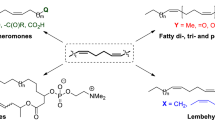
Research from the last 20 years on the use of ozonolysis of cyclic and acyclic mono- and dienes and aromatic compounds in various steps of the total synthesis of insect pheromones and juvenoids was reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Rampant use of pesticides has destroyed the natural ecological equilibrium such that the application of compounds that act selectively on the insect hormonal system remains critical in the battle with insect pests.
Pheromones are produced by insects for intraspecies communication and can be used to observe and control their population. Juvenoids are natural juvenile hormone analogs and affect insect development in the larval stage and subsequent metamorphosis into adults. Insect pheromones and juvenoids are harmless to mammals, including man, and are broadly used as ecologically safe agents for battling agricultural pests [1–6].
Aliphatic compounds with functional groups at the ends of the carbon chains are usually required to synthesize pheromones and juvenoids for many insect species. Ozonolysis of olefins is one of the most promising synthetic pathways to such synthons [7–9]. Ozone is an effective and ecologically friendly oxidant that is broadly used in organic synthesis, including in targeted synthesis of biologically active compounds, because of the capability to transform selectively the α,ω-bifunctional oxygen-containing compounds that are formed during ozonolysis of olefins.
Research from the last 20 years on the use of ozonolytic transformations of cyclic and acyclic mono- and dienes and aromatic compounds in various steps of the total synthesis of insect pheromones and juvenoids was reviewed. This review continued a previous one [10].
1. Selective Ozonolysis of Cyclic and Acyclic Dienes and Aromatic Compounds in the Synthesis of Insect Pheromones and Juvenoids
Oxidative cleavage of olefins became especially useful owing to the success of metal-complex catalysis, as a result of which cyclic and acyclic oligomers and co-oligomers of 1,3-dienes became available.
Products from selective ozonolysis of cyclic butadiene oligomers are widely used to synthesize insect pheromones. Partial ozonolysis of available cyclic oligomers and co-oligomers of butadiene [(1Z,5Z)-cyclooctadiene (1) and (1E,5Z)-cyclodecadiene (2)] represented a new approach to the synthesis of 1,8-octane- (3) and 1,10-decanediol (4), selective bromination of which followed by condensation of the obtained bromides (5 and 6) with lithium acetylenide synthesized 7 and 8 with a terminal triple bond. Then, alkylation and hydrogenation of the disubstituted alkynes (9 and 10) produced the target (9E)-dodecen-1-ylacetate (11), which was identified, like the corresponding alcohol, in the sex pheromone of Sparganothis pilleriana, and (11E)-tetradecen-1-ylacetate (12), the sex pheromone of Loxostege sticticalis [11] (Scheme 1).

Scheme 1
Ozonolysis of monocyclopropanation product 13, which was synthesized via reaction of 1,5-cyclooctadiene (1) with dichlorocarbene using phase-transfer catalysis or ultrasonic irradiation, produced convenient synthons containing a gem-dichlorocyclopropane ring. Depending on the reductant, α,ω-dialdehyde 14, which was characterized as dimethylacetal 15, or diol 17, which was converted through tosylate 18 into corresponding bromide 19, was isolated. Horne–Wadsworth–Emmons olefination of 14 with a stabilized phosphonate produced the potential juvenoid dienoate 16 as a mixture of E- and Z-isomers in an 85:15 ratio. Reaction of bromide 19 with potassium 2,4-dichlorophenoxide in the presence of tetrabutylammonium bromide gave 1,3-bis-[3-(2,4-dichlorophenoxy)propyl]-2,2-dichlorocyclopropane (20), which exhibited juvenoid activity. Another juvenoid 21 was prepared by treating diol 17 with 2,4-dichlorophenoxyacetylchloride. The yields in all steps of the proposed syntheses were ~40–60%. Therefore, the overall yields of target juvenoids 20 and 21 were low [12] (Scheme 2).

Scheme 2
Another butadiene cyclodimerization product [13], vinylcyclohexene (22), was also used to synthesize low-molecularweight insect bioregulators. Thus, a new approach based on exhaustive ozonolysis of 22 was proposed [14] for synthon 23 for furan-containing analogs of native juvenile hormones and more effective juvenoids with a 2,4-dienoate system (24) (Scheme 3).

Scheme 3
Partial ozonolysis of 1,4-cyclohexadiene (25) synthesized (10E,12Z)-hexadecadienol (bombykol) (35) and (7E,9Z)-dodecadien-1-ylacetate (36), sex pheromones of Bombix mori [15] and Lobesia botrana [16, 17]. Wittig olefination of the reductive ozonolysis product 26 gave the corresponding methyl esters of (4E,6Z)-decane- (27) and (4E,6Z)-nonane-4,6-dienoic acid (28), which then were reduced by LiAlH4 to alcohols 29 and 30. The key step was extension of the carbon chain by reacting the oxidation products of alcohols 29 and 30 with the corresponding Grignard reagents. Resulting alcohols 31 and 32 were de-oxygenated by LiAlH4 through the mesylate intermediates. Chemo- and regiospecific hydroboration by 9-BBN followed by H2O2 oxidation converted trienes 33 and 34 into target alcohol 35 and then acetate 36 (Scheme 4).

Scheme 4
The key step in the synthesis of (2S)-acetoxytridec-10-ene (40) (sex pheromone of Mayetiola destructor) was catalyzed cross-coupling of chiral 41 and achiral 39 block synthons. The latter was obtained from phenoxy derivative 38, the partial ozonolysis product of butadiene–phenol diene telomer 37 [18] (Scheme 5).

Scheme 5
One approach to synthesizing terpenoid insect pheromones with a tri-substituted double bond was to use regularly constructed oligomers, co-oligomers, and isoprene telomers, the preparation of which is now well developed, in addition to natural terpenoids with tri-substituted double bonds as starting materials. If this approach was used, the problem consisted of choosing selective methods for the required transformation of the starting material so that the carbon skeleton required for the pheromone could be constructed using the resulting functional block synthon. The starting cyclic isoprenoid was transformed into the α,ω-bifunctional synthon by opening the ring. A suitable method for converting a starting acyclic compound with the given geometry of the double bonds into an intermediate that was convenient for further use in preparing the target pheromone was selected [10].
The most available isoprene cyclooligomer is (1Z,5Z)-dimer 42, selective ozonolysis of which produced unsaturated ketoacetal 43, which was used to synthesize insect pheromones [10], e.g., (±)-3,7-dimethylpentadec-2-ylacetate (diprionylacetate) (49) [19, 20], which was a racemic analog of sex pheromones from four species of pine sawflies in the genera Diprion and Neodiprion. The key synthetic step was Wittig olefination of saturated ketone 44, the product from catalytic hydrogenation of enone 43, which gave an ~4:1 mixture of the Z- and E-isomers of 1,1-dimethoxy-4,8-dimethyldec-8-ene (45). Construction of the carbon skeleton of pheromone 49 was completed by Li2CuCl4-catalyzed cross-coupling of n-amylmagnesiumbromide and tosylate 47, which was obtained via selective transformations of unsaturated acetal 45 along the path 46 → 47 → 48. The secondary acetoxy group was introduced by regiospecific hydration using an organoboron intermediate followed by acetylation (Scheme 6).

