011517.2) Forward: 5′-TCA GAG CCA GAG AAT GAA AG-3′; Reverse: 5′-CTG CTG AGT TTC CAT CAT AAC-3′, 163 bp; TH2B (GeneBank No.NM_175663.1) Forward: 5′- CGG TAA AGG GTG CTA CTA T-3′; Reverse: 5′-CACTTGTTTCAGCACCTTA-3′, 137 bp. The reaction products were separated and visualized by 1.0 % agarose gel electrophoresis.
Proliferation assay
The SSCs were treated with 5 mg/ml 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) for 4 h at 37 °C. Then the supernatant was discarded and DMSO (dimethyl sulfoxide) was added to dissolve the blue-violet substances (formazan) in cells. Subsequently, the SSCs were placed in shaker for 20 min and the absorbance of cells (OD490) was measured at 490 nm.
Statistical analysis
For culture assays of mouse SSCs with growth factors of GDNF and LIF in vitro, each data point represents the means of three separate experiments ± standard deviation. Statistical significance among the proliferation of cells was determined by using ANOVA and subsequent pair-wise analysis by Duncan test (least significant difference) of the SPSS statistical software (SPSS16 for Windows; SPSS, Chicago, IL, USA). p < 0.05 indicated statistical significance.
Results
Isolation and purification of mouse SSCs
To obtain high purity SSCs, SSCs were separated by two-step differential plating method. Cell colonies appeared at 6 d, whose morphology were round and small, similar with mouse SSCs (Fig. 1). That was different for the Sertoli cells which were irregular form (Fig. 1).
Alkaline phosphatase (AKP) assay
AKP is a pluripotency cell marker (Neri et al. 2007; Park et al. 2008). AKP positive cells have the properties of stem cells that can be stained into blue violet or brown. Twenty-four hours after transfer into a new flask, most of the round shaped SSCs began to divide and proliferate. AKP staining of SSCs was positive and SSCs clusters were stained brown or bluish violet (Fig. 2).
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis
The NGN3 gene is expressed specifically in undifferentiated spermatogonia (Yoshida et al. 2004). It was shown that Oct4 keeps ES cells from differentiating and governs pluripotency, self-renewal, genome surveillance and cell fate determination (Loh et al. 2006). It was reported that beta 1- and alpha 6- integrin are surface markers of mouse SSCs (Shinohara et al. 1999). The expression of TH2B and Scyp3 are considered as markers of differentiated spermatogonial stem cells (Lim et al. 2010). In Fig. 3, SSC colonies expressed Oct4, integrin alpha 6, integrin beta 1, β-actin and Ngn3, while the Sertoli cells only expressed the β-actin (not shown).
CD9 expression on the cell surface of the mouse SSCs
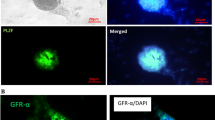
It is commonly considered that the As (a single) and Apr (a paired) spermatogonia have stem-cell properties. CD9 is the surface maker of the As (a single) and Apr (a paired) spermatogonia. It is shown in Fig. 4 that the cell colonies expressed CD9 on the cell surface after culture of 3–5 days. The cells showed a large spherical nucleus with a thin rim of cytoplasm (Fig. 4a, c), whose morphology was similar to that of mouse SSCs.
Effects of GDNF on mouse SSCs proliferation in vitro
In order to analyze the effect of GDNF on the proliferation of mouse SSCs in vitro, SSCs were cultured in the presence of a range of doses of GDNF. The cell proliferation capacity was detected by MTT assay and the value of OD490 was measured at 3 d, 5 d and 7 d respectively, in the presence of GDNF. The results are shown in Table 2. At day 3, the proliferation capacity of mouse SSCs in the 20 ng/ml GDNF group and the 40 ng/ml GDNF group was significantly greater than that of the control group and the 10 ng/ml GDNF group (p < 0.05), but there was no significant difference between the 20 ng/ml GDNF and the 40 ng/ml GDNF group. At days 5 and 7, the proliferation capacity of the mouse SSCs in the 20 ng/ml GDNF group was the highest compared with the other groups (p < 0.05). No difference was observed for the proliferation capacity between the control and 10 ng/ml GDNF group (p > 0.05).
