For the estimation of donor/acceptor character of conjugated heterocyclic compounds, the φ0 index is used. This parameter is determined by the relative positions of the frontier molecular orbital energy levels. It is shown that φ0 value of 0.5 means that the donor and acceptor properties in the conjugated molecule are balanced, while an increase of the index (φ0 > 0.5) corresponds to increasing of the donor strength, and, conversely, its lowered value (φ0 < 0.5) points to increased acceptor strength. In this work, a series of widely known heterocyclic compounds, as well as derivatives of oxazole and nucleobases are analyzed in detail. It is shown that change in φ0 index is connected to the biological activity. As an example, the influence of the conjugated substituents is studied and it is found that the oxazole derivatives with acceptor substituents inhibit cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Properties and applications of conjugated heterocycles are largely determined by the common system of π-electrons in their molecular structure. Conjugated heterocycles are widely used as terminal groups in some linear conjugated molecules: polymethine dyes and related compounds, polyenes.1,2,3 Conjugated heterocycles containing one or more nitrogen atoms are particularly important; they are well known as components of biologically and pharmaceutically active molecules (pharmacophores).4,5,6 Also, some derivatives of polynitrogen heterocycles pyrimidine and purine are the key constituent parts of nucleic acids (NA): nucleobases guanine, adenine, cytosine, thymine, and uracil.7 Obviously, the biological properties are connected with the electronic structure features of the conjugated heterocycles. Presupposing the formation of the stable complex between the heterocyclic part of the pharmacophore and the interacting part of the active center of the protein by the stacking mechanism,8 it may be assumed that the stacking interaction should stabilize such complex if both interacting moieties have π-electron systems. For example, it was established that nucleobases form the stable chain not only by hydrogen bonds, but also by stacking interaction between π-electron systems of such bases; similar interactions work in biological processes.11,12,13,14,15
Likewise, besides the stacking interaction, hydrogen bonds and hydrophobic intermolecular interaction can possibly stabilize the complex pharmacophore – active site. In this work, we propose to treat the total sum of the similar interactions in such complex as a biological affinity of the heterocycle. In referring to the estimated π-electron contribution to this affinity in conjugated heterocycles we will call such property the π-electron component of biological affinity.
Undoubtedly, the π-electron characteristics should essentially depend on donor-acceptor properties of the conjugated heterocycles, and thus on the relative position of their frontier molecular orbital energy levels. The molecular orbital energy level position or/and total π-energy in any conjugated molecule is determined by its molecular topology. Numeral indexes based on the graph theory have been proposed for estimation of the characteristics of molecular topology.2, 16, 17 It was shown that in the Hueckel approximation, energies of molecular orbitals (MOs) coincide with the eigenvalues of the bond matrix of the so-called topological graph representing the molecule;16 such graph takes correctly into consideration all π-bonds, their types, conjugated system branching, number of cycles, type and position of heteroatoms.17
A new stage in the study of conjugated heterocycles was provoked by developments of recent quantum-chemical methods. Nevertheless, the use of topological indices remains a convenient and effective approach for estimation of donor/acceptor strength or, in general, relative positions of the frontier MO levels.2 As the donor ability of molecule is commonly connected with the energy of the highest occupied MO (HOMO) and the acceptor ability with the lowest unoccupied MO (LUMO), we propose to apply the frontier MO energies as proxies for the π-electron component of total biological affinity.
Investigating the influence of the terminal heterocyclic residues on the electronic properties of linear conjugated systems,2, 18 we have proposed to estimate the donor/ acceptor properties by the frontier MO energy level shift. Such approach is employed also in the current work.
1. Quantum-chemical estimation of donor/acceptor affinity. Changes in the donor or/and acceptor ability of neutral π-electron molecules could be estimated quantitatively by changes of the relative positions of their frontier MO levels. Earlier,18 we have proposed to characterize the relative positions of the frontier MO energy levels in any conjugated molecules by the following index φ0:
LUMO energy, while εHOMO is the HOMO energy, and α is the energy of non-bonding π-level, which corresponds to the energy of the 2pz electron in an sp2-hybridized carbon atom.