Scheme 6
2,6-Dimethyl-(2E)-octene-1,8-diol diethers (60 and 61), which were active against Culex mosquito larvae, were synthesized in ~16% overall yield from the product of exhaustive ozonolysis of 1,5-dimethyl-1,5-cyclooctadiene (42), i.e., the dimethylacetal of levulinic aldehyde (50) [21]. Petersen olefination of 50 added ethoxycarbonylmethylene to give unsaturated ethyl ester 51, subsequent reduction of the ester of which by Li in liquid NH3 was accompanied by simultaneous hydrogenation of the ∆2-bond to afford alcohol 52. Phenyl ether 54 was prepared by treating its 6-tosyl derivative (53) with sodium phenoxide. Methyl ether 55 was prepared by alkylating alcohol 52 with methyliodide through an intermediate sodium alkoxide. Acid hydrolysis of acetals 54 and 55 followed by olefination of the free aldehydes by isopropylidenetriphenylphosphorane gave 1-phenoxy- (56) and 1-methoxy-3,7-dimethyl-6-octene (57), allylic oxidation of which gave 8-phenoxy- (58) and 8-methoxy-2,6-dimethyl-(2E)-octen-1-ol (59), which were transformed into target diethers 60 and 61, respectively (Scheme 7).

Scheme 7
Partial ozonolysis of dihydromyrcene (62), which occurred preferentially at the tri-substituted double bond, produced alcohol 63 (R = OH) or aldehyde 64, which are widely used to synthesize insect pheromones, depending on the decomposition conditions for the intermediate peroxides (Scheme 8) [22, 23].

Scheme 8
Thus, the synthetic capabilities of unsaturated alcohol 63 were studied using preparation of the three insect pheromone components 70, 72, and 73 as examples [24, 25]. One of the proposed synthetic approaches was based on catalytic crosscoupling of tosylate 65 with n-octylmagnesiumbromide. Successive Wacker–Tsuji oxidation of alkene 66 and Baeyer–Villager oxidation of the obtained (S)-3-methyltetradecan-2-one (67) gave Drosophila mulleri pheromone 70 in 40% overall yield. An alternative and less effective (20% overall yield) synthetic route to chiral acetate 70 consisted of Wacker–Tsuji oxidative transformation of vinyl tosylate 65 into ketone 68 and then Baeyer—Villager oxidation into acetoxytosylate 69, which underwent chemoselective cross-coupling with a magnesium-cuprate reagent generated from n-octylbromide. Reduction of diester 69 by LiAlH4, which occurred with configuration retention of the chiral center, produced enantiomerically enriched (S)-pentan-2-ol (71), which could be readily converted into components of the Rhyzopertha dominica aggregation pheromone, i.e., dominicalur-1 (72, 94%) and dominicalur-2 (73, 95%) (Scheme 8).
The terminal vinyl group in (S)-(+)-dihydromyrcene (62) had to be cleaved in order to synthesize a component of the smaller yellow ant Acanthomyops claviger sex pheromone (75). Diene 62 was epoxidized in order to protect the isopropylidene group, which was more sensitive to electrophiles. Further ozonolysis and treatment of the peroxide with Et3N–Ac2O gave key epoxyester 74 in high yield [26] (Scheme 8).
A synthesis of intermediates 93 and 95 was developed [27] in order to prepare (−)-dihydroactinidiolide (94) and (−)-anastrephin (96), pheromone components of Solenopsis invicta Buren and Anastrepha suspense Loew, respectively. Starting geraniol 76 [28] was converted by Sharpless asymmetric epoxidation in the presence of L-(+)-DET and subsequent silylation into epoxysilyl ether 77. Rearrangement of 77 through the action of methylaluminum bis(4-bromo-2,6-di-t-butylphenoxide) gave (S)-aldehyde 78 (ee 95%), which was transformed by several sequential reactions first into the two unsaturated esters 79 and 80 and then into 81 and 82. Removal of the protection in the last two and Swern oxidation produced the corresponding aldehydes, which underwent Noyori acetalization to form esters 83 and 84, which were then converted to amides 85 and 86. Treatment of 85 and 86 with trifluoroacetic anhydride in the presence of collidine in refluxing benzene caused 1,2-asymmetric induction during [2+2]-cycloaddition to form after hydrolysis a chromatographically separable mixture of cyclobutanone diastereomers 89/90 and 91/92 in 5:1 and 3:1 ratios, respectively. The configuration of the new asymmetric center (C-1) was established using the NOE-effect between the C-2 methyl and the C-1 methine proton in principal diastereomers 89 and 90. According to the researchers, the diastereoselectivity of the cycloaddition was explained by ketenimine cyclization through eight-membered enamine intermediates 87 and 88 (Scheme 9).

Scheme 9
Baeyer–Villager oxidation of 89 gave a lactone, which was hydrolyzed to form 93, which was transformed into (−)-dihydroactinidiolide (94) as before [29]. Also, 90 was transformed into lactone 95, a key intermediate in the synthesis of (−)-anastrephin (96) [30], by successive Baeyer–Villager oxidation, acid hydrolysis, and olefination (Scheme 10).

Scheme 10
Juvenoids often include aromatic moieties in their structures. Therefore, ozonolytic cleavage of substrates containing aromatic systems was used to synthesize them.
Ozonation of naphthalene (97) in MeOH formed primarily aldehydoester 98, regardless of the reductant. Horner–Wittig olefination of 98 produced mono- (99) and diene (101) esters, which were intermediates in the synthesis of bis-phenyl ethers 100 and 102, aromatic analogs of Culex and Pectinophora gossypiella juvenile hormones [31, 32] (Scheme 11).

Scheme 11
Ozonolysis by various methods of 1,4-dihydronaphthalene (103), a readily available product from partial Birch reduction of naphthalene, produced functionalized benzene derivatives 104–107, which were used to synthesize juvenoids [33] (Scheme 12).

Scheme 12
N-(2,6-Difluorobenzoyl)-N′-arylureas (113) are highly touted as effective insect chitin biosynthesis inhibitors. The most convenient pathways for synthesizing them were the reaction of 2,6-difluorobenzamide (111) with arylisocyanates or the reaction of 2,6-difluorobenzoylisocyanate (112) with arylamines. An approach to the synthesis of 112 was developed based on 2,6-difluorobenzoic acid (109), which was synthesized via O3–O2 oxidation of 2,6-difluorotoluene (108) in the presence of Co(OAc)2. Transformation of 109 into acid chloride 110 and treatment of 110 with NH4OH gave 2,6-difluorobenzamide (111), which was converted to isocyanate 112 by reaction with oxalylchloride [34] (Scheme 13).

Scheme 13
An alternative synthetic route to benzamide 111 [33] was based on alkylation of available m-difluorobenzene (114) by the action of methylallylchloride on its sodium derivative, which was generated using sodium amide in liquid NH3. Subsequent transformations of the alkylation product, 1-(2-methyl-2-propen-1-yl)-2,6-difluorobenzene (115), gave eventually amide 111 with high yields in each step. Olefin 115 isomerized readily on heating in benzene in the presence of TsOH to styrene derivative 1-(2-methyl-1-propen-1-yl)-2,6-difluorobenzene (116), ozonolysis of which and subsequent treatment of the peroxide ozonolysis product with NH2OH∙HCl gave 2,6-difluorobenzaldoxime (117), which was transformed under Beckmann reaction conditions into benzamide 111. The overall yield of 111 from difluorobenzene (114) was 80%. Ozonation of alkene 116 in AcOH followed by oxidative decomposition of the ozonide in the presence of SeO2 gave 2,6-difluorobenzoic acid (109), which was converted into amide 111 by the usual route (Scheme 14).

