Abstract
Plant hybrid zones receive little conservation attention, yet they may be centers of diversity and evolutionary opportunity for dependent species. In previous studies, cottonwood hybrid zones have been shown to be important drivers of biological diversity and herbivore evolution. Despite these findings, no studies have examined whether hybrid host use drives herbivore genetic divergence across a broad geographic range. Here, we examined the role of Populus hybridization on the evolution of the eriophyid mite, Aceria parapopuli, using ITS1 sequence differentiation. We found support for the hypothesis that Populus hybridization has driven genetic divergence in mites in multiple hybrid zones. Furthermore, our data suggest that hybrid host use has followed at least two instances of mite genetic divergence. Our findings have several important conservation implications. First, they suggest that cottonwood hybrid zones can be important drivers of evolutionary divergence in a dependent herbivore. Second, different hybrid zones represent different ecological environments, and provide independent opportunities for local adaptation and divergence. Although hybrid plants are not considered a high priority for conservation management, and in some cases viewed as “evolutionary dead ends”, our results suggest that new consideration ought to be given to plant hybrid zones. As shown here, natural hybrid zones provide unique ecological and evolutionary opportunities, and essential habitat for dependent species, all of which deserve conservation attention and increased protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant hybridization is common, naturally occurring, and important to plant evolution. It has been suggested that 30–70 % of land plants may have arisen from ancestral hybridization (Grant 1971; Stace 1987; Rieseberg et al. 1996; Mallet 2007; Soltis and Soltis 2009). Plant hybridization also has strong impacts on plant traits such as defensive chemistry, growth, and morphology (Dungey et al. 2000; Rehill et al. 2005; Bangert et al. 2006; Travis et al. 2008; Cheng et al. 2011), which in turn can be associated with herbivore preference, performance, and distribution (Whitham 1989; Floate and Whitham 1993; Whitham et al. 1994, 1999; Gange 1995; Fritz 1999; Fritz et al. 1999; Orians 2000; Hochwender and Fritz 2004; Hochwender et al. 2005; Bailey et al. 2009; Carmona et al. 2011). These effects can be so pronounced that they affect soil microbes (Schweitzer et al. 2004), the understory plant community (Lamit et al. 2011) and aquatic insects that feed on leaves in streams (LeRoy et al. 2006).
Despite the importance of plant hybridization for plant evolution and its strong influence on herbivore ecology, there has been little focus on the evolutionary impacts of plant hybridization on dependent organisms. In a previous study, Floate and Whitham (1993) proposed the hybrid bridge hypothesis as a mechanism for arthropods to shift hosts through intermediate hybrids, which emphasized the importance of plant hybrids in arthropod host use evolution as stepping-stones between parental plant species. Alternatively, the hybrid sink hypothesis (Whitham 1989) suggested that hybrids could be evolutionary sinks for herbivores by virtue of their increased susceptibility, shifting herbivores away from parental species to hybrid plants. However, while these hypotheses address plant hybridization as influencing herbivore evolution, neither tests whether hybrid plants themselves are unique resources for herbivores, which may diverge onto hybrid hosts.
Recently, it has been shown that hybridizing cottonwoods can drive herbivore divergence in the same way that different plant species drive the formation of genetically differentiated arthropod lineages (Evans et al. 2008). Adaptation of herbivores to alternative host plant species may lead to reproductive isolation and associated genetic differentiation (host-associated differentiation, or HAD), and has been suggested as one reason for the diversity of plant-associated arthropods (Dres and Mallet 2002; Stireman et al. 2005; Dickey and Medina 2010). Just as different plant species can drive HAD, hybrids between species may be sufficiently different from parental plant species to promote adaptation, reproductive isolation, and HAD in dependent herbivores. For example, along the Weber River, UT Moran and Whitham (1988) demonstrated that the complex life cycle (host alternation) of the aphid, Pemphigus betae, has evolved in association with Populus hybridization. Evans et al. (2008) and McIntyre and Whitham (2003) examined neutral locus genetic differentiation and adaptation in the eriophyid mite, Aceria parapopuli, and demonstrated that mites have adapted to and are genetically differentiated on Populus angustifolia and P. angustifolia x P. fremontii hybrids to the level of potential cryptic species.