The parameter α can be calculated as a midgap in the long polyenes or polyacenes; it depends only on the applied quantum-chemical approximation. In conjugated molecules that will be used as reference, the donor and acceptor properties are mutually balanced (φ0 0.5), i.e., energy gap is situated symmetrically in respect to the virtual level α. A calculation by the nonempirical approximation HF/6-31(d,p) (package Gaussian 0919) for the comparatively long polyene C12H24 gives: εLUMO –1.296 eV; εHOMO –5.747 eV; therefore α –3.524 eV; and, consequently, φ0 0.5. In this case, the symmetrical disposition of the frontier levels is schematically presented in Figure 1b. In such approach, the shift of energy gap up (and hence increase of the parameter φ0 > 0.5) points to the primary donor nature of the conjugated molecule; this case is pictured in Figure 1c. On the contrary, if φ0 < 0.5, then the frontier levels are shifted down (Fig. 1a), which attests to the predominately acceptor nature.
Thus, the parameter φ0 obtained by quantum-chemical calculations could be applied for quantitative estimation of the donor/acceptor π-electron component of the biological affinity of conjugated nitrogen-containing heterocycles. Hereinafter, all required calculations were performed by HF/6-31(d,p) method.
2. Simplest conjugated nitrogenic heterocycles. There are only four simplest neutral monosubstituted cyclic nitrogen-containing heterocycles, presented by formulae 1–4 (Fig. 2).
It is to be noted that 4- and 6-membered cycles (compounds 1 and 3) are electron-balanced π-systems, while 5- and 7-membered cycles (compounds 2 and 4) are electronexcessive systems; in both cases, the replacing of CH group by the more electronegative nitrogen atom should cause the shifting of the frontier levels down and hence the increasing of acceptor properties. The calculated MO energies of heterocycles 1–4 and indices φ0 are collected in Table 1.
Indeed, the acceptor heterocycles 1 and 3 have φ0 values < 0.5; on the contrary, both electron-sufficient heterocycles 2 and 4 are donors and have φ0 > 0.5.
Azete (1) is antiaromatic, and it is still unknown as a component of biologically active nitrogen-containing heterocyclic molecules. Therefore, we will restrict the further analysis only to the conjugated derivatives of pyrrole (2) and pyridine (4); both heterocycles are main components of well-known pharmacophores.
3. Chemical constitution variations .
3a. Introducing of additional heteroatoms. Introduction of additional nitrogen atoms to the molecular skeleton of the model heterocycles may be considered as elementary method of chemical constitution modification without changing the π-electrons in electron shell. Additionally, the replacement of carbon atom (CH group) in 1-methylpyrrole (2) by oxygen or sulfur atom (at position 3) should be investigated, since both 1,3-oxazole and 1,3-thiazole are well known as important components of pharmacophores.5, 6 The estimation results for the donor/acceptor parameter φ0 change upon similar chemical transformation for the main compounds 5–10 (Fig. 3) together with calculated MO energies are presented in Table 2.
The replacing of the CH group in position 3 of the electron-sufficient 1-methylpyrrole (2) by the more electronegative nitrogen atom causes a negligible shifting down of the HOMO, whereas the LUMO shifts up because of the shortening of two N=CH bonds in comparison to CH=CH bonds. As a result, the energy gap shifts and increases essentially in cycle 5a containing two heteroatoms, so that the index φ0 decreases, i.e., the donor property decreases if compared with the reference compound 2. Introducing the second nitrogen atom (2 → 6a), as it can be seen from Table 2, is accompanied by further decreasing of the parameter φ0. It must be noted that going from imidazole 5a to 1,3-oxazole 5b or 1,3-thiazole 5c decreases additionally the value of index φ0. Similarly, the parameter φ0 decreases in the series of heterocycles with three heteroatoms going from 1,2,4-triazole (6a) to 1,3,4oxadiazole (6b) and then to 1,3,4-thiadiazole (6c).