Scheme 14
2. Synthesis of Pheromones and Juvenoids Based on Ozonolysis Products of Cyclic and Acyclic Alkenes
Ozonolysis of monoenes afforded saturated oxygen-containing compounds that were of interest for synthesizing pheromones and juvenoids.
The trail pheromone (pharanal) of Monomorium pharaonis, a food pest and dangerous infection vector, was identified as 3,4,7,11-tetramethyldeca-(6E,10Z)-dienal (123) although natural pharanal has the (3S,4R)-configuration [35]. A synthesis of 123 starting from cis-dimethylcyclohexene (118) was proposed. Asymmetric cleavage of meso-epoxide 119 using (S)-(pyrrolidin-2-ylmethyl)pyrrolidine (124) gave allyl alcohol 120, reductive ozonolysis of which and protection of the C-1 and C-2 hydroxyls in the obtained triol followed by replacement of the C-6 OH gave iodide 121. Condensation of 121 with a lithium derivative produced enantiomerically pure acetal 122, hydrolysis of which and subsequent periodate oxidation completed the synthesis of target 123 in 12.5% yield (Scheme 15).

Scheme 15
An alternative pathway for preparing racemic (±)-pharanal (123) starting from cis-dimethylcyclohexene (118) was proposed [35]. The key synthon of the proposed synthesis was iodide 129. Ozonolysis of 118 followed by Jones oxidation produced diacid 125, pyrolysis of which in the presence of Ba(OH)2 afforded cyclic ketone 126. Baeyer–Villager oxidation of 126 to lactone 127 and treatment with anhydrous HBr in EtOH gave bromoester 128, which then was transformed into key iodide 129. Further condensation of 129 with Li-derivative 130b gave tetrahydropyranyl ether 131, which was transformed by standard reactions into (±)-pharanal (123) in 18% overall yield from iodide 129 (Scheme 16).

Scheme 16
The most important components of queen substance and royal jelly of Apis mellifera L., 9-oxo- (140) and 10-hydroxy-2E-decenoic acid (143), were synthesized starting with an ozonolytic cleavage product of methylcyclohexene (132), 7-hydroxyheptan-2-one (133) [36]. The key synthon for both target acids 140 and 143 was unsaturated acetate 138, which was synthesized by standard transformations of ketoalcohol 133 along the route 133→134→135→136→137→138. Further transformation of alkenylacetate 138 that was directed at oxoacid 140 consisted of single-step Wacker–Tsuji transformation into ketoacetate 139. Building block 142 for hydroxyacid 143 was constructed from intermediate monoester 141 using chemoand regioselective oxidative hydroboration (Scheme 17).

Scheme 17
1,1-Dimethoxy-6-oxoheptane (144), another reductive ozonolysis product of 1-methyl-1-cyclohexene (132), was used to synthesize a racemic mixture of echinolone Z- and E-isomers (146), which exhibited higher juvenile hormone activity than each of the isomers. Transformation of ketoacetal 144 into vinyl alcohol 145 and condensation of the latter with the complementary phosphorane generated from phosphonium tosylate 147 gave target compound 146 [37] (Scheme 18).

Scheme 18
Ozonolytic ring opening of optical isomers of silylenol ethers 148a or 148b followed by NaBH4 reduction and treatment with MeOH in the presence of TMSCl gave ω-hydroxyesters 149a and 149b, which were used to synthesize 154 and 155, two female sex pheromones of Lyonetia prunifoliella, a pest endemic to the eastern regions of North America [38]. The carbon chain of 149a was lengthened from the side of the primary hydroxyl in three steps, i.e., transformation into tosylate 150, chemoselective hydride reduction of the ester to alcohol 151, and final cross-coupling with an excess of n-propylmagnesiumbromide in the presence of stoichiometric amounts of CuBr∙SMe2 to branched dimethylalcohol 152. The last was transformed into tosylate 153, which underwent CuBr∙SMe2-catalyzed reaction with 6-heptenyl- or n-hexylmagnesiumbromide to form target pheromones 154 and 155, respectively (Scheme 19).

Scheme 19
The availability of starting compounds in addition to the preparation of chemically and optically pure target compounds in yields as high as possible and the economic feasibility and ease of carrying out the proposed pathway often remain problematical for planning targeted organic syntheses. The problem is often solved in practice by using renewable natural raw material that is available from essential oils, turpentine, and other sources.
Ozonolysis of α-pinene (156), which was isolated from turpentine from sap of various coniferous Pinus species [28], was used to demonstrate [39] its synthetic capabilities for synthesizing pheromones. (+)-cis-(1R)-2,2-Dimethyl-3-acetylcyclobutanylethanol (157), a product from ozonolysis of α-pinene [(+)-156] followed by NaBH(OAc)3 [40, 41] or NaBH4–NaOH–H2O [42] reduction, was proposed for synthesizing several structural analogs (158a-h) of a Planococcus citri (Risso) pheromone via acylation and Wittig olefination (Scheme 20).

Scheme 20
A convenient synthesis in a few steps of a Planococcus citri (Risso) sex pheromone (162) that was based on ozonolysis of an α-pinene oxidation product (156), verbenone [(1R,5R)-159], in MeCN was proposed [43]. Reaction with an excess of ozone at −40°C caused simultaneous cleavage of the double bond in 159 and oxidative degradation of the side chain, which resulted in ketoacid 160, which was converted to ketoester 161. Olefination of the last, hydride reduction, and acylation completed the synthesis of 162 (Scheme 21).

Scheme 21
Optically pure pentanolide 169, an intermediate in the synthesis of aggregation pheromone components of Tribolium flour beetles [(4R,8R)- and (4R,8S)-stereoisomers of 4,8-dimethyldecanal (181)], was synthesized from l-menthol (163), the principal component of Mentha piperita [44–46]. Lactone 169 was obtained by intramolecular re-esterification of 168, a product from exhaustive Baeyer–Villager oxidation of chiral diketone 167. The last was synthesized using reductive ozonolysis of (3R)-methylmenthene (166), which was prepared in turn by regiospecific acidic dehydration of Grignard coupling intermediate products [165a and b (94:6)] of menthone (164) and methylmagnesiumiodide (Scheme 22).

Scheme 22
Pheromones (4R,8R)- and (4R,8S)-181 were approached using acidic opening of lactone 169 to acetoxyacid 170 [47], oxidative decarboxylation to unsaturated acetate 171, oxidative hydroboration in acetate buffer to give quantitatively alcohol 172, and PCC oxidation into one of the required fragments, acetoxyaldehyde 173. The second required structural unit, phosphonium salt 176, was synthesized from the same acetate 171 by saponification into alcohol 174 and standard two-step transformation of the latter into bromide 175 without isolating the intermediate tosylate (Scheme 22).
Phosphonium salt 176 was used to construct monoterpene fragment 177 of target (4R,8R)-181 from key synthons 173 and 176 according to Wittig without affecting the chiral centers. Catalytic hydrogenation of 177 followed by saponification of the intermediate saturated acetate gave alcohol 178, which was transformed in high yield into bromide 179. The concluding step in the synthesis of pheromone (4R,8R)-181 involved extending the carbon chain of 179 by a CH2CHO fragment, which was achieved in two steps by CuI-catalyzed condensation of a Grignard reagent prepared from 179 and allylbromide and subsequent ozonolysis of olefin 180. The overall yield of (4R,8R)-181 was 10% from starting acid 170. The (4R,8S)-isomer was synthesized analogously (Scheme 23).