Despite these studies, it is unclear if plant hybrid zones, in general, can drive herbivore evolution and if so, whether hybrid host use has evolved once, or multiple times in multiple locations. Similar patterns of genetic differentiation in multiple locations would provide evidence of the role of host plants in arthropod evolution, because in the absence of host plant effects, geographic isolation would lead to mite genetic differentiation among hybrid zones, but not among mites on different host plants within zones. Alternatively, if different populations inhabiting similar ecological environments exhibit independent instances of genetic differentiation, it is strong evidence that natural selection has driven divergence and reproductive isolation through repeated and independent events, in response to similar environmental selection pressures (Rundle et al. 2000; Nosil et al. 2002; Rundle and Schluter 2004). Whether an arthropod differentiated among plant hosts once with subsequent spread among rivers, or multiple times, independently in different rivers, it would argue for acknowledging plant hybridization as an important, but previously unrecognized process driving herbivore evolution.
Plant hybrids have not typically been given protection under the United States Endangered Species Act because of a prevailing view that hybrids are ecologically and evolutionarily mal-adapted, contributing to the extinction of species (O’Brien and Mayr 1991; Whitham and Maschinski 1996; Allendorf et al. 2001; Haig and Allendorf 2006). However, as noted above, natural hybridization has been shown to have important ecological and evolutionary roles for both plants and dependent organisms, and therefore deserves consideration in conservation guidelines (Whitham et al. 1991, 1999; Whitham and Maschinski 1996; Allendorf et al. 2001; Evans et al. 2008). Furthermore, if different hybrid zones between the same pair of species represent unique ecological and evolutionary opportunities for dependent organisms, then each instance may warrant added protections as distinct entities driving evolutionary processes. Because hybrid zones are often found in restricted areas of overlap between parental species (e.g., Whitham et al. 1994; Martinsen et al. 2001; Tovar-Sanchez and Oyama 2006) they are particularly vulnerable to habitat destruction (Whitham and Maschinski 1996), and thus may deserve special attention for conservation management.
To examine whether plant hybridization arose once as a single evolutionary event, or multiple times, we examined genetic divergence in A. parapopuli Kiefer (Acari: Eriophyidae) across three different Populus hybrid zones, using newly collected data and data from a previous study (Evans et al. 2008). Specifically, we hypothesized that A. parapopuli would be genetically differentiated among parental Populus species and their hybrids in multiple hybrid zones. Evidence in support of this hypothesis would argue that the genetic differentiation of dependent species in plant hybrid zones may not be a rare event and should be studied in other systems. We next tested whether hybrid host use by A. parapopuli has followed multiple, independent instances of genetic differentiation. Confirmation of this hypothesis would argue that hybrids represent important and predictable evolutionary pathways for dependent species. Support for host-associated differentiation in multiple hybrid zones would provide ample justification for developing conservation strategies that emphasize the importance of plant hybridization as essential habitat for herbivore evolution.
Materials and methods
Aceria parapopuli is a single nominal species that forms woody, cauliflower-like galls by attacking the buds of all North American species of Populus (Kiefer 1940; Drouin and Langor 1992; Amrine and Stasny 1994; Baker et al. 1996). Mites disperse via wind and crawling along twigs. Windborne dispersal studies (Zhao and Amrine 1997; Bergh 2001) and population genetic studies along a single river (Evans et al. 2008) indicate that mites are capable of long-distance dispersal. Previous studies (Evans et al. 2012) have shown that A. parapopuli is genetic differentiated among species of Populus hosts (Evans et al. 2012)
Hybridization is a common feature among North American species of Populus (Eckenwalder 1984). Cottonwoods are dominant riparian species, but riparian habitat is the most endangered habitat in the western United States (Noss et al. 1995), despite supporting the greatest biodiversity in the region (Finch and Ruggiero 1993). Furthermore, hybrids themselves typically occur only in narrow bands between parental species, which are themselves distributed across an elevation gradient (Martinsen et al. 2001, Floate 2004), a characteristic similar to other systems where hybrid plants occur only in narrow contact zones [e.g., Eucalyptus (Whitham et al. 1994), Quercus (Tovar-Sanchez and Oyama 2006)]. Both hybrid cottonwoods and their parental species are susceptible to A. parapopuli (Kalischuk et al. 1997; Whitham et al. 1999). Susceptibility to mites is genetically based (Kalischuk et al. 1997; McIntyre and Whitham 2003), and yearly abiotic variation has minimal effects on gall number relative to tree genetic effects (Evans et al. 2012).