In a manner similar to that of 5-membered electronexcessive cycles, replacing of the methine CH group by the nitrogen atom in the 6-membered electron-balanced cycle leads to shift down of both frontier MO energy levels; such shift of the energy gap decreases parameter φ0, i.e., these cycles become more potent acceptors, although this effect is small-scale and is approximately equal for all three isomers (compare the values φ0 for compounds 7, 8, and 9 in Table 2). At last, the effect of the third nitrogen atom was shown by the calculation to be somewhat more pronounced (for example: 8 → 10).
3b. Enhancement of π-electron system by ring fusion. Here, we will consider the topological modification, but the chemical modifications will be not treated. The simplest enhancement of the conjugated system of the initial heterocycles 2, 4, 5, 7–9 is a conjunction of a benzene cycle (annulation or fusing). Some well-known nitrogencontaining heterocycles are presented by structures 11–18 (Fig. 4). The calculated electron and topological effects for compounds 11–18 are presented in Table 3.
At first, the modification of the starting model heterocycles by ring fusion causes a decrease of the index φ0, i.e., enhances the acceptor property of all fused compounds. However, the quantitative effects differ appreciably in the 5-membered electron-excessive cycles 11–13 and in the 6-membered electron-balanced cycles 14–18. Attention should be paid to the fact that the benzo-fusing of imidazole, 1,3-oxazole, and 1,3-thiazole (5a → 13a, 5b → 13b, 5c → 13c) produces smaller effect than that of pyrrole (2 → 11). The replacing of the heteroatom X in series NH → O → S, as seen from Table 3, is accompanied by a decrease of the fusing effect. The addition of the second benzene cycle causes an additional decrease of the parameter φ0, but the effect is no additive.
Thus, applying the topological index φ0 enables us to estimate quantitatively the changes of the donor/acceptor properties of conjugated heterocycles or, more correctly, their π-electron affinity upon the modification of their structure.
4. Biologically active conjugated heterocycles. As it was written above, many of the nitrogen-containing heterocycles exhibit biological activities, and it is a reason for them to be tested as effective pharmacophores. They have been employed as scaffolds for the construction complex drug substances. For example, indole core 11 is a prominent structural motif for numerous compounds possessing anti-inflammatory, analgesic, antifungal, insecticidal, or anticancer activity.5
Biologically active compounds may contain two or more cycles of the different types.5, 6 Also purine nucleobases, adenine and guanine, are constructed from two rings: the 6-membered acceptor pyrimidine, and 5-membered donor pyrazole.7 Besides, pharmacophores may contain additionally various exocyclic substituents.
Here, we will consider and examine both types of the structure complications of initial nitrogen-containing heterocycles.
4a. Condensed nitrogen-containing bicycles. At first, we would like to estimate the donor/acceptor properties and π-electron affinity of simplest bicyclic nitrogen-containing heterocycles 19–22, substituted with either methyl or phenyl groups in both 6-membered cycle and 5-membered cycle. Compounds with a similar heterocyclic core have shown biological activities; the close structural similarity of pyrazolo[1,5-a][1,3,5]triazines to biogenic purines allows for targeting purinergic signalling receptors and enzymes.20 Being the focus of active research as bioactive molecules with promising therapeutic potential, this heterocyclic system has been established as a privileged scaffold in medicinal chemistry.21,22,23,24 Pyrazolo[1,5-a][1,3,5]triazines have shown promise for treating proliferative disorders such as cancer, as well as other kinase-associated conditions including inflammation, pain, and certain immunological disorders.
We have investigated the π-electron affinity of phenylsubstituted pyrazolo[1,5-a][1,3,5]triazines 19–22 (Fig. 5). Calculated HOMO and LUMO energies of compounds 19–22 as well as the parameter φ0 are presented in Table 4.