Scheme 23
Another method for functionalizing l-menthol (163) through (−)-menthone (164) ended with the conversion of the latter into (R)-4-menthenone (183) and was based on halogenation–dehydrohalogenation of the corresponding enolacetate 182 [48, 49] (Scheme 24).

Scheme 24
(R)-4-Menthenone (183) was transformed ozonolytically into acetalester 184 [50] in order to use 183 as a substrate for synthesizing optically pure biologically active compounds. Compound 184 was a promising bifunctional synthon for synthesizing several optically active pheromones and juvenoids [51–53] (Scheme 24).
A key step in the synthesis of (4R,8R)-dimethyldecanal (181) and its (4R,8S) stereoisomer was cross-coupling of bromide (R)-187 and tosylate 192, products of chemoselective transformations of chiral synthon 184 [54, 55]. The first block [(R)-187] was synthesized from acetalester 184 via Huang–Minlon deoxygenation of intermediate aldehydoester 185. Hydrolysis of the ester occurring during this allowed key bromide (R)-187 to be obtained after Hunsdiecker decarboxylation of 186 (Scheme 24).
The second block 192 was synthesized using a product from hydride reduction of 184, i.e., hydroxyacetal 188, in which the hydroxyl had to be protected for further transformations. This was achieved by converting it into benzyl ether 189, deprotection of the oxo group in which gave aldehyde (S)-190. Sequential reduction and then esterification of mono-substituted diol 191 gave the required methyl-branched synthon 192 (Scheme 25).

Scheme 25
Benzyl ether 193 was prepared in the key step via alkylation of the tosyl group in 192 by the Grignard reagent of bromide (R)-187. The carbon chain was lengthened by converting alcohol 194 to bromide 195 followed by formylation of the corresponding Grignard reagent to complete the synthesis of (4R,8R)-181 (Scheme 26).

Scheme 26
The (4R,8S)-181 isomer could be synthesized analogously using chiral synthon (S)-187, which was obtained from (S)-4-menthen-3-one [(S)-183], instead of the (R)-187 isomer [55] (Scheme 26).
Optically pure (S)-(+)-hydroprene (199), an juvenile hormone analog from insects with incomplete metamorphosis [56], was synthesized from methyl (3S)-3,7-dimethyl-5-oxooctanoate (196), an ozonolysis product of (R)-4-menthen-3-one (183) in the presence of Py or Et3N in CH2Cl2–MeOH (1:1) [57, 58]. Subsequent Huang–Minlon deoxygenation of 196, which was accompanied by saponification of the ester, gave (3S)-3,7-dimethyloctanoic acid (197), which was converted by sequential hydride reduction and Corey oxidation into aldehyde 198. Condensation of the last with phosphonate 200 in the presence of KOH and [n-Bu4N]OH as before [59] formed target (S)-(+)-hydroprene (199) as a 9:1 mixture of the (2E,4E)-199a and (2Z,4E)-199b isomers in 32% overall yield from ester 196 (Scheme 27).

Scheme 27
The conjugated enone system in (R)-4-menthenone (183) enabled it to undergo regioselective 1,2-addition of organometallic reagents. Condensation of enone (R)-183 with ethyllithium produced tertiary allylic alcohol 201 [60], oxidation of which by Cr(VI) with allylic rearrangement gave 5-ethylmenthenone (202). Huang–Minlon reduction of the oxo group in ketoester 203, which was obtained by ozonolysis of enone 202, to a methylene was accompanied by hydrolysis of the ester to afford (S)-3-methylheptanoic acid (204), a Coleoptera scarabaeidae pheromone. Its reduction product, alcohol 205, was converted to bromide (S)-206, which was transformed by standard reactions into (4S)-methyloctanoic acid (208), a component of an Oryctes rhinoceros beetle aggregation pheromone. Alkylation of bromide (S)-206 by a Grignard reagent generated from 10-undecenylbromide resulted in (S)-14-methyloctadecene [(S)-207], a Lyonetia clerkella sex pheromone [61, 62] (Scheme 28).

Scheme 28
Derivatives of R-(+)-pulegone [(R)-209], which is available from Mentha pulegium L. essential oil [28], are often used to synthesize insect pheromones. The ring of (R)-209 was contracted using Favorskii rearrangement of the corresponding isomer of dibromo-derivative 210 to produce the (1S,2R)-isomer of unsaturated cyclic ester 211 [63] (Scheme 29).

Scheme 29
This process was employed to synthesize 1,8-dimethyl-4-(1′-methylethenyl)spiro[4, 5]dec-7-ene [(+)-acoradiene] (214), a stereoisomer of which is an aggregation pheromone of the flour beetle Gnatocerus cornutus [64]. Ozonolytic cleavage of the double bond of unsaturated ester (1S,2R)-211 followed by simple transformations produced conjugated ketone 212, which underwent a Diels–Alder reaction to construct the required structure with spiro-connected five- and six-membered rings. The regiospecific but not stereospecific course of the last reaction explained the formation of an equimolar mixture of stereoisomers of both the resulting spiroketone 213 and acoradiene (214) obtained from it (Scheme 29). The resulting mixture of 214 stereoisomers was separated using preparative GC. The two isomers with retention times closest to that of the natural compound were selected for convergent synthesis.
Citronellic acid (215) was formed in two steps via recyclization of pulegone [(R)-209] [65]. It could be reduced to (R)-citronellol [(R)-218] by LiAlH4 or DIBAH after conversion into ester 216 or 217. Alcohol (R)-218 was readily transformed into citronellal (219), citronellylacetate (220), citronellyltosylate (221) [65], and other derivatives used to synthesize insect pheromones. (R)-218 and its oxidation products (aldehyde 219 and acid 215) were natural compounds in citrus and eucalyptus essential oils [28] (Scheme 30).

Scheme 30
A synthesis based on citronellic acid (215) was proposed for synthesizing the principal component of Cantao parentum abdominal gland secretion, which was identified as (2S,4R,6R,8S)-trimethyl-1,7-dioxaspiro[5.5]undecane (233a). This was the first example of a branched spiroacetal in insects [66]. This unique compound was synthesized enantioselectively [67] through the intermediate acetonide of unsaturated ketone 226. Successive ozonolysis of the double bond of 226 and reduction of the peroxide products gave hydroxyketal 227, dehydration of which through the corresponding iodide 228 gave ketoolefin 229 after acid hydrolysis. The required carbon chain was constructed by alkylation of the lithium derivative of the corresponding hydrazone (230) using optically active protected iodohydrin (S)-224, which was prepared from intermediate hydroxyester (S)-223 [68]. The (R)-enantiomer of the last was synthesized by hydrolysis of poly[(R)--hydroxybutyrate] (222); (S)-223, by enzymatic reduction of ketoester 225 using Candida cylindracea lipases or porcine pancreas lipase (PPL) as before [69]. Furthermore, (S)-223 was obtained from asymmetric hydrogenation of ketoester 225 on catalysts modified with chiral ligands [70] (Scheme 31).

