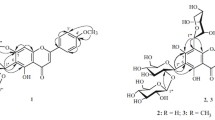
Two new acylated apigenin glucosides that were identified as apigenin-7-O-(4″-malonyl)-β-Dglucopyranoside (1) and apigenin-7-O-(4″-malonyl-6′′-acetyl)- β-D-glucopyranoside (2) were isolated from edge flowers of Matricaria chamomilla [Chamomilla recutita], variety Podmoskovnaya. The effects of stomach and intestinal juices and cellular microflora on the stability of 1 and 2 were studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Matricaria chamomilla L. [Chamomilla recutita (L.) Rauschert] (Asteraceae) is a medicinal plant that is widely used in official medical practice. Chemical studies of M. chamomilla flowers isolated >40 flavonoids. Edge flowers of this species accumulated characteristically derivatives of the flavones apigenin [1–7], luteolin [1, 2], and chrysoeriol [2] whereas tubed flowers were dominated by derivatives of the flavonols kaempferol [8, 9], quercetin [1, 2, 10], isorhamnetin [2, 11], and quercetagetin [8, 12, 13]. Apigenin derivatives are a biologically active group of compounds that were responsible for the anti-inflammatory, antioxidant, and antitumor activity of M. chamomilla preparations [14].
Two new apigenin glycosides (1 and 2) and 18 known compounds were isolated from M. chamomilla variety Podmoskovnaya during the present study. The known compounds were identified as trans/cis-spiroether (3/4), gerniarin (5), apigenin (6), apigenin-7-O-(6″-acetyl)-β-D-glucopyranoside (7), apigenin-7-O-(4″-acetyl)-β-D-glucopyranoside (8), cosmosiin (9), cynaroside (10), umbelliferone (11), daphnetin (12), skimmin (13), daphnin (14), trans/cis-2-hydroxy-4-methoxycinnamic acid 2-O-β-D-glucopyranoside (15/16), apigenin-7-O-(4″-acetyl-6″-malonyl)-β-D-glucopyranoside (17), apigenin-7-O-(6″-malonyl)-β-D-glucopyranoside (18), 3-O-caffeoylquinic acid (19), and 3,5-di-O-caffeoylquinic acid (20).

Compound 1 had the formula C24H22O13 according to HR-ESI-MS (m/z 519.421 [M + H]+; calcd 519.443) and PMR data. ESI-MS spectra showed the protonated molecular ion with m/z 519 [M + H]+ and apigenin ion (m/z 271) in addition to an ion with m/z 433 that resulted from loss of malonyl (C3H2O3). The spectral properties of 1 were similar to those of apigenin-7-O-(6″-malonyl)-β-D-glucopyranoside (18) that was isolated earlier from M. chamomilla [6]. However, 1 had a characteristic HPLC retention time (t 11.10 min) that was shorter than that of 18 (t 11.48 min).
NMR spectra showed a weak-field shift of glucopyranose H-4″ (δ 4.57) and C-4″ (δ 71.4) relative to their positions in apigenin-7-O-β-D-glucopyranoside (9) (δH 3.25 and δC 69.5, respectively). This indicated that the C-4″ position was substituted (Table 1). Correlations between resonances of H-4″ (δ 4.57) and malonyl C-1″′ (δ 167.0) in the HMBC spectrum confirmed this. Thus, 1 was apigenin-7-O-(4″-malonyl)-β-D-glucopyranoside.
Based on HR-ESI-MS and NMR data, 2 had the formula C26H24O14. The UV and mass spectra of 2 were similar to those of the known flavonoid apigenin-7-O-(4″-acetyl-6″-malonyl)-β-D-glucopyranoside (17) [6] although the retention time of 2 under the HPLC conditions was longer (t 14.78 min) than that of 17 (t 14.51 min). NMR spectra were similar to those of 1 except for additional acetyl resonances (δH 2.04, δC 21.6, 172.6). The weak-field shifts of the glucopyranose H-6″ (δH 4.29, 4.46) and C-6″ (δC 64.2) resonances and HMBC correlations between H-6″ and the acetyl carbonyl C atom (δC 172.6) indicated that the last was located on glucopyranose C-6″. Therefore, 2 was apigenin-7-O-(4″-malonyl-6″-acetyl)-β-Dglucopyranoside.