As it seen from Table 4, compound 19 with a donor N-5 nitrogen atom (providing lone electron pair in conjugated system) and three acceptor-type nitrogen atoms should exhibit cumulative acceptor property, despite of three donor methyl substituents. Replacement of one methyl group by the phenyl substituent is accompanied by decreasing of the parameter φ0. It is to be noted that the effect of such chemical modification in position 4 (compound 20) is almost twice more than it is in position 7 (compound 21). However, the calculations do not give the addition effect of two phenyl groups in molecule 22: the calculated change of the index φ0 is lower than the sum of the effects of phenyl group in compounds 20 and 21.
4b. 1,3-Oxazoles with conjugated substituents. The donor/ acceptor properties could be tuned by introducing conjugated substituents in the core heterocycle. Study of similar substituted heterocyclic systems showed that they may demonstrate biological activity. In particular, 1,3-oxazoles play a significant role as potential biologically active compounds: brain-derived neurotrophic factor inducers,25 analgesic,26 trypanocidal agents,27 antimitotic agents with pro-apoptotic activity,28 antibacterial and antituberculosis agents,29 fungicides,30 anti-inflammatory agents,31 etc.
Also, 1,3-oxazole could be considered as perspective moiety in the design and further synthesis of novel biologically active compounds that exert anticancer activity.32,33,34
Taking into consideration planar constitution of conjugated nitrogen-containing heterocycles, it may be assumed that the increasing of acceptor properties should increase an affinity to the donor biological active centers of enzymes. We could also suppose that introducing effective conjugated substituents will increase such affinity and as a result will stabilize complex of the potential pharmacophores with the active centers of biomolecules.
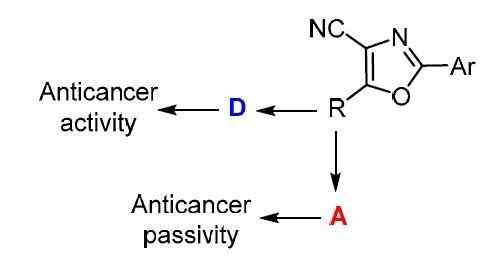
The influence of some donor and acceptor substituents on the biological activity of substituted 1,3-oxazoles was also studied in vitro on the example of compounds 23a–j (Table 5) with donor and acceptor substituents, which have been screened for anticancer activity in the 60-cell panel in accordance with the protocol of the NCI, USA.35,36,37,38 The results of anticancer tests (Fig. 6) of compounds 23f,g40 and 23h–j41 have been published previously, those for compounds 23a–e are described in the Supplementary information file to the present article. The results of the single dose (10 μM) testing are presented as the percent growth of the treated cells when compared to the untreated control cells. The negative growth values show the reduction of the measured amount of the protein at the end of the drug treatment as compared to that at the beginning indicating a net loss of cells following the treatment.
The analysis of relationship between pharmacological activity and molecular structure shows unambiguously that compounds with the substituents with high acceptor properties (SO2R) at position 5 (compounds 23f–j) have demonstrated appreciably higher level of the inhibition of the cell growth than the analogous 1,3-oxazole derivatives 23a–e which contain the donor substituents (NHR, SR) at position 5 and virtually do not affect cell growth, i.e., the biological activity depends evidently on donor/acceptor properties.
Similar results were obtained by QSAR modeling: introduction of the acceptor substituents into 1,3-oxazole cycle increases its inhibitor properties, whereas the donor substituents causes the growth of the tested cancer cells.39
At the same time, the parameter φ0 was calculated for model substituted 1,3-oxazoles 24a–j with some obvious donor/acceptor substituents. The calculated results for compounds 24a–j are presented in Table 6.