Scheme 31
The hydroxyl was introduced by oxidizing the double bond of 231a using the chiral osmium reagent AD-mix . The appearance in the precursor of alcohols in the δ and δ′ positions relative to the oxo group was accompanied by simultaneous ketalization to give hydroxyketal 232, deoxygenation of which gave target spiroketal 233 (Scheme 32). Other diastereomers of 233 that were minor components in the insect isolates were also obtained [70].

Scheme 32
The key step in the synthesis [71] of (2S,4R,5S)-2,4,6-trimethyl-5-heptanolide (240), a sex pheromone component of Macrocentrus grandii, a larval parasite of Ostrinia nubilaris, was stereoselective bromolactonization of unsaturated acid 238, which was obtained from alcohol 234, an ozonolysis product of methylcitronelloate 216. The carbon chain of 234 was grown using a Grignard reagent (after protecting the hydroxyl). Dehydration of tertiary alcohol 235 produced a mixture of required 236 and its regioisomer 237, from which it was separated by chromatography. The second methyl was introduced into lactone 239 using MeI in the presence of LDA. Pheromone 240 was separated from side product 241 using HPLC (Scheme 33).

Scheme 33
Pheromones that were isolated from Aphthona flava and Phyllotreta cruciferae included (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene (242), (5R,5aS)-1,1,5,8-tetramethyl-1,2,3,4,5,6,5a-heptahydrobenzo[1,2-a][7]annulene (243), and (R)-1,1,5,8-tetramethyl-1,2,3,4,5-pentahydrobenzo[a][7]annulene (244). All these compounds were prepared from one precursor, (1S,2R)-2,2,6-trimethylbicyclo[5.4.0]undec-7-en-9-one (245) [72].
Ozonolytic cleavage of the double bond in ethylcitronelloate (217) and Horner–Emmons olefination of resulting aldehyde 246 produced diester 247, Dieckmann condensation of which created the required seven-membered ring. Subsequent Robinson annelation of ketone 248 introduced the six-membered ring of 245 [72]. Wittig olefination converted the last into component 242. Compound 243 was the product from migration of the double bond in 242. Component 244 was obtained via aromatization of the six-membered ring by chloranil (tetrachlorobenzoquinone) (Scheme 34).

Scheme 34
(R)-Citronellol (218) was used to prepare both synthons 253 and 255 in a convergent synthesis of (11R,17S)-dimethyltriacontane (257), a Camponotus vagus communication pheromone [73]. Reductive ozonolysis of alcohol (R)-218 in the synthesis of phosphonium salt 253 gave a mixture of hydroxyaldehyde 249 and hemiacetal 250, Wittig olefination of which produced (Z)-unsaturated alcohol (R)-251, which was transformed through bromide 252 into salt 253. The second synthon 255 was obtained from ozonolytic cleavage of intermediate tosylate 254, the esterified alkylation product of n-dodecylmagnesiumbromide and (R)-219, which was prepared via oxidation of citronellol (R)-218 [65] or incubation of racemic citronellal (219) with baker’s yeast enzymes [74]. Resulting aldehyde (R)-255 underwent Wittig olefination by the phosphorane from phosphonium salt 253. Reduction of tosyloxydiene 256 completed the synthesis of target pheromone 257 (Scheme 35).

Scheme 35
3,13-Dimethylheptadecane (267) was identified in 1993 as the principal female sex pheromone component of Nepytia freemani [75], a conifer pest that is broadly distributed in the northwestern USA and in southeastern Canada. The key step in the synthesis of all stereoisomers of 3,13-dimethylheptadecane (267a–d) was alkylation of phenylsulfones 262, which had active α-methylenes [76]. The aforementioned organosulfur compounds were synthesized by first alkylating citronellyltosylate enantiomers 221 with EtMgBr using Grignard–Schlosser cross-coupling. The double bond of synthesized alkene 258 underwent ozonolytic cleavage. The resulting alcohol 259 was transformed into iodide 260, which was alkylated by the lithium derivative of 4-pentyn-1-ol. Exhaustive hydrogenation followed by phenylthiylation of 261 and oxidation by m-CPBA gave the corresponding (R)- and (S)-isomers of 262 (Scheme 36).

Scheme 36
The second building block, 2-methylbutyliodide (265) [77], was synthesized using the required enantiomer of methyl 2-methyl-3-hydroxybutanoate (264), the (R)-isomer of which was a product of microbiological oxidation of isobutanoic acid (263) by Candida rugosa IFO 0750 [78]. Its antipode (S)-264 was produced by Pseudomonas putida ATCC 21244 [79] (Scheme 37).

Scheme 37
Various combinations of lithium derivatives of sulfone 262 (R and S) and iodide 265 (R and S) isomers cross coupled to give sulfone 266 with two optically pure chiral centers. Desulfurization and hydrogenation completed the synthesis of target pheromones 267 (RS, RR, SR, SS) (Scheme 37).
The absolute configuration of the Hesperophylax occidentalis sex pheromone was elucidated using simple syntheses of both enantiomers (S or R)-270. For this, citronellyltosylate (R)-221 was treated with Me2CuLi and transformed into alkene 268, ozonolysis of which gave aldehyde 269. Treatment of the last with EtMgBr and subsequent oxidation completed the synthesis of (S)-ketone 270. (S)-Citronellyltosylate [(S)-221] was transformed analogously into the (R)-enantiomer of 270 [80] (Scheme 38).

Scheme 38
Both stereoisomers of 7-methylheptadecane (272), a Lambdina athasaria and L. pellucidaria sex pheromone, were synthesized and tested [81]. The approach was based on two sequential Schlosser reactions of the (R)- or (S)-enantiomer of citronellyltosylate (221) with Me(CH2)7MgBr and tosylate 271 with Me(CH2)2MgBr. Biological tests showed that the (S)-isomer of 272 was active (Scheme 38).
A concise and effective synthesis of (4R,8R)- or (4S,8R)-181, components of the Tribolium confusium and T. castaneum aggregation components, was proposed using ozonolysis in the final step to transform the isopropylidene into an aldehyde [82, 83]. The key synthetic step was the Li2CuCl4-catalyzed cross-coupling of tosylates (R)- and (S)-221 with (R)-2-methyl-1-bromobutane (187). This led to olefins (6R,10R)- and (6S,10R)-273, ozonolysis of which produced pheromones (4R,8R)- and (4S,8R)-181 (Scheme 38).
Ozonolysis of mono-unsaturated carboxylic acids and their derivatives represented a convenient route to α,ω-bifunctional reagents that were used to synthesize insect pheromones.
(S)-2,5-Dimethylheptadecane (276) is a minor component of the Lambdina fiscellaria lugubrosa sex pheromone. Ozonolytic fragmentation of (R)-2,6,9-trimethyldec-1-ene (274), which was obtained from (R)-citronellic acid (215), gave (4R,7)-dimethyloctanal (275), coupling of which with n-nonylidenetriphenylphosphorane followed by catalytic hydrogenation led to target pheromone 276 [84] (Scheme 39).

