Two new isoflavones, 6-hydroxy-7-(3-hydroxypropyl)-4′-methoxyisoflavone (1) and 6,4′-dihydroxy-7-(3-hydroxypropyl)-isoflavone (2), were isolated from the flowers of Rosa damascena. Their structures were elucidated by spectroscopic methods, including extensive 1D and 2D NMR techniques. Compounds 1 and 2 were evaluated for their anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) activity. The results revealed that compounds 1 and 2 showed weak inhibition with IZD of 11.6 ± 1.2 and 11.2 ± 1.0 mm, respectively. Compounds 1 and 2 were also tested for their antioxidant activity and showed good activity with an IC50 value of 4.2 and 4.0 μg/mL, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Rosa is one of the most important ornamental plants in the world. Some species of Rosa are renowned for their beautiful flowers and fine fragrance [1, 2]. Rosa damascena Mill. is a rose hybrid, derived from Rosa gallica and Rosa moschata. Its flowers are renowned for their fine fragrance and are commercially harvested for rose oil (either “rose otto” or “rose absolute”) used in perfumery and to make rose water and “rose concrete” [3, 4]. The flower petals are also edible, and are often used to flavor food, as a garnish, as an herbal tea, and preserved in sugar as gulkand, or used as medicine for treating stomachache, diarrhea, and women′s diseases [2, 5].
Previous phytochemical researches have revealed that tannins [5,6,7,8], flavonoids [7,8,9,10,11,12], as well as terpenoids [13,14,15] are major components isolated from the genus of this plant. In our continuing studies on multipurpose utilization of the genus Rosa and to identify bioactive natural products from this plants, in this paper, we carry out a phytochemical investigation of R. damascena. As a result, two mew isoflavones (1 and 2) were isolated from this plant. The structure elucidation and the evaluation of the anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) activity and antioxidant activity of compounds 1 and 2 are described in this paper.
A 70% aq. methanol extract prepared from the flowers of R. damascena was subjected repeatedly to column chromatography on Si gel, MCI-GEL, Sephadex LH-20, RP-18, and preparative HPLC to afford two new isoflavones, 6-hydroxy-7-(3-hydroxypropyl)-4′-methoxyisoflavone (1) and 6,4′-dihydroxy-7-(3-hydroxypropyl)-isoflavone (2).

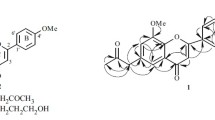
Compound 1 was obtained as an orange-yellow gum. It has the molecular formula C19H18O5 from HR-ESI-MS (m/z 349.1046 [M + Na]+, calcd 349.1052), with 11 degrees of unsaturation. Its IR spectral data showed the presence of hydroxy group (3416 cm–1), carbonyl group (1655 cm–1), and aromatic ring (1614, 1563, and 1439 cm–1). The UV absorptions at 338, 260, and 210 nm also showed a substituted aromatic ring. The 1H and 13C NMR spectrum of 1 (Table 1) along with analysis of the DEPT spectra displayed 19 carbon signals and 18 proton signals, respectively, corresponding to a 1,2,4,5-tetrasubstituted aromatic ring (C-5–C-10; H-5 and H-8), a 1,4-disubstituted aromatic ring (C-1′–C-6′; H-2′, 6′ and H-3′, 5′), an α,β-unsaturated ketone (C-2–C-4; H-2), a hydroxypropyl group (C-1′′–C-3′′; H2-1′′–H2-3′′) [16], a methoxy groups (δC 56.4 q, δH 3.79 s), and a phenolic hydroxy group (δ 10.76 s). The typical NMR signal of two aromatic rings and an α,β-unsaturated ketone indicated that 1 should be a isoflavone [17]. This was also supported by the HMBC correlations (Fig. 1) from H-2 to C-3, C-4, C-9, C-1′, from H-5 to C-4, C-9, C-10, from H-8 to C-9, C-10, and from H-2′, 6′ to C-3. Since the nucleus of the compound has been determined, the additional signals (hydroxypropyl, methoxy, and phenolic hydroxy groups) were accounted for by the remaining substituents. The HMBC correlations of the methoxy protons (δ 3.79) with C-4′ (δ 160.3) suggested the attachment position of this methoxy group at C-4′. The hydroxypropyl group located at C-7 was supported by the HMBC correlations from H2-1′′ (δ 2.72) to C-6 (δ 150.2), C-7 (δ 130.3), C-8 (δ 116.7), from H2-2′′ (δ 1.87) to C-7 (δ 130.3), and from H-8 (δ 6.70) to C-1′′ (δ 28.9). The phenolic hydroxy group located at C-6 was supported by the HMBC correlations of the phenolic hydroxyl proton (δ 10.76) with C-5 (δ 114.1), C-6 (δ 150.2), and C-7 (δ 130.3). Furthermore, the typical protons signals (H-5, H-8, H-2′, 6′, and H-3′, 5′) also confirmed that ring A is 6,7-disubstituted and ring B is 4′-monosubstituted on the isoflavone nucleus. The structure of 6-hydroxy-7-(3-hydroxypropyl)-4′-methoxyisoflavone (1) was therefore established as shown in Fig. 1.