For the starting model 2-phenyloxazole 24a, the index φ0 is close to the middle value, which means that its donor and acceptor properties are balanced. Introducing of the acceptor groups (CN or CO2Me, compounds 24b,c) decreases parameter φ0, while dimethylamino group possessing the donor properties (compound 24d) increases it. Molecules 24g,h contain both donor and acceptor substituents, which leads to a slight decrease of the index φ0, but the donor nature prevails. On the other hand, simultaneous presence of two acceptor substituents in one molecule 24i,j should cause substantial increasing of the acceptor property as far as φ0 < 0.4.
When considering the stability of the complex pharmacofore–active site, we could assume that conjugated substituents of acceptor type should additionally stabilize such complex, in contrast to the donating conjugated groups which hence, should, decrease its biological activity. Such assumptions and calculated data are in accordance with in vitro and in silico (QSAR) performed investigations discussed above.
5. Heterocyclic nucleic bases as derivatives of heterocyclic systems. At last, let us consider the π-electron affinities of nucleobases, which are typical heterocyclic conjugated systems with exocyclic conjugated substituents In general, the bases in the nucleic acids (NA) play the role of the polymer subunits that are connected consistently with five-carbon sugar (ribose or deoxyribose) and phosphate residues. However, here we will describe the model heterocylic bases 25–29, when the sugar/phosphate parts are replaced by methyl group (Fig. 7).
The calculated frontier level energies and other characteristics of the studied purine and рyrimidine bases 25–29 are presented in Table 7.
The performed calculations clearly show the dependence of the donor/acceptor character on the chemical constitution of the nucleobases. Thus, three nucleobases cytosine (C), thymine (T), and uracil (U), like also their respective methyl derivatives 27–29, consist of 6-membered ring with the exocyclic oxygen atom giving one 2pz electron in total conjugated system, whereas cytosine have an exocyclic amino group with two electrons included in the π-electron system, so that each molecule has a stable electron shell. Because of the electronegative atoms, the heterocyclic bases C and T should demonstrate the principally acceptor properties. As regards U, it contains two oxygen atoms (giving two electrons in the total π-electron system); two nitrogen atoms give four electrons at the expense of their lone electron pairs (LEPs). But although bases U, C, T are electron-sufficient systems, they play a role of acceptors, due to the presence of the electronegative nitrogen atoms in their structures.
In contrast, the heterocyclic bases adenine (A) and guanine (G), represented by the respective methyl derivatives 25 and 26, contain a 5-membered cycle with the nitrogen atom (with its LEP) which makes these molecules to a greater extent donor π-electron systems. Thus, their φ0 parameters are higher than the middle value 0.5.
It is known that in DNA chain molecules, nucleobases make up the base pairs connected by two or three hydrogen bonds.11 Besides, the pyrimidine bases form pairs with the purine bases (A–T and G–C) while possible pairs are less stable.
We could suppose that donor/acceptor properties expressed by index φ0 are related to the charge distribution within molecule and hydrogen bonding strength. Then it is interesting to compare the index φ0 of each nucleobase, as well as compare the difference of the indices of the pair of the corresponding bases connected by the hydrogen bonding.
One can see that the bonding base pairs are formed when one of the bases has the index φ0 > 0.5 (purine) while φ0 < 0.5 for the second base (pyrimidine). It may be pointed out that the base pairs with maximum or minimum difference of indexes Δφ0 are likely less or not stable.
Thus, the proposed index φ0 allows to estimate quantitatively the donor/acceptor properties of conjugated heterocyclic compounds. The connection of this index with the position of the frontier MO enables to treat it as the π-electron affinity. It was shown that this parameter depends on the chemical constitution of the heterocycles and their conjugated substituents. Taking into consideration that the π-electron index φ0 is a component of the general biological affinity allows to explain the opposite influence of the donor and acceptor substituents on the biological activity of oxazole derivatives, particularly, on the inhibition of cancer cell growth. The proposed approach can also explain the donor/acceptor properties of nucleobases.