Scheme 39
10-Undecenoic acid (277), which is available via destructive distillation of castor oil isolated from castor beans [85], was used for a series of syntheses of octadeca-2E,13Z-dienylacetate (283) [86, 87], a pheromone component of the hazardous garden pests Synanthedon tipuliformis and Zeuzera pyrina. Ozonolysis of enyne 278 occurred selectively at the double bond because the acetylene group was less reactive toward ozone than the vinyl group. Reduction of the peroxide products by Me2S gave hexadec-11-ynal (279) in high yield. Use of the Doebner reaction allowed the required carbon chain to be constructed and the 2E-double bond to be introduced. Resulting acid 280 was converted to the chloride (281), which was reduced by LiAlH4 to octadec-2E-en-13-yn-1-ol (282). Catalytic hydrogenation of the triple bond in 282 and subsequent acetylation completed the synthesis of target diene pheromone 283 in 16% overall yield from starting acid 277 (Scheme 40).

Scheme 40
Alkaline dehydrohalogenation at 150°C of dibromo-derivative 284 of acid 277 synthesized with high regioselectivity 9-undecynoic acid (285), reductive ozonolysis of the olefin analog of which (286) gave aldehydoester 287, a key compound for (9Z)-hexadecenal (288), a minor component of the Heliothis armigera sex pheromone [88] (Scheme 40).
13-Hydroxy-2-oxotridecane (292) that was isolated from fruit extracts of Evodia hupehensis Dode was an active attractant for honeybees. New approaches to the synthesis of this attractant were developed. The first was based on selective transformations of the monoalkylation product of acetoacetic ester by bromide 289. Decarbethoxylation of unsaturated ketoester 290 gave the key intermediate tetradec-13-en-2-one (291), ozonolysis of which followed by NaBH(OAc)3 reduction, which reduced selectively existing or formed aldehydes without affecting the ketones, produced target compound 292. An alternative version used reductive ozonolysis of 1-methylcyclododecene (293). The yield of attractant in this instance was 89% [89, 90] (Scheme 41).

Scheme 41
The sex pheromone of Grapholitha molesta, a pest of peaches, apples, pears, and apricots, was identified as (Z)-8-dodecylacetate (299a). It was observed that an impurity (up to 10%) of the (E)-isomer (299b) did not inhibit its activity. A short synthetic scheme [91] for a mixture (72.5:27.5) of acetates 299a and b started from 10-hydroxydecanoic acid (294) and used ozonolysis of 9-nonenylacetate (297) to prepare aldehyde 298 and Wittig olefination of the last. In turn, the carbon chain of starting hydroxyacid 294 was shortened by dehydrobromination of 296, which was obtained by Hunsdiecker decarboxylation from acetoxyacid 295 (Scheme 42).

Scheme 42
A new approach to the synthesis of 9-oxo-2E-decenoic acid (140), the queen substance of Apis mellifera L., started from available methylallylchloride 300, coupling of which with 5-(2-tetrahydropyranyloxy)pentanemagnesiumbromide (305) gave the 2-tetrahydropyranyl ether of 7-methyl-7-octen-1-ol (301) in high yield [92, 93]. The ketone in terminal alkene 301 was generated by reductive ozonolysis. The resulting tetrahydropyran ether 302 was hydrolyzed to hydroxyketone 303, oxidation of which by PCC gave the key ketoaldehyde 304, which was transformed as usual into acid 140 in 24% overall yield (Scheme 43).

Scheme 43
Ozonolytic cleavage of dibenzyl ether 306 followed by reduction of the peroxide products by Me2S and reaction of intermediate aldehyde 307 with EtMgBr under Grignard conditions produced alcohol 308, further oxidation of which by 2-iodoxybenzoic acid into ketone 309 gave (+)-iso-exo-brevicomin (310), a Dendroctonus ponderosae aggregation pheromone, after removal of the benzyl protection [94] (Scheme 44).

Scheme 44
A total asymmetric synthesis of the aggregation pheromone (vittatalactone) 317 of Acalymma vittatum, the principal grain and melon pest in North America, was proposed [95, 96]. Key synthon 314 was obtained by repeating the reaction sequence of Cu-catalyzed allyl substitution to the corresponding syn-S N 2′ products, their ozonolysis followed by borohydride reduction, bromination of the intermediate alcohols, conversion of bromides 312 and 313 to the Grignard reagents, and coupling of them with the corresponding ethers 311 and 318. Transformation of intermediate 314 toward target pheromone 317 concluded with stereoselective Sharpless epoxidation, conversion of epoxide 315 into 1,3-diol 316 by treatment with cyanodimethylcuprate, chemo- and stereoselective oxidation of the primary alcohol to produce the corresponding -hydroxyaldehyde, which was oxidized to the -hydroxyacid and then converted into target lactone 317 (Scheme 45).