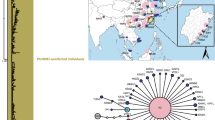
To test whether A. parapopuli exhibits hybrid host associated differentiation in multiple hybrid zones, we examined mite genetic differentiation in three different hybrid zones (Fig. 1). We collected galls from hybrid zones in Indian Creek, UT [N37.9933°, W109.5234°] and San Miguel River, CO [N38.3621°, W108.7214°], and drew upon previously published data and findings from mites along the Weber River, UT [N41.1716°, W111.9974°] (Evans et al. 2008). In each zone, we collected galls from Populus angustifolia, P. fremontii, and their F1 hybrids. In the four-corners region, authorities have disputed the classification of broadleaf cottonwoods in the section Aigeiros as P. fremontii or P. deltoides (Eckenwalder 1977, 1984; Ford 2004), and preliminary molecular evidence suggests that it is an area of hybridization between these two Aigeiros cottonwoods (Max et al., unpublished data). For this study, we have therefore classified all mites from Aigeiros cottonwoods as collections from a single “broadleaf” cottonwood host. Tree classification (e.g., broadleaf Populus, P. angustifolia, or F1 hybrid) was based on leaf morphology and tree architecture (Floate and Whitham 1995; Floate 2004; Floate, Whitham, and Isabel, unpublished manuscript). Morphological categorization can reliably identify parental species v. F1 hybrid cottonwood (Keim et al. 1989; Floate and Whitham 1995; Martinsen et al. 2001). Morphological discrimination has been used to accurately identify hybrid Populus in multiple systems, including the Weber River, one of our collection sites (e.g., Keim et al. 1989; Floate and Whitham 1995; Martinsen et al. 2001). We note that in the previous studies, a low proportion of the morphological P. angustifolia x P. fremontii F1 hybrids were in fact early generation backcross hybrids towards P. angustifolia, which support arthropod communities similar to true F1 hybrids (Floate and Whitham 1995; Floate 2004; Floate, Whitham, and Isabel unpublished manuscript). Furthermore, recent work (Hersch-Green et al., unpublished manuscript), has suggested that the incidence of backcross hybridization to either parental species in the San Miguel River (another one of our collection sites) is relatively rare (only 2 % of the trees sampled in that study). While this raises the possibility that some of the mites we collected from morphological F1 hybrids were in fact from early generation backcross hybrids, it is unlikely and furthermore would not invalidate the hypothesis that tree hybridization (F1 or early generation backcross) leads to herbivore genetic differentiation and evolution.
We sampled one to two mites on each individual tree, totaling 9–24 mites per host type at each location (Table 1). Plant host types were geographically sympatric at each site. Data for the Weber River, UT (where mites are found on F1 hybrid trees and P. angustifolia, but not P. fremontii; Whitham et al. 1999) were taken from Evans et al. (2008). To maintain similar sample sizes among all three rivers, we randomly chose two mite sequences from each host tree sampled in the Weber River by Evans et al. (2008).
To examine host-driven population genetic structure of A. parapopuli we sequenced the Internal Transcribed Spacer 1 (ITS1) region of nuclear ribosomal DNA using individual mites and following the extraction, amplification, and sequencing methods of Evans et al. (2008). We found 17 variable positions in the 499 bp region. Some individuals were heterozygous at multiple sites; therefore, we phased haplotypes using PHASE v. 2.1 (Stephens et al. 2001) as implemented in DnaSP (Librado and Rozas 2009), and found 18 unique haplotypes (Table 1; Genbank Accessions JF792213-JF792237). Ploidy differs between male and female eriophyid mites; males are haploid while females are diploid (arrhenotokous parthenogenesis; Helle and Wysoki 1996). As sexing individuals requires clearing mites for microscopy, we were unable to determine haploid males from homozygous females before DNA extraction. Therefore, to avoid bias from ploidy level differences of individuals, we randomly chose one haplotype per individual (from PHASE output) for analysis (as in Carew et al. 2004; Evans et al. 2008).