Experimental
IR spectra were recorded on a Vertex-70 spectrometer in KBr pellets. 1H and 13C NMR spectra were recorded on a Varian Mercury 400 spectrometer (400 and 100 MHz, respectively, compound 23a) or Varian Mercury 500 spectrometer (500 and 125 MHz, respectively, compound 23b) with residual solvent signal as internal standard (δ 2.50 ppm (DMSO-d6) for 1H and 39.9 (DMSO-d6) and 77.0 ppm (CDCl3) for 13C nuclei). Mass spectra were recorded on an Agilent 1100 Series LC-MS system, equipped with diode array and mass selective detector Agilent LC/MSD SL (atmospheric pressure chemical ionization). Elemental analysis was performed in the Analytical Laboratory of the Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine. Melting points were determined on a Fisher-Johns apparatus. The reactions were followed by TLC (silica gel, aluminum sheets 60 F254, Merck). Reagents and solvents from commercial sources were used. Syntheses of compounds 23b–j have been described previously.35,36,37,38,39,40
5-[(2-Hydroxyethyl)(methyl)amino]-2-(4-methylphenyl)1,3-oxazole-4-carbonitrile (23a). To a solution of N-(2,2dichloro-1-cyanovinyl)-4-methylbenzamide (2.55 g, 0.01 mol) in THF (40 ml), Et3N (3.08, 0.022 mol) and 2-(methylamino)ethanol (0.8 ml, 0.01 mol) were added. The mixture was stirred at room temperature for 12 h. All volatiles were removed in vacuo and water was added. The precipitate was filtered off and purified by recrystallization from PhMe. Yield 1.80 g (70%), white solid, mp 104–106°C. IR spectrum (KBr), ν, cm–1: 1048, 1428, 1634, 2198 (CN), 3446 (OH). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): 2.34 (3H, s, CH3); 3.21 (3H, s, NCH3); 3.54–3.57 (2H, m, CH2); 3.62–3.65 (2H, m, CH2); 4.92 (1H, t, J = 5.2, OH); 7.29 (2H, d, J = 8.0, H Ar); 7.71 (2H, d, J = 8, H Ar). 13C NMR spectrum (CDCl3), δ, ppm: 21.5; 38.0; 54.0; 59.9; 84.8; 116.8; 123.1; 125.4; 129.5; 140.5; 150.9; 160.5. Mass spectrum, m/z: 258 [M+H]+. Found, %: C 65.28; H 5.86; N 16.35. C14H15N3O2. Calculated, %: C 65.36; H 5.88; N 16.33.
5-{[2-(4-Chlorophenyl)-2-(piperidin-1-yl)ethyl]amino}2-phenyl-1,3-oxazole-4-carbonitrile (23b). To a solution of N-(2,2-dichloro-1-cyanovinyl)benzamide (2.41 g, 0.01 mol) in THF (40 ml), Et3N (3.08 ml, 0.022 mol) and 2-(4-chlorophenyl)-2-(piperidin-1-yl)ethanamine (2.39 g, 0.01 mol) were added. The mixture was stirred at room temperature for 12 h. All volatiles were removed in vacuo and water was added. The precipitate was filtered off and purified by recrystallization from EtOH. Yield 2.77 g, (68%); yellow solid; mp 174–176 °C; IR spectrum (KBr), ν, cm–1: 1012, 1092, 1447, 1485, 1634, 2206 (CN), 3340 (NH). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): 1.20–1.21 (2H, m, CH2), 1.36 (4H, br. s, 2CH2), 2.20 (2H, br. s, CH2), 2.41 (2H, br. s, CH2), 3.50–3.54 (1H, m, CH2), 3.68–3.72 (1H, m, CH), 3.95–3.98 (1H, m, CH2), 7.29–7.47 (7H, m, H Ar), 7.78 (2H, d, J = 8.0, H Ar). 8.23 (1H, s, NH). 13C NMR spectrum (DMSO-d6), δ, ppm: 24.6; 26.4; 44.6; 50.9; 68.6; 84.1; 116.3; 125.5; 126.3; 128.3; 129.5; 130.5; 131.0; 132.5; 136.1; 149.8; 162.0. Mass spectrum, m/z: 407 [M+H]+. Found, %: C 67.85; H 5.69; Cl 8.69; N 13.69. C23H23ClN4O. Calculated, %: C 67.89; H 5.70; Cl 8.71; N 13.77.
