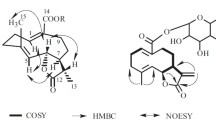
A new sesquiterpene, 1β-hydroxy-4(5),7(8),11(12)-triene-6,9-dione-7,8-furanoeudesmane (1), was isolated from the root extract of Chloranthus multistachys C. Pei. The structure of the new compound was characterized by means of spectroscopic methods, including 1D and 2D NMR and HR-ESI-MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Chloranthus, belonging to the Chloranthaceae family, consists of 17 species in the world, distributed mainly in tropical and temperate regions of Asia; there are 13 species and five varieties found in China [1]. Members of the genus Chloranthus have been used as traditional Chinese medicine to treat hypertension, breast cancer, wounds, and arthritis [2]. Sesquiterpenes are the major constituents of the Chloranthus genus, and a series of sesquiterpenes was isolated from this genus previously [3,4,5]. Chloranthus multistachys C. Pei, called “Siyexixin” in China, is a perennial herb growing under the hillside forest and in the grass beside the gullies and is widely distributed in China. The roots of C. multistachys are common Chinese folk medicines for treating bruises and rheumatic joint pain [6].
In our continuous search for new and bioactive sesquiterpenes from Chinese traditional medicine, a phytochemical investigation of C. multistachys was carried out, and a new sesquiterpene, 1β-hydroxy-4(5),7(8),11(12)-triene-6,9-dione-7,8-furanoeudesmane (1), along with five known sesquiterpenes (2–6), was isolated. Herein we describe the isolation and structural determination of the new compound 1.
Compound 1 was obtained as a yellowish oil. The molecular formula was deduced to be C15H16O4 on the basis of the [M + H]+ peak at m/z 261.1129 (calcd for C15H17O4, 261.1121) and the [M + Na]+ peak at m/z 283.0949 (calcd for C15H16O4Na, 283.0941) in its HR-ESI-MS, and further supported by evidence from 13C NMR combined with DEPT experiments (15 carbons as 3 × CH3, 2 × CH2, 2 × CH, 8 × C). The IR spectrum exhibited absorptions at 3506 cm–1 (hydroxyl) and 1670 cm–1 (α,β-unsaturated ketone). In the 1H NMR, the presence of three methyls (δ 1.44, 2.03, 2.32) and an olefinic proton (δ 7.50, d, J = 1.2 Hz) showed that compound 1 is a furanoeudesmane sesquiterpene [7, 8]. The 1H, 13C NMR, and HSQC spectra of 1 also showed the presence of several typical functions, such as an oxygenated CH group [(δH 4.27 (dd, J = 4.2, 12.0 Hz) and δC 70.0], two persubstituted double bonds [(δC 148.0 (C-4), 134.6 (C-5), 133.1 (C-7), 150.8 (C-8)], one trisubstituted double bond [(δC 121.5 (C-11), 146.5 (C-12), and two carbonyl signals (δC 186.6, 193.3).
In addition, the 1H–1H COSY spectral data showed the spin system of H-1/H-2/H-3. Moreover, in the HMBC spectrum (Fig. 1), the signal at δH 4.27 (1H, dd, J = 4.2, 12.0 Hz, H-1) showed cross peaks with C-2 (δ 24.6), C-3 (δ 33.1), and C-14 (δ 22.3), which suggested that the OH group was attached at C-1. The correlations of H-15 with C-3 (δ 33.1), C-4 (δ 148.0), and C-5 (δ 134.6) revealed the presence of a Δ4 double bond in 1. The multiple correlations of H-13 (δ 2.32) with C-7 (δ 133.1), C-11 (δ 121.5), and C-12 (δ 146.5), as well as H-12 (δ 7.50) with C-7, C-8 (δ 150.8), and C-11, in the HMBC data further revealed the establishment of the furan ring with a methyl attached at C-11. The location of the two carbonyl groups was deduced from the correlations of H-1 and H-14 (δ 1.44) with C-9 (δ 193.3) and H-15 (δ 2.03) with C-6 (δ 186.6). On the basis of these observations, the planar structure of 1 was established to be 1-hydroxyfuranoeudesma-4,7,11-triene-6,9-dione. The relative configuration of 1 was established from the NOESY spectroscopic data. Biogenetically, Me-14 was tentatively considered to be β-oriented in eudesmane sesquiterpenoids [8, 9]. Thus, the configuration of the hydroxy group at C-1 was deduced to be in the β-orientation from the cross-peaks between H-14 (δ 1.44) with H-2β (δ 1.74) and H-2α (δ 1.88) with H-1 in the NOESY spectrum. This is further evidenced by the broadened double doublet at δH 4.27 (dd, J = 4.2, 12.0 Hz, H-1), suggesting that H-1 was in α-orientation [10, 11]. Hence, the new compound 1 was elucidated as shown in Fig. 1 and identified as 1β-hydroxy-4(5),7(8),11(12)-triene-6,9-dione-7,8-furanoeudesmane.
