Chemical investigation of the stems and leaves of Passiflora edulis Sims led to the isolation of a pair of phenylethanoid glycosides (1, 2), two benzyl alcohol glycosides (3, 4), and two cyanogenic glycosides (5, 6). Compounds 1 and 2 were an inseparable mixture in a 1:2 ratio. The structures were identified by spectroscopic analysis and comparison with literature data. Compound 1 was a new phenylethanoid alloside, and compound 2 was found in this plant for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Passiflora edulis Sims (Passifloraceae), also known as passion fruit, is widespread in tropical and subtropical regions of the world and is popular for its nutritional balance and health benefits [1]. P. edulis has two main and common varieties: purple (P. edulis Sims) and yellow (P. edulis var. flavicarpa Degener) [2]. Although the stems and leaves have long been used in traditional folk medicine to treat many neurogenic disorders in Europe and America [3], most of them are discarded as waste in China and are not comprehensively utilized. Previous chemical investigations of the aerial part of this species have reported on the isolation of cycloartane triterpenoids [4, 5], flavonoids [2, 6], cyanogenic glycosides, and aromatic glycosides [7, 8]. As part of our ongoing study on the comprehensive utilization of subtropical fruits, the present paper reports the isolation and structural elucidation of a new phenylethanoid glycoside (1) and five known glycosides (2–6) from the stems and leaves of P. edulis Sims.

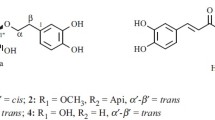
The HR-ESI-MS for the mixture of 1 and 2 showed a molecular ion peak at m/z 307.1165 [M + Na]+ (calcd 307.1152), corresponding to the molecular formula C14H20O6 (5 degrees of unsaturation). Although the mixture was not liable to separation by HPLC under various conditions, its NMR spectra contained a pair of well-separated signals, indicating the presence of a pair of isomers (1 and 2) in a 1:2 ratio. The 1H and 13C NMR data for 1 were readily assigned in Table 1 based on the 2D NMR spectra and the published data [7,8,9]. The 1H NMR data of 1 indicated the characteristic signals belonging to a phenethyl moiety [a monosubstituted phenyl ring at δ 7.25 (4H, m), 7.16 (1H, m); two methylenes at δ 2.92 (2H, m, H-7), 3.77, 4.04 (1H each, m, H-8)], and a sugar moiety [δ 4.66 (1H, d, J = 7.8 Hz)]. The HMBC correlations (Fig. 1) of H-8 (δ 3.77, 4.04) with C-7 (δ 37.3), C-1 (δ 140.1), and C-1′ (δ 101.9), of H-7 (δ 2.92) with C-8 (δ 71.7), C-1, and C-2, 6 (δ 129.3), of H-1′ (δ 4.66) with C-8, which confirmed the presence of the phenethyl moiety linked to C-1′ of the sugar unit. In the 1H NMR spectrum, a small coupling constant (J = 3.0 Hz) of the equatorial H-3′ indicated the presence of D-allose for compound 1 [8]. Notably, the NMR signals due to the sugar moiety were also superimposable with those of benzyl-O-β-D-allopyranoside (3) and (2R)-β-D-allopyranosyloxy-2-phenylacetonitrile (5) isolated from the same plant [7, 8]. The coupling constant (J = 7.8 Hz) of the anomeric H-atom suggested that the allose residue was β-form. Therefore, the structure of compound 1 was elucidated as phenylethyl-O-β-D-allopyranoside.
The known compounds were identified as phenylethyl-O-β-D-glucopyranoside (2) [9], benzyl-O-β-D-allopyranoside (3) [7], benzyl-O-β-D-glucopyranoside (4) [8], (2R)-β-D-allopyranosyloxy-2-phenylacetonitrile (5) [7], and (2R)-prunasin (6) [8] by comparison with the reported NMR data.
EXPERIMENTAL
General Experimental Procedures. Sample separation was carried out on MCI (75–150 μm, Mitsubishi Chemical Corporation, Japan) and silica gel (100–200 mesh, Qingdao Marine Chemical Factory, China) column chromatography. Semipreparative HPLC was performed on an Agilent 1200 liquid chromatograph with an Eclipse XDB-C18 (5 μm, 9.4 × 250 mm). HR-ESI-MS data were obtained using a LC/MS-IT-TOF mass spectrometer. NMR spectra were measured on an Avance III HD 500 spectrometer. Fractions were monitored by TLC and spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in EtOH. All solvents were distilled prior to use.