References
Bricks, J. L.; Kachkovskii, A. D.; Slominskii, Y. L.; Gerasov, A. O.; Popov, S. V. Dyes Pigm. 2015, 121, 238.
Kachkovsky, A. D. Russ. Chem. Rev. 1997, 66, 647. [Usp. Khim. 1997, 66, 715.]
Hünig, S.; Berneth, H. Top. Curr. Chem. 1980, 1, 92.
Saini, M. S.; Kumar, A.; Dwivedi, J.; Singh, R. Int. J. Pharma Sci. Res. 2013, 4, 66.
Sharma, V.; Kumar, P.; Pathak, D. J. Heterocycl. Chem. 2010, 47, 491.
Al-Mulla, A. Pharma Chem. 2017, 9, 141.
Zaenger, W. Principles of Nucleic Acid Structure; SpringerVerlag: New-York, Berlin, Heidelberg, Tokyo, 1984, p. 584.
Bissantz, C.; Kuhn, B.; Stahl, M. A. J. Med. Chem. 2010, 53, 5061.
Schneider, H. J. Acc. Chem. Res. 2015, 248, 1815.
Cauet, E.; Rooman, M.; Wintjens, R.; Lievin, J.; Biot, C. J. Chem. Theory Comput. 2005, 1, 472.
Desiraju, G. R.; Stainer, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press, Inc.: New York, 1999.
Grabowski, S. J. J. Phys.Org. Chem. A 2001, 105, 10739.
Zhang, C.; Bell.; Harger, M.; Ren, P. J. Chem. Theory Comput. 2017, 13, 666.
Šponer, J.; Riley, K. E.; Hobza, P. Phys. Chem. Chem. Phys. 2008, 10, 2595.
Marsili, S.; Chelli, R.; Schettino, V.; Procacci, P. Phys. Chem. Chem. Phys. 2008, 10, 2673.
Trinajstić, N. In Semi-empirical Methods of Electron Structure Calculation; Part A: Techniques; Segal, G. A., Ed.; Plenum Press: New York, London, 1977, p. 1.
Kachkovsky, A. D.; Dekhtyar, M. L. MATCH 1995, 32, 127.
Shablykin, O.; Kobzar, O.; Prostota, Y.; Kachkovsky, O.; Brovarets, V.; Vovk, A.; Merzhyievskyi D.; Obernikhina, N. Electronics and Nanotechnology (ELNANO-2018): Proc. of 38th Int. Sci. Conf. (April 24–26 2018, Kyiv, Ukraine); Kyiv, 2018, p. 449.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Peralta, J. E., Jr.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox D. J. Gaussian 09; Gaussian, Inc.: Wallingford, 2009.
Dolzhenko, A. V.; Dolzhenko, A. V.; Chui, W.-K. Heterocycles 2008, 75, 1575.
Burnstock, G.; Verkhratsky, A. Acta Physiol. 2009, 195, 415.
Lim, F. P. L.; Dolzhenko, A. V. Eur. J. Med. Chem. 2014, 85, 371. 23. Nie, Z.; Perretta, C.; Erickson, P.; Margosiak, S.; Lu, J.; Averill, A.; Almassy, R.; Chu, S. Bioorg. Med. Chem. Lett. 2008, 18, 619.
Bettayeb, K.; Sallam, H.; Ferandin, Y.; Popowycz, F.; Fournet, G.; Hassan, M.; Echalier, A.; Bernard, P.; Endicott, J.; Joseph, B.; Meijer, L. Mol. Cancer Ther. 2008, 7, 2713.