The 1H and 13C NMR spectra of 6,4′-dihydroxy-7-(3-hydroxypropyl)-isoflavone (2) (Table 1) were similar to those of 1. The marked differences between them were due to the absence of a methoxy signal and appearance of a phenolic hydroxyl signal (δ 10.98 s) in compound 2. This change indicated that the methoxy group in 1 was replaced by a phenolic hydroxy group in compound 2. In addition, the obvious chemical shift differences of the upfield shift of C-4′ from δ 160.3 to δ 157.5 ppm suggested that the substituent groups should be varied at C-4′. This was also supported by the HMBC correlations of the phenolic hydroxy proton signal (δ 10.98) with C-3′, 5′ (δ 116.0) and C-4′ (δC 157.5). In addition, the other substituent positions were also determined by further analysis of its HMBC correlations. Thus, the structure of 2 was determined as shown.
Compounds 1 and 2 were screened for anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) activity according to an arbitrary criterion [18] with diameter of inhibition zone (IZD) as follows: very weak inhibition (IZD 6–8 mm), weak inhibition (IZD 8–12 mm), good inhibition (IZD 12–16 mm), and strong inhibition (IZD > 16 mm). The IZD of the positive control was 32 mm, and that of the negative control was zero. Compounds 1 and 2 showed weak inhibition with IZD of 11.6 ± 1.2 and 11.2 ± 1.0 mm, respectively.
Compounds 1 and 2 were also tested for their antioxidant activity by the 2′,7′-dichlorofluorescin diacetate (DCFH–DA) method reported previously [19]. The results revealed that compounds 1 and 2 show good antioxidant activity with an IC50 values of 4.2 and 4.0 μg/mL, respectively.
Experimental
General. UV spectra were obtained using a Shimadzu UV-2401A spectrophotometer. IR spectra were obtained in KBr disc on a Bio-Rad Win-IR spectrophotometer. ESI-MS were measured on a VG Auto Spec-3000 MS spectrometer. 1H, 13C, and 2D NMR spectra were recorded on a Bruker DRX-500 instrument with TMS as internal standard. Column chromatography was performed on silica gel (200–300 mesh) or on silica gel H (10–40 μm, Qingdao Marine Chemical Inc., China). An Agilent 1100 HPLC equipped with a ZORBAX-C18 (21.2 mm × 250 mm, 7.0 μm) column and DAD detector was used for a second separation.
Plant Material. The flowers of Rosa damascena Mill. were collected in Pingyin County, Shandong Province, and harvested in June 2016. The species was identified by Prof. Y. J. Chen. A voucher specimen (YNNI 16-9-56) was deposited in the Herbarium of the Yunnan Minzu University.
Extraction and Isolation. The dried samples (3.6 kg) were crushed to 30 mesh, and the powders were extracted with 95% aqueous MeOH (4 × 5 L) at room temperature and filtered. The filtrate was evaporated under reduced pressure and then extracted with EtOAc. The EtOAc-soluble materials (52.6 g) were applied to a silica gel (150–200 mesh) column eluted with a chloroform–methanol (CHCl3–MeOH) gradient (20:1, 9:1, 8:2, 7:3, 6:4, 5:5) to afford six fractions (Frs. A–F). Further separation of fraction B (9:1, 22.5 g) by silica gel column chromatography eluted with chloroform–acetone (1:0–1:2) yielded subfractions (Subfrs. B1–B7). Subfraction B4 (7:3, 2.86 g) was separated on a silica gel column eluted with petroleum ether–EtOAc, followed by semipreparative HPLC (55% MeOH, flow rate 20 mL/min) to give 1 (14.8 mg) and 2 (18.2 mg).