It has been argued that HAD should result in multi-locus differentiation of arthropods (Scheffer and Hawthorne 2007). Here, we focus on a single locus, the ITS1 region, for several reasons. First, eriophyid mites have relatively few genomic resources (Cruickshank 2002), but the ITS1 locus can be reliably amplified and sequenced. Second, ITS1 differentiation has led to morphological revision of sibling species (e.g., Fenton et al. 1993, 2000; Amrine et al. 1994) and has been considered a reliable marker for taxonomic purposes, and has reflected patterns of other loci for eriophyid mites using SSRs and ITS1 (Carew et al. 2004) and nrDNA and mtDNA (Navia et al. 2005). Third, primers for mtDNA (Navia et al. 2005) do not reliably amplify in A. parapopuli (Evans, unpublished data), possibly due to major structural rearrangements with the mite genome (Yuan et al. 2010). Fourth, because of the small size of mites (~150 μm), non-Acari DNA is extracted along with A. parapopuli, and using non-Acari-specific marker methods is unreliable as attempts (with subsequent cloning and sequencing of the amplification product) have yielded bacterial, fungal, and Populus DNA (Evans, unpublished data). An additional complication is that multiple copies of ITS1 exist within individuals due to duplications, and intra-individual variation in ITS1 has been observed (e.g., Buckler et al. 1997). However, if this exists in our sample it would only increase the total variance, not the partitioning of that variance via AMOVA (see below), and we have attempted to control for ploidy level in the analyses via randomly choosing one haplotype from each individual (as in Carew et al. 2004; Evans et al. 2008).To test our first hypothesis, that A. parapopuli is differentiated among Populus host types (parental species v. F1 hybrid trees) in each of three rivers, we performed an Analysis of Molecular Variance (AMOVA) of mites on host types within each hybrid zone separately and among all pairwise comparisons, as implemented in Arlequin v.3.1 (Excoffier et al. 2005).
Upon confirming that mites are differentiated among host types in all three rivers, we then used a series of AMOVA models to test the hypothesis that F1 hybrid host use followed multiple instances of genetic divergence in A. parapopuli using the Akaike Information Criterion (with small sample size correction, AICC; Burnham and Anderson 1998) method of Halverson et al. (2008). We tested multiple alternative models using AMOVA. The first model strictly suggests that host use arose only once for each host associated mite lineage, with subsequent differentiation among rivers within each host type. The second model strictly proposes that mites differentiated first among rivers, with subsequent and independent differentiation of host type use in each river. The model that explains the most variation in the highest level (host types for the strict single origin model, rivers for the strict multiple origins model) is the preferred model, quantified as the smallest AICC as described by Halverson et al. (2008):
where n is the number of observations, σ2 = SSR/n where SSR is the total sum of squares for all levels except the highest, and K is the number of parameters. Here, K = 3 and n = 119. A difference between models in AICC of more than 10 indicates substantially more support for the model with the smaller AICC value (Burnham and Anderson 1998). Little support for any given model would suggest a complex history of hybrid host use in A. parapopuli.
Because we were interested in the evolution of hybrid host use, we next tested multiple models of mite differentiation on F1 hybrid hosts. These alternative models treated mites on F1 hybrid hosts as subpopulations of mites on the parental Populus species. We tested models that had one, two, or three mite populations on F1 hosts as subpopulations within either the broadleaf Populus group or the P. angustifolia mite group. A brief description of each model is found in Table 4. Previous work has shown that mites on each of the parental Populus species are strongly differentiated from one another, regardless of geographic proximity (Evans et al. 2012); therefore, we expected a single origin for mites on each parental species, and did not test alternative models of host use evolution on the parental species. We evaluated these models for each of the host type pairs using AICC as above, and identified the best-supported model of hybrid host use as the model with the lowest AICC value.
