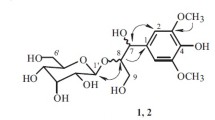
The new natural compound salicyl alcohol 7-O-β-D-(6′-O-benzoyl)glucopyranoside, which we called isopopulin, and the known compounds gaulterin, gallic acid, quercetin, spireoside, and astragalin were isolated from the aerial part of Filipendula ulmaria (L.) Maxim. The chemical structures of the isolated phenolic compounds were studied using UV, 1H and 13C NMR spectroscopy; mass spectrometry; and chemical transformations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Filipendula ulmaria (L.) Maxim. (Rosaceae) is broadly distributed in European Russia and western and eastern Siberia [1, 2]. Currently, the herb of F. ulmaria is included in pharmacopoeias of many countries [3]. Flowers of F. ulmaria are the only pharmacopoeial type of raw material in the Russian Federation (Pending Monograph 42-1777-87, Meadowsweet flowers) [4].
Preparations based on F. ulmaria raw material possess broad spectra of biological activity, including anti-inflammatory, antimicrobial, hepatoprotective, antioxidant, and nootropic [2,3,4,5,6,7,8,9,10]. The chemical composition of the aerial part of F. ulmaria is represented by phenols (gaulterin, spirein, salicylic acid, gallic acid, ethyl gallate, 4-methoxybenzoic acid, salicylaldehyde), a coumarin (esculetin), flavonoids (kaempferol, quercetin, isoquercitrin, avicularin, spireoside, quercetin 4′-O-β-Dgalactopyranoside, rutin), and triterpene saponins (oleanolic and ursolic acids) [2, 4,5,6, 11, 12].
The aim of the present work was to study the constituent composition of the aerial part of F. ulmaria collected in Alekseevka, Samara Region, in July 2021 during flowering.
Chromatographic studies isolated from the aerial part of F. ulmaria the phenolic compounds 1–6.
The 1H NMR spectrum of 1 exhibited resonances for nine aromatic protons of salicylic alcohol and a benzene ring at 7.44 ppm (2H, dd, J = 2.0, 8.0, H-2′′, H-6′′), 7.26–7.35 (4H, m, H-3, H-3′′, H-4′′, H-5′′), 7.13 (1H, dt, J = 2.0, 8.0, H-4), 6.68 (1H, d, J = 8.0, H-2), and 6.48 (1H, d, J = 8.0, H-5). Also, the 1H NMR spectrum of 1 had two 1H doublets at 5.25 and 5.10 ppm with spin−spin coupling constant (SSCC) 13.0 Hz for CH2OH groups of salicylic alcohol. A resonance for glucose C-6′ at 66.53 ppm in the 13C NMR spectrum of 1 confirmed that the glucose CH2OH group (C-6) was esterified by benzoic acid. A free phenolic group in 1 was confirmed by a 1H singlet for the phenolic OH of salicylic alcohol at 9.95 ppm in the 1H NMR spectrum of 1. This indicated that the glucose was bonded to the CH2OH group of salicylic alcohol.

This conclusion was confirmed by 1 having a free phenolic OH on C-1, in contrast to populin, and appearing as an orange spot under TLC conditions upon treatment by a basic solution of diazobenzenesulfonic acid. Therefore, 1 had the structure salicylic alcohol 7-O-β-D-(6′-O-benzoyl)glucopyranoside and was a new natural compound that we called isopopulin. Previously, populin with a structure close to that of isopopulin was isolated and had a glucose bonded to the phenolic OH group of salicylic alcohol. It is interesting that 1 and populin had significantly different melting points of 269–272°C and 179–180°C, respectively.
Compounds 2 and 3 were identified using UV, NMR, and mass spectral data as gaulterin and gallic acid, which were reported from F. ulmaria [2, 5, 6, 11]; flavonoids 4–6, as quercetin (4), spireoside (5), and astragalin (6), which were isolated by us for the first time from the aerial part of F. ulmaria.
Experimental
The chemical structures of the compounds isolated from the aerial part of F. ulmaria were studied using UV, 1H and 13C NMR spectroscopy. 1H and 13C NMR spectra were taken on a JNM-ECX 400 instrument (399.78 MHz for 1H; 100.52 MHz for 13C). High-resolution mass spectra were recorded on a Bruker maXis impact instrument using electrospray ionization (ESI). Spectra were recorded in 10-mm cuvettes on a Specord 40 spectrophotometer (Analytik Jena AG, Germany) in the range 190–500 nm. Acid hydrolysis of phenolic and flavonoid glycosides 1, 2, 5, and 6 used HCl (2%) on a boiling-water bath for 2 h. Enzymatic hydrolysis of flavonoids 5 and 6 used an aqueous solution of β-glucosidase (Sigma). Monosaccharides in acid hydrolysates of the glycosides were identified by paper chromatography using the solvent system n-BuOH–glacial AcOH–H2O in a 4:1:2 ratio (anilinium phthalate reagent).