Another four apigenin derivatives containing malonyl residues are known in addition to 1 and 2. These are apigenin-7-O-(6″-malonyl)-β-D-glucopyranoside (18) [15], apigenin-7-O-(4″-acetyl-6″-malonyl)-β-D-glucopyranoside (17) [6], apigenin-7-O-(2″-β-D-apiofuranosyl-6″-malonyl)-β-D-glucopyranoside [16], and apigenin-7-O-β-D-glucopyranoside-4′-O-(6″-malonyl)-β-D-glucopyranoside [17].
Factors such as pH, temperature, light, etc. are also known to affect the chemical stability of flavone malonyl-glucosides [14]. It was shown earlier that apigenin-7-O-(4″-acetyl-6″-malonyl)-β-D-glucopyranoside (17) could decompose into apigenin-7-O-(4″-acetyl)-β-D-glucopyranoside (8) and apigenin-7-O-(6″-malonyl)-β-D-glucopyranoside (18) if the pH was lowered or raised [6]. Considering this, the behaviors of 1 and 2 under the influence of simulated physiological media were investigated. It was found that 1 was not altered significantly chemically in stomach or intestinal juices. The table below describes the reactions of 1 and 2 with simulated physiological media (Tr = trace):
Medium | Compound | |||
1 | 2 | |||
Stomach juice | 9 (Tr.) | 1, 7, 9 (Tr.) | ||
Intestinal juice | 9 (Tr.) | 1, 7, 8, 9 (Tr.), 17, 18 | ||
Intestinal microflora | 6 | 6. |
Constituent 2 in stomach juice formed a mixture consisting of 1, apigenin-7-O-(6″-acetyl)- β-D-glucopyranoside (7), and traces of apigenin-7-O-β-D-glucopyranoside (9). Additional compounds including 8, 17, and 18 were formed by intestinal juice. Thus, the pH affected the stability of 2 more than that of 1. The only transformation product of 1 and 2 by intestinal microflora was apigenin (6). This was probably characteristic of all apigenin glycosides.
Experimental
Plant raw material of M. chamomilla variety Podmoskovnaya was grown in the open at experimental plantations of the IGEB SB RAS (June 2014) from authenticated seeds obtained from the N. V. Tsitsin Main Botanical Garden, RAS (Moscow, Russia). Edge flowers were collected manually and dried in a microwave oven to moisture content ≤5%. Column chromatography (CC) used SiO2, RP-SiO2, polyamide (Sigma-Aldrich, St. Louis, MO, USA), and Sephadex LH-20 (GE Healthcare, Little Chalfont, UK). Spectrophotometry was carried out on a SF-2000 spectrophotometer (OKB Spectr, St. Petersburg, Russia). MS analysis used a high-resolution MAT 8200 mass spectrometer (Thermo Finnigan LLC, San Jose, CA, USA). NMR spectra were recorded on a VXR 500S NMR spectrometer (Varian, Palo Alto, DA, USA). Preparative HPLC was performed on a Summit liquid chromatograph (Dionex, Sunnyvale, CA, USA); analytical HPLC, on a Milichrom A-02 microcolumn liquid chromatograph (EcoNova, Novosibirsk, Russia).
Extraction and Fractionation. A weighed portion of dried and ground raw material (1 kg) was extracted (2×) with EtOH (50%, 1:20) in an ultrasonic bath (100 V, 35 kHz) at 40°C for 90 min. The resulting extract was filtered off and concentrated in vacuo (40°C) to an aqueous residue that was extracted with hexane and n-BuOH. The solvent was removed to afford hexane (CF-1, 25 g) and n-BuOH fractions (CF-2, 254 g). Fraction CF-2 (250 g) was separated over polyamide (CC, 2 kg) with elution sequentially by H2O (fraction CF-2/1, 91 g), EtOH (80%) (CF-2/2, 112 g), and NH3 (0.5%) in EtOH (90%) (CF-2/3, 34 g). Fraction CF-2/2 (95 g) was chromatographed over SiO2 (CC, 10 × 60 cm) using gradient elution by hexane–EtOAc (100:0→70:30) to afford subfractions CF-2/2-01–CF-2/2-07.
