The preparation of perfluoroalkylated 1,3-dithietanes and synthetic use of 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane are considered in this review, with the emphasis on (2+2) cycloaddition, (2+4) cycloaddition, [2+2+2] cycloaddition, and alkylation.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
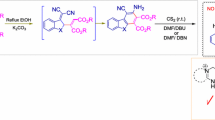
Synthesis of 2-perfluoroalkyl-1,3-dithietanes

Perfluoroalkylated 1,3-dithietanes 1 are reactive sulfur-containing heterocycles with diverse substituents that can be considered as masked thiocarbonyl compounds. An example of compound 1 was obtained for the first time in 1958 by a thermal reaction of 2-iodoperfluorohexane with HgS and by a thermal reaction of perfluoro-2-hexyl polysulfides in the presence of HgS.1 The reaction of trifluorothioacetamide (2, RF = CF3) with acetyl chloride in acetonitrile at –20°C in the presence of pyridine afforded 1,3-dithietane 1 as a mixture of two stereoisomers.2
The reaction of compound 2 with 5-hydroperfluoropentanoyl chloride was carried out by heating at 100°C in the absence of a base, affording derivative 3, which slowly dimerized into a mixture of 1,3-dithietane 1 stereoisomers.2 1,3-Dithietane 1 with the hydrogen atom in the α-position (R1 = H) was obtained for the first time by treatment of sulfinyl chloride 4 with Et3N.3 Bubbling of hexafluoropropene (5) into a mixture of dry DMF containing KF and sulfur afforded the dimer of hexafluorothioacetone 1 (HFTA).4 – 7


[2+2+2] Cycloaddition

Quadricyclane is reactive component in [2π+2σ+2σ] cycloaddition reactions with electron-deficient compounds. The reaction of 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (1a) with quadricyclane was used for the synthesis of polyfluorinated sulfur-containing polycyclic compound 6.8 , 9


 Volodymyr Ogurok was born in Lviv, Ukraine in 1990. He graduated from the Lviv Polytechnic National University, obtaining a Master's degree in 2012. Currently he is a PhD student at the Institute of Organic Chemistry of National Academy of Sciences of Ukraine, at the Department of organic sulfur compounds chemistry headed by Prof. Yu. G. Shermolovich. His scientific interests include chemistry of polyfluorinated derivatives of sulfinic acids.
Volodymyr Ogurok was born in Lviv, Ukraine in 1990. He graduated from the Lviv Polytechnic National University, obtaining a Master's degree in 2012. Currently he is a PhD student at the Institute of Organic Chemistry of National Academy of Sciences of Ukraine, at the Department of organic sulfur compounds chemistry headed by Prof. Yu. G. Shermolovich. His scientific interests include chemistry of polyfluorinated derivatives of sulfinic acids.
(2+2) Cycloaddition

The reaction of 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (1a) with vinyl-containing compounds led to the formation only of one (2+2) cycloadduct 7.5 , 6 , 8 , 10 , 11 The reaction proceeded in polar solvent in the absence of a catalyst at elevated temperature or in the presence of CsF at room temperature. In the case of reaction with styrenes in DMF medium with CsF catalyst, a mixture of cycloadducts was obtained due to the competition of (2+2) and (2+4) cycloadditions.12


(2+4) Cycloaddition

The reaction of 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (1a) with non-conjugated (1Z,5Z)-cycloocta-1,5-diene stopped at the stage of conjugated cycloocta-1,3-dienes 8a,b. Compounds 8a,b did not undergo further cycloaddition reactions with hexafluorothioacetone.8 , 13


Alkylation of azoles

2,2,4,4-Tetrakis(trifluoromethyl)-1,3-dithietane (1a) was used as a good alkylation reagent for the introduction of hexafluoroisopropyl group in the reactions with azoles 9.7 It is interesting that two processes took place in the case of N-substituted imidazoles 10: alkylation of the second nitrogen atom in the molecule of imidazole and formation of imidazole-2-thione.14 The reaction of indoles 11 with 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (1a) in the presence of CsF as catalyst led to formation of indoles 12 bearing a SCH(CF3)2 group at position 3. When the same reaction was carried out in the absence of catalyst, it led to indoles 13 bearing a C(CF3)2S2CH(CF3)2 group at position 3. It was found that the pyrrole ring was more reactive toward 1,3-dithietane 1a, compared to furan and thiophene, which did not react with 1,3-dithietane 1a even at elevated temperature.15


References
Hauptschein, M.; Braid, M. J. Am. Chem. Soc. 1958, 80, 853.
Mykhaylychenko, S. S.; Pikun, N. V.; Shermolovich, Yu. G. J. Fluorine Chem. 2012, 140, 76.
Shermolovich, Yu. G.; Barashenkov, G. G.; Dubinina, T. N.; Markovski, L. N. Russ. J. Org. Chem. 1989, 25, 2591.
England, D. C. J. Org. Chem. 1981, 46, 153.
Petrov, V. A.; Marshall, W. J. J. Fluorine Chem. 2009, 130, 780.
Petrov, V. A.; Marshall, W. J. Fluorine Chem. 2012, 133, 61.
Petrov, V. A.; Marshall, W. J. J. Fluorine Chem. 2013, 156, 262.
Petrov, V. A.; Marshall, W. J. Fluorine Chem. 2015, 179, 56.
Petrov, V. A.; Krespan, C. G.; Marshall, W. J. J. Fluorine Chem. 2005, 126, 1332.
Petrov, V. A.; Marshall, W. Beilstein J. Org. Chem. 2013, 9, 2615.
Petrov, V. A.; Marshall, W. J. Fluorine Chem. 2012, 143, 220.
Petrov, V. A.; Marshall, W. J. Fluorine Chem. 2011, 132, 783.
Petrov, V. A.; Marshall, W. J. J. Fluorine Chem. 2007, 128, 729.
Petrov, V. A.; Marshall, W.; Dooley, R. J. Fluorine Chem. 2014, 167, 159.
Petrov, V. A.; Dooley, R.; Marchione A. A.; Marshall, W. J. Fluorine Chem. 2016, 182, 12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(4), 211–212
Rights and permissions
About this article
Cite this article
Ogurok, V.M. 2,2,4,4-Tetrakis(trifluoromethyl)-1,3-dithietane in organic synthesis (microreview). Chem Heterocycl Comp 52, 211–212 (2016). https://doi.org/10.1007/s10593-016-1863-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1863-1