Results
Aceria parapopuli was strongly differentiated between hybrid and parental tree species in all three rivers (Tables 1, 2), supporting our hypothesis that Populus hybridization drives mite differentiation in hybrid zones of multiple rivers. Furthermore, the strong pairwise differentiation of mites found on different host types (e.g., F1 hybrid vs. P. angustifolia) supports the hypothesis that Populus hybridization drives mite genetic differentiation within rivers (Table 3).
Neither the strict single origin of hybrid host use model, nor the strict multiple origins model of host use was the best-supported model (Table 4). Instead, the best supported model of host use was one in which the Indian Creek and San Miguel River populations of mites on F1 hybrid hosts were grouped with populations of mites on broadleaf Populus (Tables 4, 5). This model had very strong support, with the next-best supported model having an AICC > 15 larger.
Additional support for variation among hybrid host mite populations was found in the pairwise FST estimates (Table 3). We found mite differentiation among rivers within each host type (mean FST = 0.28), and much higher differentiation among host types (mean FST = 0.62). However, differentiation of mites on F1 hybrid trees between Indian Creek and San Miguel was minimal and not significant, while differentiation of each compared to mites on F1 hybrid trees in the Weber River was very high (FST = 0.60 and 0.42, respectively). Additionally, mites on Indian Creek and San Miguel F1 hybrid trees were much less differentiated from those on broadleaf Populus compared to those on F1 hybrid trees in the Weber River.
Overall, our AMOVA results suggest a single, divergence for mites found on P. angustifolia and mites found on broadleaf Populus, and that mites on hybrid hosts have a more complex history. The best-supported model included mites on Indian Creek and San Miguel River F1 hybrid trees as subpopulations of mites on broadleaf Populus, while mites on Weber River F1 hybrid trees were distinct from mites on either parental species. These results are consistent with a hypothesis of at least two independent instances of hybrid host associated differentiation, but with different host-associated evolutionary histories.
Discussion
Mite differentiation on hybrid hosts in multiple hybrid zones
We have shown that A. parapopuli on F1 hybrid cottonwoods are strongly differentiated from mites on parental cottonwood species in three rivers (Tables 1, 2 and 3). Furthermore, mites on hybrid hosts in different rivers are genetically differentiated from one another, as supported by AMOVA and AICC analysis (Tables 3, 4). The history of hybrid host use may be evolutionarily complex, and AMOVA and AICC analysis suggest that mites on hybrid hosts in different rivers may have genetically differentiated from different parental hosts (Tables 3, 4), implying that different hybrid zones impose different evolutionary trajectories on dependent herbivores. Together, these results support our hypothesis that hybrid host-driven A. parapopuli divergence has occurred in multiple hybrid zones, and are suggestive of multiple origins of hybrid host use.
The multiple occurrences of Populus hybrid host use by A. parapopuli indicate that adaptation to host trees has led to reproductive isolation and genetic differentiation of mites (as shown in Evans et al. 2008), because replicated patterns of differentiation in multiple locations is strong evidence that natural selection has driven adaptation and reproductive isolation (Rundle and Schluter 2004; Nosil et al. 2002). Our analysis suggests that F1 hybrid host use in A. parapopuli may have followed independent instances of genetic divergence, which supports the hypothesis that tree hybridization drives herbivore evolution. The geographical isolation of hybrid zones may have facilitated the differentiation of mites on hybrids in the Weber River from those in the San Miguel and Indian Creek, which are similar to one another (Fig. 1). The latter two, geographically near, host genetically similar mites on hybrid trees (Table 3), and it may be that their proximity facilitates gene flow or a more recent divergence and colonization of both, apparently from the broadleaf Populus hosts (Table 4). Being isolated, and without nearby mites on broadleaf Populus hosts (Whitham et al. 1999), mites in the Weber River may have a unique evolutionary history, potentially from the P. angustifolia hosts. Therefore, both geographical and host context may determine the evolutionary dynamics of A. parapopuli.
Although the role of plant hybridization has received little focus in studies of herbivore evolution (e.g., Orians 2000; Dres and Mallet 2002), our results show that it can be a repeatable phenomenon with evolutionary effects of the same scale as different plant species (Table 3). While there are a growing number of studies investigating herbivore HAD among plant species (e.g., Dres and Mallet 2002; Stireman et al. 2005; Dickey and Medina 2010), our studies suggest that greater emphasis should be given to the role of plant hybridization on herbivore evolution.