Subfraction CF-2/2-02 was rechromatographed over SiO2 (CC, 2 × 30 cm, petroleum ether–Me2CO, 100:0→90:10) to isolate trans-spiroether (E-en-yn-bicycloether, 44 mg, 3) and cis-spiroether (Z-en-yn-bicycloether, 12 mg, 4) [18]. CC over SiO2 (3 × 40 cm, hexane–EtOAc, 100:0→75:25) and RP-SiO2 (2 × 20 cm, H2O–MeCN, 100:0→50:50) of subfractions CF-2/2-03–CF-2/2-04 isolated gerniarin (5, 27 mg) [19], apigenin (6, 14 mg), apigenin-7-O-(6″-acetyl)-β-D-glucopyranoside (7, 29 mg) [2], and apigenin-7-O-(4″-acetyl)-β-D-glucopyranoside (8, 18 mg) [10].
Subfraction CF-2/2-05 was separated under analogous conditions to isolate cosmosiin (apigenin-7-O-β-Dglucopyranoside, 9, 8.6 g), cynaroside (luteolin-7-O-β-D-glucopyranoside, 10, 11 mg) [20], umbelliferone (11, 10 mg), daphnetin (12, 14 mg), skimmin (umbelliferone-7-O-β-D-glucopyranoside, 13, 9 mg), and daphnin (daphnetin-7-O-β-D-glucopyranoside, 14, 37 mg) [21].
Subfraction CF-2/2-06 was separated by CC over RP-SiO2 (3 × 30 cm, H2O–MeCN, 100:0→0:100) to afford trans-2-hydroxy-4-methoxycinnamic acid 2-O-β-D-glucopyranoside (E-GMCA, 15, 29 mg) and cis-2-hydroxy-4-methoxycinnamic acid 2-O-β-D-glucopyranoside (Z-GMCA, 16, 87 mg) [22].
Fraction CF-2/3 (30 g) was separated in portions (1 g) using preparative HPLC conditions 1. Subfractions with retention times 15–19 min were combined after preparative HPLC and separated over Sephadex LH-20 (CC, 2 × 60 cm, MeOH–H2O, 100:0→0:100) and then rechromatographed by preparative HPLC to afford 1 (34 mg) and apigenin-7-O-(6″-malonyl)-β-D-glucopyranoside (18, 110 mg) [6].
Subfractions with retention times 32–37 min were combined after preparative HPLC and worked up analogously to separate 2 (20 mg) and apigenin-7-O-(4″-acetyl-6″-malonyl)-β-D-glucopyranoside (17, 208 mg) [6]. Subfractions with retention times 8–12 min after preparative HPLC were chromatographed (CC) over Sephadex LH-20 (1 × 70 cm, EtOH–H2O, 50:50→20:80) to isolate 3-O-caffeoylquinic acid (19, 27 mg) and 3,5-di-O-caffeoylquinic acid (20, 31 mg) [23].
Apigenin-7- O -(4″-malonyl)- β -D-glucopyranoside (1). C24H22O13. HR-ESI-MS, m/z: 519.421 ([M + H]+; calcd 519.443). +ESI-MC, m/z: 519 [M + H]+, 433 [(M – C3H2O3) + H]+, 271 [(M – C3H2O3 – C6H10O5) + H]+. UV spectrum (MeOH, λmax, nm): 256, 335. For 1H and 13C NMR , see Table 1.
Apigenin-7- O -(4″-malonyl-6″-acetyl)- β -D-glucopyranoside (2). C26H24O14. HR-ESI-MS, m/z: 561.312 ([M + H]+; calcd 561.481). +ESI-MC, m/z: 561 [M + H]+, 475 [(M – C3H2O3) + H]+, 271 [(M – C3H2O3 – C2H2O – C6H10O5) + H]+. UV spectrum (MeOH, λmax, nm): 256, 335. For 1H and C NMR , see Table 1.