By comparison of the NMR data with those of the corresponding compounds in the literature, the known compounds were identified as atractylenolide III (2) [12], lasianthuslactone A (3) [13], 9α-hydroxy-5α-H-eudesma-4(15),7(11)-dien-8α,12-olide (4) [14], 6α-hydroxyeudesma-4(15),7(11),8(9)-triene-8,12-olide (5) [9], and 4β-hydroxyeudesma-11-en-1-one (6) [15]. Compounds 4–6 were isolated from C. multistachys for the first time.
EXPERIMENTAL
General. Optical rotations were recorded in MeOH using a Perkin Elmer 241 polarimeter. IR spectra were recorded on a Nicolet NEXUS 670 FT-IR spectrometer, and UV detections were measured on a Shimadzu UV-1800 spectrometer. NMR spectra were acquired using an Advance III-600 spectrometer (Bruker, Bremerhaven, Germany) and an INOVA-400 FT-NMR spectrometer (Varian, USA) with TMS as internal standard. Semipreparative HPLC was performed on a prep-HPLC manufactured by Hanbon Sci & Tech of China using a Cosmosil C18 column (250 × 10 mm). Silica gel (200–300 mesh) used for column chromatography and silica gel (GF254) for TLC were supplied by the Qingdao Marine Chemical Factory in China. TLC was conducted at 254 nm, and spots were visualized by spraying with 10% H2SO4 in ethanol (v/v) followed by heating.
Plant Material. The plant material was collected from Zhashui County, Shaanxi Province of P. R. China and was identified by Prof. Xiaofeng Niu, School of Pharmacy, Xi′an Jiaotong University, Xi′an, P. R. China. A voucher specimen (No. 2012-06-23) has been deposited at the Herbarium of the School of Pharmacy, Xi′an Jiaotong University.
Extraction and Isolation. The air-dried roots of Chloranthus multistachys C. Pei (6.5 kg) were powdered and extracted three times with 95% EtOH (each for 7 days) at room temperature. The crude extract was suspended in water and extracted successively with petroleum ether (PE) (60–90°C), EtOAc, and BuOH. The EtOAc extract (120 g) was subjected to column chromatography (CC) on silica gel, eluting with PE–EtOAc (40:1, 20:1, 15:1, 10:1, 8:1, 5:1, 2:1, 1:1), to yield eight fractions (Frs. 1–8). Fraction 2 (4.5 g) was further subjected over silica gel column (PE–EtOAc, 8:1) and then purified by preparative HPLC eluted with CH3OH–H2O (70:30) to yield compounds 2 (14 mg) and 6 (3 mg). Repeated chromatography of Fr. 3 (3.2 g) over a silica gel column (PE–EtOAc, 10:1; PE–Me2CO, 8:1) yielded pure 4 (7 mg) and 5 (43 mg). Fraction 4 (2.4 g) was subjected to silica gel column chromatography and eluted with PE–Me2CO (15:1) and CHCl3–EtOAc (100:1) to obtain 1 (4 mg) and 3 (21 mg).