Scheme 45
Thus, the literature review indicated that ozonolysis of unsaturated compounds in various steps of total syntheses of insect pheromones and juvenoids has great potential and often determines the success of implementing the selected pathway.
References
V. V. Plemenkov, Chemistry of Isoprenoids [in Russian], Izd. Altaiskogo Univ., Kaliningrad, Kazan, Barnaul, 2007, 322 pp.
M. Jacobson, Insect Sex Pheromones, Academic Press, New York, 1972.
K. V. Lebedeva, V. A. Minyailo, and Yu. B. Pyatnova, Insect Pheromones [in Russian], Nauka, Moscow, 1984, 268 pp.
Yu. A. Ovchinnikov, Bioorganic Chemistry [in Russian], Prosveshchenie, Moscow, 1987, 815 pp.
V. N. Odinokov and E. P. Serebryakov, Synthesis of Insect Pheromones [in Russian], Gilem, Ufa, 2001, 372 pp.
V. N. Odinokov, V. N. Burov, and O. S. Kukovinets, Semiochemicals in the Protection of Grain and Grain Products from Harmful Insects [in Russian], Gilem, Ufa, 2005, 232 pp.
T. I. Zvereva, V. G. Kasradze, O. B. Kazakova, and O. S. Kukovinets, Zh. Org. Khim., 46, 1431 (2010).
G. Yu. Ishmuratov, Yu. V. Legostaeva, L. P. Botsman, and G. A. Tolstikov, Zh. Org. Khim., 46, 1591 (2010).
V. N. Odinokov, Mendeleev Commun., 217 (2005).
G. Yu. Ishmuratov, R. Ya. Kharisov, V. N. Odinokov, and G. A. Tolstikov, Usp. Khim., 63, 580 (1994).
V. N. Odinokov, L. P. Botsman, and E. V. Gladysheva, Chem. Nat. Compd., 32, 381 (1996).
A. G. Kukovinets, T. A. Kargapol’tseva, O. S. Kukovinets, F. Z. Galin, V. V. Zorin, F. A. Shakhova, and G. A. Tolstikov, Zh. Org. Khim., 36, 237 (2000).
N. A. Plate and E. V. Slivinskii, Principles of Monomer Chemistry and Technology: Student Aide [in Russian], Nauka: MAIK Nauka/Interperiodika, Moscow, 2002, 696 pp.
T. I. Zvereva, O. S. Kukovinets, V. G. Kasradze, M. I. Abdullin, and F. Z. Galin, Bashk. Khim. Zh., 14, 30 (2007).
O. S. Kukovinets, V. G. Kasradze, E. V. Chernukha, V. N. Odinokov, A. V. Dolidze, F. Z. Galin, L. V. Spirikhin, M. I. Abdullin, and G. A. Tolstikov, Zh. Org. Khim., 35, 1185 (1999).
O. S. Kukovinets, V. G. Kasradze, E. V. Chernukha, V. N. Odinokov, F. Z. Galin, M. I. Abdullin, P. I. Fedorov, and G. A. Tolstikov, Zh. Org. Khim., 36, 234 (2000).
O. S. Kukovinets, V. G. Kasradze, E. V. Salimova, V. N. Odinokov, F. Z. Galin, and P. I. Fedorov, Chem. Nat. Compd., 35, 358 (1999).
G. Yu. Ishmuratov, R. Ya. Kharisov, and I. M. Muslimova, in: Proceedings of the 2nd All-Russian Convention “Wood Chemistry and Organic Synthesis” [in Russian], Syktyvkar, 1996, p. 24.
V. N. Odinokov, V. R. Akhmetova, and R. G. Savchenko, Chem. Nat. Compd., 34, 96 (1998).
V. N. Odinokov, V. R. Akhmetova, G. Yu. Ishmuratov, L. P. Botsman, and G. A. Tolstikov, Zh. Org. Khim., 22, 953 (1986).
V. N. Odinokov, O. S. Kukovinets, R. A. Zainullin, V. G. Kasradze, A. V. Dolidze, and G. A. Tolstikov, Zh. Org. Khim., 28, 1178 (1992).
V. G. Kasradze, Candidate Dissertation, Inst. Org. Chem., Ufa Sci. Cent., RAS, Ufa, 1994, 21 pp.
O. S. Kukovinets, V. G. Kasradze, V. N. Odinokov, L. V. Spirikhin, and G. A. Tolstikov, in: Proceedings of the 2nd All-Russian Convention “Wood Chemistry and Organic Synthesis” [in Russian], Syktyvkar, 1996, p. 25.
G. Yu. Ishmuratov, R. Ya. Kharisov, O. V. Botsman, V. V. Zorin, and G. A. Tolstikov, Izv. Akad. Nauk, Ser. Khim., 1929 (2000).
V. N. Odinokov, O. S. Kukovinets, V. G. Kasradze, A. V. Dolidze, V. R. Akhmetova, E. P. Serebryakov, and G. A. Tolstikov, Zh. Org. Khim., 29, 39 (1993).
O. S. Kukovinets, V. G. Kasradze, V. N. Odinokov, and G. A. Tolstikov, Khim. Interesakh Ustoich. Razvit., 20, 1 (2012).
O. Irie and K. Shishido, Chem. Lett., 53 (1995).
M. I. Goryaev, Characteristics of Chemical Compounds in Essential Oils [in Russian], Izd. Akad. Nauk Kaz. SSR, Alma-Ata, 1953, 371 pp.
K. Mori and Y. Nakazono, Tetrahedron, 42, 283 (1986).
K. Tadano, Y. Isshiki, M. Minami, and S. Ogawa, J. Org. Chem., 58, 6266 (1993).
O. S. Kukovinets, M. I. Kislitsyn, R. A. Zainullin, M. I. Abdullin, and F. Z. Galin, Russ. J. Org. Chem., 42, 396 (2006).
M. I. Kislitsyn, R. V. Kunakova, and R. A. Zainullin, in: Proceedings of the Republic Scientific-Practical Conference of Young Scientists “Young Scientists of the New Millennium [in Russian], Ufa, 2000, p. 150.
E. A. Lozhkina, Candidate Dissertation, Inst. Org. Chem., Ufa Sci. Cent., RAS, Ufa, 1995, 16 pp.
G. A. Tolstikov, V. N. Odinokov, O. S. Kukovinets, V. Zh. Bikulova, E. A. Lozhkina, G. Yu. Ishmuratov, N. V. Volchkov, and O. M. Nefedov, SU Pat. No. 1,671,657, Aug. 23, 1991; Byull. Izobret., No. 31, 2 (1991).
N. Y. Grigorieva and P. G. Tsiklauri, Russ. Chem. Rev., 69, 573 (2000).
G. Yu. Ishmuratov, L. P. Botsman, and Yu. V. Legostaeva, in: Proceedings of the All-Russian Scientific Conference “Biostimulators in Medicine and Agriculture” Dedicated to the 10th Anniversary of the Department of Bioorganic Chemistry of Bashkir State Univ., Ufa, RITs BashGU, 2011, p. 58.
V. N. Odinokov, O. S. Kukovinets, R. A. Zainullin, V. G. Kasradze, and G. A. Tolstikov, Zh. Org. Khim., 31, 103 (1995).
R. P. van Summeren, S. J. W. Reijmer, B. L. Feringa, and A. J. Minnaard, Chem. Commun., 1387 (2005).
E. C. Wayne, C. W. Jerome, and M. M. Vasilios, Tetrahedron Lett., 47, 2217 (2006).
G. Yu. Ishmuratov, R. Ya. Kharisov, M. P. Yakovleva, O. V. Botsman, R. R. Muslukhov, and G. A. Tolstikov, Izv. Akad. Nauk, Ser. Khim., 198 (1999).
G. Yu. Ishmuratov, R. Ya. Kharisov, M. P. Yakovleva, O. V. Botsman, R. R. Muslukhov, and G. A. Tolstikov, Zh. Org. Khim., 37, 49 (2001).
F.-C. Liu, W.-X. Li, Y.-C. Wang, and J. Lin, Synth. Commun., 25, 3837 (1995).
O. S. Kukovinets, T. I. Zvereva, V. G. Kasradze, F. Z. Galin, L. L. Frolova, A. V. Kuchin, L. V. Spirikhin, and M. I. Abdullin, Chem. Nat. Compd., 42, 216 (2006).