Anti-MRSA Agar Disc Diffusion Assay. The MRSA strain ZR11 was clinically isolated from infectious samples of critically ill patients in the Clinical Laboratory of the First People′s Hospital of Yunnan Province and confirmed by standard cefoxitin disk diffusion test following CLSI standard procedures [18]. The anti-MRSA activity of the compounds was evaluated via the disc diffusion method. The ZR11 strain was inoculated in Mueller Hinton Broth and incubated at 37°C for 24 h. The turbidity of the bacterial suspension was adjusted to 0.5 McFarland standard, which equals 1.5 × 108 colony-forming units (CFU)/mL. Sterile filter paper discs (6 mm) were impregnated with 20 μL (50 μg) of each compound and placed on inoculated Mueller Hinton agar containing the bacterial suspension adjusted to 0.5 McFarland standard. Commercially available discs containing 30 μg vancomycin were used as positive control, whereas discs without samples (5% DMSO) served as negative control. The inhibition zones, including the diameter of the disc (mm), were measured and compared after incubation at 37°C for 24 h. The tests were carried out in triplicate for each sample.
Antioxidant Assay. The antioxidant activity was tested using the 2′,7′-dichlorofluorescin diacetate (DCFH–DA) method reported previously [19]. Myelomonocytic HL-60 cells (1 × 106 cells/mL, ATCC) were suspended in RPMI 1640 medium with 10% FBS and antibiotics at 37°C in 5% CO2:95% air; 125 μL of the cell suspension was added to each well of a 96-well plate. After treatment with a different concentrations of the test material for 30 min, the cells were stimulated with 100 ng/mL phorbol 12-myristate 13-acetate (PMA, Sigma) for 30 min. Then the cells were incubated for 15 min after the addition of 5 μg/mL DCFH-DA (Molecular Probes). DCFH-DA is a nonfluorescent probe that diffuses into cells. Cytoplasmic esterases hydrolyze DCFH-DA to 2′,7′-dichlorofluorescin (DCFH), and the reactive oxygen species (ROS) generated within HL-60 cells oxidize DCFH to the fluorescent dye 2′,7′-dichlorfluorescein (DCF). The ability of the test materials to inhibit exogenous cytoplasmic ROS-catalyzed oxidation of DCFH in HL-60 cells was measured by PMA treated control incubations with and without the test materials. The levels of DCF were measured using a CytoFluor 2350 fluorescence measurement system (Millipore) with an excitation wavelength at 485 nm (bandwidth 20 nm) and emission at 530 nm (bandwidth 25 nm).
6-Hydroxy-7-(3-hydroxypropyl)-4′-methoxyisoflavone (1), C19H18O5, obtained as an orange-yellow gum. UV (MeOH, λmax, nm) (log ε): 338 (3.28), 260 (3.64), 210 (4.31). IR (KBr, νmax, cm–1): 3416, 3052, 2930, 1655, 1614, 1563, 1480, 1439, 1148, 1065. 1H and 13C NMR data (CDCl3, 500 and 125 MHz, respectively), see Table 1. ESI-MS m/z 349; HR-ESI-MS m/z 349.1046 [M + Na]+ (calcd for C19H18NaO5, 349.1052).
6,4′-Dihydroxy-7-(3-hydroxypropyl)-isoflavone (2), C18H16O5, obtained as an orange-yellow gum. UV (MeOH, λmax, nm) (log ε): 335 (3.34), 258 (3.72), 210 (4.18). IR (KBr, νmax, cm–1): 3446, 3057, 2924, 1652, 1610, 1555, 1472, 1434, 1142, 1059. 1H and 13C NMR data (CDCl3, 500 and 125 MHz, respectively), see Table 1. ESI-MS m/z 335; HR-ESI-MS m/z 335.0898 [M + Na]+ (calcd for C18H16NaO5, 335.0895).