Effect of LIF on mouse SSCs proliferation in vitro
To detect the effect of LIF on the proliferation of mouse SSCs in vitro, an experiment similar to that for studying the effect of GDNF on mouse SSCs proliferation was performed. The results are shown in Table 3. However, Table 3 shows that there were no significant differences between the LIF treatment groups and the control groups in proliferation of the mouse SSCs (p > 0.05). LIF played no significant role in promoting proliferation of the mouse SSCs in vitro.
Effect of the combination of GDNF and LIF on mouse SSCs proliferation in vitro
The effect of the combination of GDNF and LIF on the proliferation of mouse SSCs was measured on days 3 and 5 (Fig. 5). Regardless of the concentration of GDNF, the proliferation capacity of the mouse SSCs cultured in 1,000 U/ml LIF was significantly higher than those cultured in 500 and 1,500 U/ml (p < 0.05). Meanwhile, regardless of the concentration of LIF, the optimal concentration of GDNF was 20 ng/ml and the OD490 value was 0.696 on day 5. The mouse SSCs cultured in the presence of 20 ng/ml GDNF and 1,000 U/ml LIF showed the highest proliferation capacity in vitro.
The combined effects of GDNF and LIF on the proliferation of mouse SSCs at 5 d. Note The different letters (a–c) indicate that there was a significant difference between the different LIF treatment groups for the same treatment group of GDNF. The different letters (x–z) demonstrate that there was a significant difference between the different GDNF treatment groups for the same treatment group of LIF
Discussion
Establishing immortalization of germ stem cell lines is a new tool to preserve genetic information of animals. For the SSCs, the immature testis is a good source as it lacks differentiated germ cells and is rich in SSCs. In particular, germ cells are separated from the basement membrane and located in the lumen of immature seminiferous tubules in the neonatal testis. The types of the germ cells present in the neonatal testis are early differentiating spermatogonia and SSCs (de Rooij 2006). Hence, SSCs can be efficiently recovered by two-step differential plating methods.
The AKP assay, RT-PCR and indirect immunofluorescence cell analysis were performed to detect SSCs. AKP is commonly expressed in pluripotent cells and is considered as a pluripotency marker (Neri et al. 2007; Park et al. 2008). In the AKP assay, AKP positive cells are stained brown, showing that the cells had the properties of stem cells. The results confirmed those of previous studies (Guan et al. 2006; Goel et al. 2007). In male mice, the expression of Oct4 gene is maintained until the beginning of spermatogenesis which is confined to type A spermatogonia (Feng et al. 2002). A previous study showed that Ngn3 was predominantly expressed in the As, Apr and Aal stages of c-Kit negative spermatogonia in adults and in the c-Kit negative fraction of the prepubertal prespermatogonia (Yoshida et al. 2004). The obtained mouse SSCs were Ngn3 positive and belonged thus to the undifferentiated A spermatogonia. The integrin alpha 6 and integrin beta 1 genes are considered surface markers of SSCs. TH2B and Scyp 3 are considered as the markers of differentiated spermatogonial stem cells (Lim et al. 2010). The cell colonies expressed Oct4, integrin alpha 6, integrin beta 1, β-actin, and Ngn3, while Sertoli cells only expressed β-actin. CD9 was selected as the surface maker of SSCs. In the Fig. 4 it was showed that the enriched cells had a large spherical nucleus with a thin rim of cytoplasm by performing indirect immunofluorescence cell analysis (Fig. 4c). The morphology of the cells was the same as the mouse SSCs. Based on these results, the obtained mouse SSCs were indeed SSCs.
In order to reliably assess the effect of exogenous growth factors on the proliferation of mouse SSCs, serum-free culture conditions were employed. It was reported that the addition of one to two growth factors could maintain cell survival in vitro (Rossi et al. 1993). In our study, GDNF or LIF alone or in combinaion were supplemented to the medium and the effects on the mouse SSCs proliferation were evaluated. GDNF or LIF alone was capable of initiating proliferation of the mouse SSCs and cell colonies appeared after culturing with GDNF or LIF alone for 7 d. The supplement of 20 ng/ml GDNF could significantly improve the SSCs proliferation in vitro. GDNF is a member of the transforming growth factor-β super-family and is the essential growth factor for SSCs self-renewal. The addition of GDNF resulted in a significant improvement in stem cell maintenance (Nagano et al. 2003). Regulation of spermatogonial proliferation by GDNF depends on Ras and PI3 kinase pathways since their activation has been shown in neurons and kidney cells. GDNF induces CREB/ATF-1 family member of phorphorylation and c-fos transcription via the Ras/ERK1/2 pathway to promote the proliferation of SSCs (He et al. 2008). It was hypothesized that the higher concentration of GDNF would inhibit cell proliferation and the lower concentration of GDNF would insufficiently stimulate cell proliferation.