Plant Material. The air-dried stems and leaves of P. edulis Sims were collected from Longsheng Various Nationalities Autonomous County, Guangxi Province, China, in December, 2019, and identified by one of the authors (Prof. Zheng-Hong Pan). A voucher specimen (CTM2019-12) was deposited at the Guangxi Key Laboratory of Plant Functional Phytochemicals and
Sustainable Utilization, Guangxi Institute of Botany, Chinese Academy of Sciences.
Extraction and Isolation. The air-dried stems and leaves of P. edulis (15.5 kg) were powdered and extracted three times with 95% EtOH (3 × 50 L) for 72 h every time at room temperature. After the solvent was evaporated in vacuo at 52°C, the residue was suspended in H2O and partitioned successively with petroleum ether (PE), EtOAc, and n-BuOH. The EtOAc partition (77 g) was subjected to a silica gel column chromatography (100–200 mesh) and eluted with a gradient of CH2Cl2–MeOH (8:1–0:1) to obtain seven fractions (A–G). Fraction F (18.9 g) was decolorized on MCI gel (eluted with 50% MeOH) and subjected to RP-18 column with MeOH–H2O (10–100%, gradient system) to yield six fractions (F1–F6). Fraction F3 (91 mg) was purified by semipreparative HPLC (CH3CN–H2O, 12:88) to obtain the mixtures 1 and 2 (4.2 mg), 3 (2.9 mg), 4 (5.8 mg), 5 (5.4 mg), 6 (4.4 mg).
Phenylethyl-O-β-D-allopyranoside (1), white amorphous powder. 1H (500 MHz) and 13C (125 MHz) NMR data, see Table 1. HR-ESI-MS m/z 307.1165 [M + Na]+ (calcd for C14H20O6Na, 307.1152).
References
X. He, F. Luan, Y. Yang, Z. Wang, Z. Zhao, J. Fang, M. Wang, M. Zuo, and Y. Li, Front Pharmacol., 11, 617 (2020).
F. Xu, C. Wang, L. Yang, H. Luo, W. Fan, C. Zi, F. Dong, J. Hu, and J. Zhou, Food Chem., 136, 94 (2013).
H. Li, P. Zhou, Q. Yang, Y. Shen, J. Deng, L. Li, and D. Zhao, J. Ethnopharmacol., 133, 1085 (2011).
K. Yoshikawa, S. Katsuta, J. Mizumori, and S. Arihara, J. Nat. Prod., 63, 1229 (2000).
C. Wang, F. Q. Xu, J. H. Shang, H. Xiao, W. W. Fan, F. W. Dong, J. M. Hu, and J. Zhou, J. Ethnopharmacol., 148, 812 (2013).
S. M. Zucolotto, S. Goulart, A. B. Montanher, F. H. Reginatto, E. P. Schenkel, and T. S. Frode, Planta Med., 75, 1221 (2009).
J. Christensen and J. W. Jaroszewski, Org. Lett., 3, 2193 (2001).
D. S. Seigler, G. F. Pauli, A. Nahrstedt, and R. Leen, Phytochemistry, 60, 873 (2002).
Z. J. Wu, Y. H. Shen, and W. D. Zhang, Chem. Nat. Compd., 49, 167 (2013).
Acknowledgment
This work was financially supported by the Special Fund for Local Science and Technology Development Guided by the Central Committee (ZY20111010), the Innovation Driven Development Special Fund Project of Guangxi (AA18118015), and the Fund of Guangxi Key Laboratory of Plant Functional Phytochemicals and Sustainable Utilization.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2023, pp. 191–192.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, GQ., Pan, ZH., Ning, DS. et al. A New Phenylethanoid Glycoside from the Stems and Leaves of Passiflora edulis. Chem Nat Compd 59, 222–224 (2023). https://doi.org/10.1007/s10600-023-03961-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-023-03961-5