1 β -Hydroxy-4(5),7(8),11(12)-triene-6,9-dione-7,8-furanoeudesmane, yellowish oil; \( {\left[\upalpha \right]}_{\mathrm{D}}^{20} \) –24.8° (c 0.20, CHCl3). UV (MeOH, λmax, nm) (log ε): 233 (4.64), 290 (4.37). IR (KBr, νmax, cm–1): 3506, 2931, 1715, 1670, 1358, 1117. HR-ESI-MS m/z: 261.1129 [M + H]+ (calcd for C15H17O4, 261.1121), 283.0949 [M + Na]+ (calcd for C15H16O4Na, 283.0941). 1H NMR (600 MHz, CDCl3, δ, ppm, J/Hz): 7.50 (1H, d, J = 1.2, H-12), 4.27 (1H, dd, J = 4.2, 12.0, H-1), 2.36 (2H, m, H-3), 2.32 (3H, d, J = 1.2, H-13), 2.03 (3H, s, H-15), 1.88 (1H, m, H-2α), 1.74 (1H, m, H-2β), 1.44 (3H, s, H-14). 13C NMR (150 MHz, CDCl3, δ, ppm): 70.0 (C-1), 24.6 (C-2), 33.1 (C-3), 148.0 (C-4), 134.6 (C-5), 186.6 (C-6), 133.1 (C-7), 150.8 (C-8), 193.3 (C-9), 53.6 (C-10), 121.5 (C-11), 146.5 (C-12), 8.7 (C-13), 22.3 (C-14), 21.9 (C-15).
References
Flora of China Editorial Committee, Flora Reipublicae Popularis Sinicae, Science Press, Beijing, 1982.
Editorial Committee of the Administration Bureau of Traditional Chinese Medicine, Chinese Materia Medica, Shanghai Science & Technology Press, Shanghai, 1998.
X. C. Wang, W. Q. Wu, S. P. Ma, J. H. Liu, and L. H. Hu, Chin. J. Nat. Med., 6, 404 (2008).
X. H. Ran, F. Teng, C. X. Chen, G. Wei, X. J. Hao, and H. Y. Liu, J. Nat. Prod., 73, 972 (2010).
Q. Q. Lu, X. W. Shi, S. J. Zheng, J. H. Zhou, X. A. Cui, and J. M. Gao, Nat. Prod. Res., 30, 2476 (2016).
Jiangsu College of New Medicine, Dictionary of Traditional Chinese and Herbal Medicine, Shanghai Science and Technology Press, Shanghai, 1977.
S. Zhang, Z. S. Su, S. P. Yang, and J. M. Yue, J. Asian Nat. Prod. Res., 12, 522 (2010).
B. Wu, S. He, X. D. Wu, and Y. J. Pan, Planta Med., 72, 1334 (2006).
L. J. Wang, J. Xiong, S. T. Liu, X. H. Liu, and J. F. Hu, Chem. Biodiv., 11, 919 (2014).
F. Bohlmann, N. Ates, R. M. King, and H. Robinson, Phytochemistry, 22, 1675 (1983).
N. Li, J. J. Chen, and J. Zhou, J. Asian Nat. Prod. Res., 7, 279 (2005).
Y. Li, X. W. Yang, J. Asian Nat. Prod. Res., 16, 123 (2014).
Y. Li, D. M. Zhang, J. B. Li, S. S. Yu, Y. Li, and Y. M. Luo, J. Nat. Prod., 69, 616 (2006).
H. H. Bokel, H. Marschall, and P. Weyerstahl, Liebigs Ann. Chem., 13, 73 (1982).
L. F. Ding, J. Su, Z. H. Pan, Z. J. Zhang, X. N. Li, L. D. Song, X. D. Wu, and Q. S. Zhao, Phytochemistry, 155, 182 (2018).
Acknowledgment
This work was supported by Natural Science Foundation of Shaanxi Province (No. 2019JM-203) and Key Research and Development Program of Gansu Herbal Medicine Biotechnology Co., Ltd. (No. 2019BC710049). We also thank the Core Facilities Sharing Platform of Xi′an Jiaotong University for providing laboratory facilities related to the characterization of natural products.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2021, pp. 888–889.
Rights and permissions
About this article
Cite this article
Liu, X., Yang, YX., Gao, X. et al. A New Sesquiterpene from the Roots of Chloranthus multistachys. Chem Nat Compd 57, 1035–1037 (2021). https://doi.org/10.1007/s10600-021-03544-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-021-03544-2