For LIF, the MTT assay indicated that the effect on the proliferation of the mouse SSCs was not significant, which was different from GDNF. The addition of LIF to serum-containing medium did not affect the proliferation of the mouse SSCs in short-term cultures. LIF has different biological activity in various tissues and cells, such as, ES cells, primordial germ cells (PGCs), liver cells and endothelial cells. Although LIF is important for ES cells and PGCs, it has no significant effect on SSCs in vitro. Nevertheless, LIF is involved in the maturation of gonocytes into spermatogonia (Kanatsu-Shinohara et al. 2007). Our results showed that supplied LIF had no significant effect on mouse SSCs proliferation in vitro and thus confirmed previous studies.
To analyze the effect of GDNF, LIF and the combination on SSCs proliferation, long- and short-term cultured SSCs had been performed and GDNF had a beneficial effect on maintenance during 7-day culture period in our study (Table 2). In the study, the combination of 20 ng/ml GDNF and 1,000 U/ml LIF has significantly enhanced the proliferation of mouse SSCs in vitro. It was reported that the inclusion of LIF in GDNF-dependent serum-free cultures did not significantly enhance the expansion of SSCs (Kubota et al. 2004), which means that SSCs derived from different strains have different requirements for growth factors. However, the addition of 500 U/ml or 1,500 U/ml LIF along with GDNF improved the maintenance of SSCs clumps and showed modest expansion of SSCs in culture. A similar but higher response was found while 1,000 U/ml LIF was added with GDNF. The degree of SSC proliferation in the culture with 20 ng/ml GDNF and 1,000 U/ml LIF was greater than that in the combination of 10 ng/ml GDNF or 40 ng/ml GDNF with different doses of LIF. These results may suggest that the effects of GDNF on the mouse SSCs were modulated by LIF. It was shown that LIF promoted the survival and proliferation of PGCs (Dorval-Coiffec et al. 2005; Jenab and Morris 1998). LIF enhances the formation of GS cell clumps in culture but does not affect their self-renewal rate during long-term culture (Kanatsu-Shinohara et al. 2007). LIF binds to a heterodimeric receptor (LIF receptor) that includes the LIF-specific binding subunit and the transmembrane signal-transducing subunit IL-6ST (Schindler and Darnell 1995). Binding of LIF to this receptor complex results in the phosphorylation of several tyrosine residues in IL-6ST by the activated Janus kinase (JAK) tyrosine kinases, which then activates signal transducer and activator of transcription (STAT) factor (Schindler and Darnell 1995; Taga and Kishimoto 1997). The LIF system may cooperate with the GDNF signaling pathway to support self-renewal of SSCs.
The success of SSCs culture in vitro has opened new possibilities in SSCs research. Although human and mouse SSCs are similar, the fact that human SSCs can not be maintained in the culture systems identical to the mouse system indicates that differences between human and mouse SSCs exist (Schmidt et al. 2011). The present study indicated that 20 ng/ml GDNF combined with 1,000 U/ml LIF was the optimal factor combination which could significantly enhance the proliferation of mouse SSCs in vitro. The culture system will be of great benefit to the research of the pluripotency of SSCs, production of genetically modified animals, gene therapy, etc.