HPLC. Conditions 1: preparative HPLC, LiChrospher PR-18 column (250 × 10 mm, ∅ 10μm, Supelco, Bellefonte, PA, USA); mobile phase H2O (A) and MeCN (B); gradient mode (%B): 0–10 min, 0–25%; 10–30 min, 25–32%; 30–60 min, 32–45%; flow rate 1 mL/min; column temperature 30°C, UV detector at λ 330 nm. Conditions 2: analytical HPLC, ProntoSIL-120-5-C18 AQ column (2 × 75 mm, ∅ 5μm, Metrohm, AG); mobile phase LiClO4 (0.2 M) in HClO4 (0.006 M) (A) and MeCN (B); gradient mode (%B): 0–6 min, 5–25%; 6–11 min, 25%; 11–17 min, 30–60%; 17–22 min, 100%, flow rate 150μL/min, column temperature 35°C, UV detector at λ 330 nm.
Stabilities of compounds were studied in a simulated gastrointestinal tract that was described by us earlier [24]. The compositions of reaction products were determined using analytical HPLC (conditions 2).
References
L. Horhammer, H. Wagner, and B. Saliner, Arzneim. Forsch., 13, 33 (1963).
R. Kunde and O. Isaac, Planta Med., 37, 124 (1979).
C. Redaelli, L. Formentini, and E. Santaniello, Phytochemistry, 19, 985 (1980).
C. Redaelli, L. Formentini, and E. Santaniello, Phytochemistry, 21, 1828 (1982).
R. Carle, B. Dolle, W. Muller, and U. Baumeister, Pharmazie, 4, 304 (1993).
V. Svehlikova, R. N. Bennett, F. A. Mellon, P. W. Needs, S. Piacente, P. A. Kroon, and Y. Bao, Phytochemistry, 65, 2323 (2004).
B. Weber, M. Herrmann, B. Hartmann, H. Joppe, C. O. Schmidt, and H.-J. Bertram, Eur. Food Res. Technol., 226, 775 (2008).
J. Exner, J. Reichling, and H. Becker, Planta Med., 39, 219 (1980).
N. Mulinacci, A. Romani, P. Pinelli, F. F. Vincieri, and D. Prucher, Chromatographia, 51, 301 (2000).
X.-Y. Xie, R. Wang, and Y.-P. Shi, Chem. Nat. Compd., 50, 910 (2014).
L.-Z. Kin and J. M. Harnly, Nat. Prod. Commun., 7, 749 (2012).
R. Hansel, H. Rimpler, and K. Walther, Naturwissenschaften, 53, 19 (1966).
J. Exner, J. Reichling, T. C. H. Cole, and H. Becker, Planta Med., 41, 198 (1981).
S. Petronilho, M. Maraschin, M. A. Coimbra, and S. M. Rocha, Ind. Crops. Prod., 40, 1 (2012).
K. R. Markham and D. R. Given, Phytochemistry, 27, 2843 (1988).
H. Eckey-Kaltenbach, Z. Sonnenbichler, E. Schafer, and J. Sandermann, Phytochemistry, 34, 687 (1993).
H. Tamura, T. Kondo, Y. Kato, and T. Goto, Tetrahedron Lett., 24, 5749 (1983).
H. Kanamori, M. Terauchi, J.-I. Fuse, and I. Sakamoto, Shoyakugaku Zasshi, 46, 384 (1992).
V. M. Malikov and A. I. Saidkhodzhaev, Chem. Nat. Compd., 34, 345 (1998).
D. N. Olennikov, N. I. Kashchenko, N. K. Chirikova, and L. M. Tankhaeva, Molecules, 20, 19172 (2015).
V. Petrulova-Poracka, M. Repcak, M. Vilkova, and J. Imrich, Food Chem., 141, 54 (2013).
H. Kanamori, M. Terauchi, J.-I. Fuse, and I. Sakamoto, Shoyakugaku Zasshi, 47, 34 (1993).
D. N. Olennikov, L. M. Tankhaeva, V. V. Partilkhaev, and A. V. Rokhin, Braz. J. Pharmacogn., 22, 490 (2012).
D. N. Olennikov, N. I. Kashchenko, and N. K. Chirikova, Nutrients, 7, 8456 (2015).
Acknowledgment
The work was sponsored by SB RAS Project No. VI.62.1.8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2016, pp. 855–858.
Rights and permissions
About this article
Cite this article
Olennikov, D.N., Kashchenko, N.I. New Acylated Apigenin Glycosides from Edge Flowers of Matricaria chamomilla . Chem Nat Compd 52, 996–999 (2016). https://doi.org/10.1007/s10600-016-1845-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-016-1845-7