Popowycz, F.; Fournet, G.; Schneider, C.; Bettayeb, K.; Ferandin, Y.; Lamigeon, C.; Tirado, O. M.; MateoLozano, S.; Notario, V.; Colas, P.; Bernard, P.; Meijer, L.; Joseph, B. J. Med. Chem. 2009, 52, 655.
Maekawa, T.; Sakai, N.; Tawada, H.; Murase, K.; Hazama, M.; Sugiyama, Y.; Momose, Y. Chem. Pharm. Bull. 2003, 51, 565.
Serrano, M. I.; Serrano, J. S.; Fernandez, A.; Sanchezcarrasco, J. M.; Fuentes, J.; Pradera, M. A.; Ortiz, M. C.; Garcia Fernandez, J. M. ChemInform Abstract 1995, 26, 22. https://doi.org/10.1002/chin.199525129
Pinto, V.; Pinto, C. M.; Pinto, M. C.; Rita, R. C.; Pezzella, C. A.; de Castro, S. L. Arzneimittelforschung 1997, 47, 74.
Uckun, F. M. Curr. Pharm. Des. 2001, 16, 1627.
Eswaran, S.; Adhikari, A. V.; Kumar, R. A. Eur. J. Med. Chem. 2010, 45, 957.
Kunes, J.; Balsanek, V.; Pour, M.; Buchta, V. Collect. Czech. Chem. Commun. 2001, 66, 1809.
Kaspady, M.; Narayanaswamy, V. K.; Raju, M.; Rao, G. K. Lett. Drug Des. Discovery 2009, 6, 21.
Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Craig, W. D.; Smee, D. F.; Grellier, P.; Lesyk, O. Eur. J. Med. Chem. 2013, 66, 228.
Semenyuta, I.; Kovalishyn, V.; Tanchyk, V.; Piyo, S.; Zyabrev, V.; Blagodatnyy, V.; Trokhimenko, O.; Brovarets, V.; Meteytsia, L. Comput. Biol. Chem. 2016, 65, 8.
Liu, X.; Bai, L.; Pan, C.; Song, B.; Zhu, H. Chin. J. Chem. 2009, 27, 1957.
Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; Gray-Goodrich, M.; Campbell, H.; Mayo, J.; Boyd, M. J. Natl. Cancer Inst. 1991, 83, 757.
Boyd, M. R.; Paull, K. D. Drug Dev. Res. 1995, 34, 91.
Boyd, M. R. In Anticancer Drug Development Guide. Cancer Drug Discovery and Development; Teicher, B. A., Ed.; Humana Press: Totowa, 1997, p. 23-42. DOI: 10.1007/978-14615-8152-9_2.
Shoemaker, R. H. Nat. Rev. Cancer. 2006, 6, 813.
Kachaeva, M. V.; Hodyna, D. M.; Semenyuta, I.; Pilyo, S. G.; Prokopenko, V. M.; Kovalishyn, V. V.; Metelytsia, L. O.; Brovarets, V. S. Comput. Biol. Chem. 2018, 74, 294.
Kachaeva, M. V.; Pilyo, S. G.; Zhirnov, V. V.; Brovarets, V. S. Med. Chem. Res. 2019, 28, 71.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file containing 1H and 13C NMR spectra of compounds 23a,b and anticancer tests data of compounds 2a–e, is available at the journal website at http://springerlink.bibliotecabuap.elogim.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(4/5), 448–454
Electronic supplementary material
ESM 1
(PDF 8277 kb)
Rights and permissions
About this article
Cite this article
Kachaeva, M.V., Obernikhina, N.V., Veligina, E.S. et al. Estimation of biological affinity of nitrogen-containing conjugated heterocyclic pharmacophores. Chem Heterocycl Comp 55, 448–454 (2019). https://doi.org/10.1007/s10593-019-02478-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02478-6