References
Abu Elhija M, Lunenfeld E, Schlatt S, Huleihel M (2012) Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl 14:285–293
Aponte PM, van Bragt MP, de Rooij DG, van Pelt AM (2005) Spermatogonial stem cells: characteristics and experimental possibilities. APMIS 113:727–742
Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T (2008) Generation of pluripotent stem cells from adult human testis. Nature 456:344–349
de Rooij DG (2006) Rapid expansion of the spermatogonial stem cell tool box. Proc Natl Acad Sci USA 103:7939–7940
Dorval-Coiffec I, Delcros JG, Hakovirta H, Toppari J, Jégou B, Piquet-Pellorce C (2005) Identification of the leukemia inhibitory factor cell targets within the rat testis. Biol Reprod 72:602–611
Feng LX, Chen YL, Dettin L, Pera RAR, Herr JC, Goldberg E, Dym M (2002) Generation and in vitro differentiation of a spermatogonial cell line. Science 297:392–395
Goel S, Sugimoto M, Minami N, Yamada M, Kume S, Imai H (2007) Identification, isolation, and in vitro culture of porcine gonocytes. Biol Reprod 77:127–137
Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W (2006) Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440:1199–1203
He ZP, Jiang JJ, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M (2008) GDNF upregulates c-fos transcription via the Ras/ERK1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells 26:266–278
Hofmann MC, Braydich-Stolle L, Dym M (2005) Isolation of male germ-line stem cells; influence of GDNF. Dev Biol 279:114–124
Jenab S, Morris PL (1998) Testicular leukemia inhibitory factor (LIF) and LIF receptor mediate phosphorylation of signal transducers and activators of transcription (STAT)-3 and STAT-1 and induce c-fos transcription and activator protein-1 activation in rat Sertoli but not germ cells. Endocrinology 139:1883–1890
Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T (2003) Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod 69:612–616
Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S (2004) Generation of pluripotent stem cells from neonatal mouse testis. Cell 119:1001–1012
Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Yoshida S, Toyokuni S, Lee J, Ogura A, Shinohara T (2007) Leukemia inhibitory factor enhances formation of germ cell colonies in neonatal mouse testis culture. Biol Reprod 76:55–62
Kubota H, Avarbock MR, Brinster RL (2004) Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA 101:16489–16494
Lim JJ, Sung SY, Kim HJ, Song SH, Hong JY, Yoon TK, Kim JK, Kim KS, Lee DR (2010) Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif 43:405–417
Loh YH, Wu Q, Chew JL, Vega VB, Zhang WW, Chen X, Bourque G, George J, Leong B, Liu J, Wong KY, Sung KW, Lee CWH, Zhao XD, Chiu KP, Lipovich L, Kuznetsov VA, Robson P, Stanton LW, Wei CL, Ruan YJ, Lim B, Ng HH (2006) The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet 38:431–440
Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M (2000) Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287:1489–1493
Nagano M, Ryu BY, Brinster CJ, Avarbock MR, Brinster RL (2003) Maintenance of mouse male germ line stem cells in vitro. Biol Reprod 68:2207–2214
Neri T, Monti M, Rebuzzini P, Merico V, Garagna S, Redi CA, Zuccotti M (2007) Mouse fibroblasts are reprogrammed to Oct-4 and Rex-1 gene expression and alkaline phosphatase activity by embryonic stem cell extracts. Cloning Stem Cells 9:394–406
Oatley JM, Avarbock MR, Brinster RL (2007) Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on src family kinase signaling. J Biol Chem 282:25842–25851
Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141–147
Rossi P, Dolci S, Albanesi C, Grimaldi P, Ricca R, Geremia R (1993) Follicle-stimulating hormone induction of steel factor (SLF) mRNA in mouse sertoli cells and stimulation of DNA synthesis in spermatogonia by soluble SLF. Dev Biol 155:68–74
Schindler C, Darnell J Jr (1995) Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 64:621–652
Schmidt JA, Abramowitz LK, Kubota H, Wu X, Niu Z, Avarbock MR, Tobias JW, Bartolomei MS, Brinster RL (2011) In vivo and in vitro aging is detrimental to mouse spermatogonial stem cell function. Biol Reprod 84:698–706
Shinohara T, Avarbock MR, Brinster RL (1999) beta(1)- and alpha(6)-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA 96:5504–5509
Taga T, Kishimoto T (1997) Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 15:797–819
Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, Nabeshima Y (2004) Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol 269:447–458
Acknowledgments
This research was supported by the Youth Extra Fund of Northwest A&F University (Z111020905), the Basic Scientific Research Expense of Sci-Tech Innovation Major Project of Northwest A&F University (QN2011061) and the Special Research Subsidy Project of Northwest A&F University (07ZR002).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, P., Suo, LJ., Wang, YF. et al. Effects of GDNF and LIF on mouse spermatogonial stem cells proliferation in vitro. Cytotechnology 66, 309–316 (2014). https://doi.org/10.1007/s10616-013-9574-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-013-9574-2