Extraction and Isolation of the Compounds. Air-dried aerial part of F. ulmaria (200 g) collected during flowering was extracted with EtOH (70%). The combined aqueous EtOH extract was evaporated under vacuum to a thick residue (60 g) that was placed onto KSK silica gel 50/100 (Russia), dried, and separated by chromatography over silica gel. The eluents were CHCl3 and CHCl3–EtOH mixtures (97:3, 95:5, 93:7, 90:10, 85:15, 80:20, 70:30, 60:40, 50:50). Eluates were divided into fractions of approximately equal volumes (200 mL each).
Fractions obtained by elution with CHCl3–EtOH (70:30) isolated crystalline 1, which was purified by rechromatography over polyamide (0.4% yield) using H2O as the eluent. Further elution of this chromatography column by 40% EtOH produced 3 (0.2% yield). Fractions obtained upon elution by CHCl3–EtOH (60:40) isolated crystalline 2, which was purified by rechromatography over polyamide (0.7% yield) using H2O as the eluent. Fractions obtained by elution with CHCl3–EtOH (93:7) isolated crystalline 4, which was purified by rechromatography (0.1% yield) over polyamide using EtOH (70%) as the eluent. Fractions obtained by elution with CHCl3–EtOH (80:20) produced a mixture of 5 and 6 that was separated by rechromatography (0.3% and 0.05% yields, respectively) over polyamide using EtOH (40% and 70%) as eluents.
Structural studies of isopopulin (1) used databases such as CAS Common Chemistry, PubChem, ChemSynthesis, and others.
Isopopulin [salicylic alcohol 7-O-β-D-(6′-O-benzoyl)glucopyranoside] (1), white crystalline compound, C20H22O8, mp 269–272°C (EtOH). UV (EtOH, λmax, nm): 233, 288. 1H NMR (400 MHz, DMSO-d6, δ, ppm, J/Hz): 9.95 (1H, s, 1-OH), 7.44 (2H, dd, J = 2.0, 8.0, H-2′′, 6′′), 7.26–7.35 (4H, m, H-3, 3′′, 4′′, 5′′), 7.13 (1H, dt, J = 2.0, 8.0, H-4), 6.68 (1H, d, J = 8.0, H-2), 6.48 (1H, d, J = 8.0, H-5), 5.27 (1H, d, J = 13.0, H-7), 5.10 (1H, d, J = 13.0, H-7), 5.01 (1H, d, J = 7.0, Glc H-1′), 2.8–4.9 (9H, m, 6H Glc and 3H of three glucose hydroxyls on C-2′, 3′, C-4′). 13C NMR (100 MHz, DMSO-d6, δ, ppm): 166.32, 155.81, 136.76, 131.72, 128.81, 128.28, 128.28, 112.39, 109.86, 106.39, 104.43, 104.38, 100.88, 77.16, 76.97, 73.91, 70.08, 66.53, 66.18. HR-ESI-MS m/z 413.2661 [M + Na]+ (calcd for C20H22O8Na, 413.3748).
Gaulterin (methylsalicylate 2-O-β-D-primeveroside) (2), C19H26O12, mp 278–280°C (EtOH). UV (EtOH, λmax, nm): 243, 286, 315 (sh). Spectral data for gaulterin agreed with those in the literature [5].
Gallic acid (3), C7H6O5, mp 219–221°C (EtOH). UV (EtOH, λmax, nm): 278. Spectral data for gallic acid agreed with those in the literature [5].
Quercetin (3,5,7,3′,4′-pentahydroxyflavone) (4), C15H10O7, mp 314–315°C (aq. EtOH). UV (EtOH, λmax, nm): 257, 268 sh, 375; + NaOAc 273, 386; + NaOAc + H3BO3 273, 390; +A1C13 273, 425; +A1C13 + HCl 270, 401. Spectral data agreed with the literature [5].
Spireoside (quercetin 4′-O-β-D-glucopyranoside) (5), C21H22O12, mp 228–230°C (aq. EtOH). UV (EtOH, λmax, nm): 260, 274 sh, 372; +NaOAc 276, 384; +NaOAc + H3BO3 276, 384; +A1C13 265, 274, 424; +A1C13 + HCl 265, 274, 424. Spectral data agreed with the literature [5].