A. M. Kim, Organic Chemistry: Student Aide for Higher Educational Institutions [in Russian], Sib. Univ. Izd., Novosibirsk, 2004, 844 pp.
G. Yu. Ishmuratov, M. P. Yakovleva, G. V. Zaripova, L. P. Botsman, R. R. Muslukhov, and G. A. Tolstikov, Chem. Nat. Compd., 40, 548 (2004).
A. M. Moiseenkov and B. A. Cheskis, Dokl. Akad. Nauk SSSR, 290, 1379 (1986).
B. A. Cheskis and A. M. Moiseenkov, Khim.-farm. Zh., 22, 597 (1988).
G. Yu. Ishmuratov, R. Ya. Kharisov, R. R. Gazetdinov, and G. A. Tolstikov, Chem. Nat. Compd., 41, 617 (2005).
G. Yu. Ishmuratov, R. Ya. Kharisov, E. R. Latypova, and R. F. Talipov, Chem. Nat. Compd., 42, 367 (2006).
R. R. Gazetdinov, Candidate Dissertation, Inst. Org. Chem., Ufa Sci. Cent., RAS, Ufa, 2004, 100 pp.
V. N. Odinokov, L. P. Botsman, and G. A. Emel’yanova, Russ. Chem. Bull. Int. Ed., 47, 2021 (1998).
R. Ya. Kharisov, R. R. Gazetdinov, O. V. Botsman, R. R. Muslukhov, G. Yu. Ishmuratov, and G. A. Tolstikov, Zh. Org. Khim., 38, 1047 (2002).
R. Ya. Kharisov, O. V. Botsman, R. R. Gazetdinov, G. Yu. Ishmuratov, and G. A. Tolstikov, Izv. Akad. Nauk, Ser. Khim., 1067 (2001).
R. R. Gazetdinov, R. Ya. Kharisov, V. V. Zorin, and G. Yu. Ishmuratov, Bashk. Khim. Zh., 10, 37 (2003).
G. Yu. Ishmuratov, R. Ya. Kharisov, R. R. Gazetdinov, and V. V. Zorin, Bashk. Khim. Zh., 11, 39 (2004).
G. Yu. Ishmuratov, V. S. Tukhvatshin, and R. F. Talipov, Butlerov. Soobshch., 36, 69 (2013).
G. Yu. Ishmuratov, A. V. Bannova, E. R. Latypova, V. S. Tukhvatshin, O. S. Kukovinets, R. R. Muslukhov, and G. A. Tolstikov, Zh. Org. Khim., 49, 52 (2013).
W. Treibs and H. Albrecht, J. Prakt. Chem., 13, 291 (1961).
G. V. Kryshtal’, G. M. Zhdankina, and E. P. Serebryakov, Izv. Akad. Nauk, Ser. Khim., 1094 (1993).
E. R. Latypova, Candidate Dissertation, Inst. Org. Chem., Ufa Sci. Cent., RAS, Ufa, 2005, 112 pp.
R. Ya. Kharisov, E. R. Latypova, R. F. Talipov, R. R. Muslukhov, G. Yu. Ishmuratov, and G. A. Tolstikov, Russ. Chem. Bull., Int. Ed., 52, 2267 (2003).
G. Yu. Ishmuratov, E. R. Latypova, R. Ya. Kharisov, R. R. Gazetdinov, A. V. Bannova, V. S. Tukhvatshin, and R. F. Talipov, Vestn. Bash. Univ., 14, 19 (2009).
J. N. Marx and L. R. Norman, J. Org. Chem., 40, 1602 (1975).
K. Mori, Molecules, 10, 1023 (2005).
C. G. Overberger and J. K. Weise, J. Am. Chem. Soc., 90, 3525 (1968).
C. J. Moore, A. Hubener, and Y. Q. Tu, J. Org. Chem., 59, 6136 (1994).
Y. Q. Tu, C. J. Moore, and W. Kitching, Tetrahedron: Asymmetry, 6, 397 (1995).
K. Mori and H. Watanabe, Tetrahedron, 40, 299 (1984).
T. Sugai and H. Ohta, Agric. Biol. Chem., 53, 2009 (1989).
R. Noyori, T. Ohkura, and M. Kitamura, J. Am. Chem. Soc., 109, 5856 (1987).
H. Kiyota and K. Mori, Biosci. Biotechnol. Biochem., 58, 1120 (1994).
S. Muto, M. Bando, and K. Mori, Eur. J. Org. Chem., 1946 (2004).
D. Pempo, J. Viala, J. L. Parrican, and M. Santelli, Tetrahedron: Asymmetry, 7, 1951 (1996).
L. Poppe, L. Novak, J. Devenyi, and C. Szantay, Tetrahedron Lett., 32, 2643 (1991).
G. Gries, G. G. S. King, R. Gries, P. D. C. Wimalaratne, T. G. Gray, R. F. Shepherd, J. Li, K. N. Slessor, and G. Khaskin, J. Chem. Ecol., 19, 1501 (1993).
H. Takikawa, Y Shirai, M. Kobayashi, and K. Mori, Liebigs Ann. Chem., 1965 (1996).
K. Mori and J. Wu, Liebigs Ann. Chem., 213 (1991).
K. Mori, Tetrahedron, 39, 3107 (1983).
D. J. Aberhard and C. T. Hsu, J. Chem. Soc. Perkin Trans. 1, 1404 (1979).
H. Takikawa, H. Tamagawa, and K. Mori, J. Indian Chem. Soc., 74, 855 (1997).
Y. Shirai, M. Seki, and K. Mori, Eur. J. Org. Chem., 3139 (1999).
V. N. Odinokov, V. R. Akhmetova, Kh. D. Khasanov, A. A. Abduvakhabov, V. R. Sultanmuratova, and G. A. Tolstikov, Zh. Org. Khim., 28, 1173 (1992).
E. M. Santangelo, A. G. Correa, and P. H. G. Zarbin, Tetrahedron Lett., 47, 5135 (2006).
K. Mori and H. Horikiri, Liebigs Ann. Chem., 501 (1996).
G. Yu. Ishmuratov, R. R. Gazetdinov, V. A. Vydrina, R. Ya. Kharisov, M. P. Yakovleva, E. G. Akhmetzyanova, G. R. Talipova, and R. F. Talipov, Vest. Bashk. Univ., 17, 1700 (2012).
G. Yu. Ishmuratov, O. V. Botsman, L. P. Botsman, M. P. Yakovleva, R. Ya. Kharisov, and G. A. Tolstikov, Chem. Nat. Compd., 36, 207 (2000).
K. Kh. Nguen, M. V. Mavrov, and E. P. Serebryakov, Bioorg. Khim., 14, 250 (1988).
S. Narasimnah and H. Mohan, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 34, 950 (1995).
G. Yu. Ishmuratov, M. P. Yakovleva, R. Ya. Kharisov, O. V. Botsman, O. I. Izibairov, A. G. Mannapov, and G. A. Tolstikov, Chem. Nat. Compd., 37, 190 (2001).
V. N. Odinokov, G. Yu. Ishmuratov, I. M. Ladenkova, and G. A. Tolstikov, Chem. Nat. Compd., 28, 235 (1992).
G. Z. Huang, J. M. Li, J. L. Lu, and H. A. Aisa, Chem. Nat. Compd., 42, 727 (2006).
V. N. Odinokov, G. Yu. Ishmuratov, I. M. Ladenkova, and G. A. Tolstikov, Chem. Nat. Compd., 22, 595 (1986).
G. Yu. Ishmuratov, R. Ya. Kharisov, and M. P. Yakovleva, Insect Endo- and Exohormones: Characteristics, Synthesis and Use [in Russian], Gos. Izd. Nauchn.-Tekh. Lit. “Reaktiv,” Ufa, 2000, 34 pp.
R. P. Kavirayani and A. Pazhamalai, Tetrahedron: Asymmetry, 18, 1419 (2007).
Y. Schmidt, L. Konrad, and U. Breuninger, J. Org. Chem., 75, 4424 (2010).
Y. Schmidt and B. Breit, Org. Lett., 11, 4767 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2015, pp. 177–196.
G. A. Tolstikov (Deceased).
Rights and permissions
About this article
Cite this article
Ishmuratov, G.Y., Legostaeva, Y.V., Garifullina, L.R. et al. Ozonolysis of Unsaturated Compounds in the Synthesis of Insect Pheromones and Juvenoids. Chem Nat Compd 51, 199–219 (2015). https://doi.org/10.1007/s10600-015-1246-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1246-3