References
The Dictionary of Roses in Colour, Ed. by S. M. Gault and P. M. Synge, Ebury Press, London, 1971.
Flora of China, Vol. 37, Ed. by L. Putian and Y. Jiang, Chinese Science Press, Beijing, 1985, p. 401.
X. H. Zhang, Z. Z. Xie, Chin. J. Shandong. Forest. Sci. Tech., 42, 5 (2012).
J. L. Lu, Chin. Flow. Hortic., 11, 26 (2012).
K. Shibata, A Cyclopedia of Useful Plant and Plant Products, Enlarged and Revised Edition, The Hokuryukan, Tokyo, 1957, p. 612.
V. Cunja, M. Mikulic-Petkovsek, F. Stampar, and V. Schmitzer, J. Am. Soc. Hortic. Sci., 139, 157 (2014).
S. Ochir, B. Park, M. Nishizawa, T. Kanazawa, M. Funaki, and T. Yamagishi, J. Nat. Med., 64, 383 (2010).
K. H. Park, K. Sung, S. E. Choi, J. H. Kwon, H. Myung, and M. W. Lee, Chem. Pharm. Bull., 58, 1227 (2010).
E. A. Porter, A. A. van den Bos, G. C. Kite, N. C. Veitch, and M. S. Simmonds, Phytochemistry, 81, 90 (2012).
E. K. Kwon, D. Y. Lee, H. Lee, D. O. Kim, N. Baek, Y. E. Kim, and H. Y. Kim, J. Agric. Food. Chem., 58, 882 (2010).
X. M. Gao, L. Y. Yang, L. D. Shu, Y. Q. Shen, Y. J. Zhang, and Q. F. Hu, Heterocycles, 85, 1925 (2012).
Y. K. Li, J. Q. Sun, X. M. Gao, and C. Lei, Helv. Chim. Acta, 97, 414 (2014).
Ali Mostafavi and Daryoush Afzali, Chem. Nat. Compd., 45, 110 (2008).
N. Zeng, Y. Shen, L. Z. Li, W. H. Jiao, P. Y. Gao, S. J. Song, W. S. Chen, and H. W. Lin, J. Nat. Prod., 74, 732 (2011).
E. S. Abdel-Hameed, S. A. Bazaid, and H. A. Hagag, J. Essent. Oil. Res., 28, 121 (2016).
M. Zhou, K. Zhou, P. He, K. M. Wang, R. Z. Zhu, Y. D. Wang, W. Dong, G. P. Li, H. Y. Yang, Y. Q. Ye, G. Du, X. M. Li, and Q. F. Hu, Planta Med., 82, 414 (2016).
L. Li, Q. P. Shen, C. B. Liu, Y. Wang, J. J. Yao, T. Zhang, F. M. Zhang, P. He, X. X. Si, Z. H. Liu, M. M. Miao, and G. Y. Yang, Phytochem. Lett., 13, 156 (2015).
S. Mulla, A. Kumar, and S. Rajdev, Clinical and Laboratory Standards Institute (CLSI), Performance Standards for Antimicrobial Susceptibility Testing: Eighteenth Informational Supplement M100-S18, 3rd edn., Wayne, Pennsylvania, 2008.
S. Takamatsu, A. M. Galal, S. A. Ross, D. Ferreira, M. A. Elsohly, A. R. Ibrahim, and F. S. El-Feraly, Phytother. Res., 17, 963 (2003).
Acknowledgment
This research was supported by the Research Foundation of China Tobacco Company (No. 110201502006), the Research Foundation of China Tobacco Yunnan Industrial Co., Ltd. (Nos. 2015539200340277, 2016JC03, and JSZX20151008-52), the National Natural Science Foundation of China (Nos. 81660717 and 21462051), and the Natural Science Foundation of Yunnan Province (No. 2014FD033).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2019, pp. 386–389.
Rights and permissions
About this article
Cite this article
Xiang, HY., Xing, HH., Li, J. et al. Two New Isoflavones from the Flowers of Rosa damascena and Their Biological Activities. Chem Nat Compd 55, 449–452 (2019). https://doi.org/10.1007/s10600-019-02711-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02711-w