Astragalin (3,5,7,4′-tetrahydroxyflavone 3-O-β-D-glucopyranoside) (6), C21H20O11, mp 173–176°C (aq. EtOH). UV (EtOH, λmax, nm): 269, 355; + NaOAc 274, 368; + NaOAc + H3BO3 272, 356; +AlCl3 and +AlCl3 + HCl 275, 306, 396. 1H NMR (400 MHz, DMSO-d6, δ, ppm, J/Hz): 12.38 (1H, s, 5-OH), 10.79 (1H, s, 7-OH), 9.50 (1H, s, 4′-OH), 8.10 (2H, d, J = 9.0, H-2′, 6′), 7.15 (2H, d, J = 9.0, H-3′, 5′), 6.41 (1H, d, J = 2.5, H-8), 6.16 (1H, d, J = 2.5, H-6), 5.34 (1H, d, J = 7.0, Glc H-1′′), 2.9–4.8 (6H, m, 6H Glc). 13C NMR (100 MHz, DMSO-d6, δ, ppm): 176.57 (C-4), 164.59 (C-7), 161.23 (C-5, 9), 156.75 (C-2), 147.29 (C-4′), 136.81 (C-3), 129.69 (C-2′, 6′), 124.91 (C-1′), 116.65 (C-3′, 5′), 103.61 (C-10), 101.93 (Glc C-1′′), 98.75 (C-6), 94.02 (C-8), 77.81 (C-5′′), 76.67 (C-3′′), 73.79 (C-2′′), 70.17 (C-4′′), 61.22 (C-6′′). HR-ESI-MS m/z 447.0930 [M – H]– (calcd 447.3701); m/z 449.1079 [M + H]+; 471.0898 [M + Na]+; 487.0846 [M + K]+.
References
P. F. Maevskii, Flora of Central European Russia [in Russian], KMK, Moscow, 2006, 292 pp.
Plant Resources of the USSR. Flowering Plants, Their Chemical Composition and Use: Families Hydrangeaceae–Haloragaceae [in Russian], Nauka, Leningrad, 1987, 326 pp.
T. L. Kiseleva and Yu. A. Smirnova, Medicinal Plants in Global Medical Practice: State Regulation of Nomenclature and Quality [in Russian], Izd. Professional′noi Assotsiatsii Naturoterapevtov, Moscow, 2009, 295 pp.
V. A. Kurkin, Pharmacognosy: Textbook for Students of Pharmaceutical Higher Educational Institutions (Faculties) [in Russian], OOO Ofort, Samara, 2019, 1278 pp.
I. V. Shilova, N. I. Suslov, and I. A. Samylina, Chemical Composition and Nootropic Activity of Siberian Plants [in Russian], Izd. Tomsk. Univ., Tomsk, 2010, 234 pp.
I. V. Shilova, I. A. Samylina, and N. I. Suslov, Development of Nootropic Agents from Siberian Plants [in Russian], Pechatnaya Manufaktura, Tomsk, 2013, 236 pp.
K. N. Sazanova, S. Kh. Sharipova, and A. V. Lyamin, Aspirant. Vestn. Povolzh′ya, 5–6, 22 (2018).
I. V. Shilova, E. A. Gereng, T. V. Zhavoronok, N. I. Suslov, and T. P. Novozheeva, Vopr. Biol., Med. Pharm. Khim., No. 2, 28 (2010).
I. V. Shilova, N. I. Suslov, N. V. Provalova, S. G. Aksimenko, and A. P. Deveikina, Vopr. Biol., Med. Farm. Khim., No. 4, 24 (2008).
I. V. Shilova, E. A. Krasnov, E. I. Koretkova, M. G. Nagaev, and A. N. Lukina, Khim.-farm. Zh., 40 (12), 22 (2006).
E. A. Krasnov and E. Yu. Avdeeva, Khim. Rastit. Syr′ya, No. 4, 5 (2012).
I. V. Shilova, I. A. Samylina, and N. I. Suslov, Farmatsiya, 61 (2), 19 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2024, pp. 557–559.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kurkin, V.A., Sokolov, N.S., Sharipova, S.K. et al. Phenolic Compounds from the Aerial Part of Filipendula ulmaria. Chem Nat Compd 60, 642–644 (2024). https://doi.org/10.1007/s10600-024-04403-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-024-04403-6