Importantly, multiple lines of evidence argue that hybrids have influenced arthropod evolution, e.g., molecular analyses in a single river (Evans et al. 2008; this paper), molecular analyses in multiple rivers (this paper), and transfer experiments and differential survival analyses within a single river (McIntyre and Whitham 2003; Evans et al. 2008). Reciprocal transfer experiments can provide powerful tests of adaptation, and future experiments of adaptation to hybrid hosts (as in Evans et al. 2008) in multiple rivers could strengthen this conclusion, as will investigations of additional hybrid zones. The level of differentiation between mites on hybrid trees versus parental species of trees is similar to levels observed among species in other arthropods (FST = 0.16–0.89; Nason et al. 2002; Abbot and Withgott 2004; Anderson et al. 2004; Blair et al. 2005), suggesting that plant host hybridization can lead to divergence and the potential for speciation in arthropods. In combination with experimental studies with aphids in which transfer experiments have shown significant evolutionary responses to hybrids (Moran and Whitham 1988), evidence is accumulating that naturally occurring hybrids can affect the evolution and speciation of dependent organisms.
Implications for conservation
Natural hybridization in plants is evolutionarily important for both plant speciation (Mallet 2007; Soltis and Soltis 2009) and dependent arthropod species (Evans et al. 2008; this study), yet plant hybrids themselves generally receive no special attention or protection (but see examples in Whitham and Maschinski 1996). Distinction between natural hybrids and those resulting from anthropogenic influences must be made, because while natural hybrids are important in an evolutionary and ecological sense, non-natural hybrids (e.g., introduced exotics hybridizing with natives) can drive species toward extinction (O’Brien and Mayr 1991; Allendorf et al. 2001; Haig and Allendorf 2006). On the other hand, taxa of natural hybrid origin should be eligible for protection because they are a natural part of evolution (Whitham et al. 1991; Allendorf et al. 2001; Haig and Allendorf 2006). Our results demonstrate that plant hybrid zones can drive genetic divergence in the herbivore A. parapopuli, and also suggest that hybrid host use has arisen at least two times in the evolutionary history of A. parapopuli. That the observed divergence in A. parapopuli is sufficiently high enough to suggest cryptic speciation underscores the fact that hybrid zones can be evolutionarily important entities for dependent arthropod evolution, worthy of increased conservation management and protection.
The fact that natural hybrids can drive herbivore evolution and provide essential habitat for dependent species and interactions also emphasizes the importance of plant hybrids for entire communities. Studies of hybridizing plants, for example, illustrate how they can influence dependent community structure and biodiversity (Whitham et al. 1999). Community structure, involving arthropods, fungi, understory plants, and vertebrates, can be affected by plant hybridization involving a wide range of plant taxa (Whitham et al. 1994, 1999; Wimp et al. 2005; Bangert et al. 2005; Bailey et al. 2009; Lamit et al. 2011). For example, in studies of Australian Eucalyptus, Whitham et al. (1994) quantified how 40 taxa responded to hybridization of E. amygdalina and the endangered species, E. risdonii. The hybrid zone had greater species richness and abundance than either pure zone, and within the hybrid zone individual hybrid trees had on average 53 % more dependent species. Relative abundances of dependent taxa on hybrids were four-fold higher than on either eucalypt species growing in pure stands. Importantly, 5 of 40 species were largely restricted to the hybrid zone, suggesting that these naturally occurring hybrids are essential habitat for rare species. Our findings suggest that multiple hybrid zones can provide unique evolutionary opportunities and essential habitat for herbivores. Given that hybrids zones are often restricted to limited areas of overlap between species (Whitham et al. 1994; Martinsen et al. 2001; Tovar-Sanchez and Oyama 2006), and can often be located where human development is rapidly occurring, (e.g., in the western United States; Whitham and Maschinski 1996), it is all the more imperative to initiate conservation management strategies designed to protect not only the hybrid zones themselves, but their associated dependent communities. Our study suggests that the protection of multiple, naturally occurring hybrid zones may be important for maintaining natural evolutionary processes for dependent community members, especially in the face of ongoing habitat loss, a primary threat to hybrid zones in the western United States (Whitham and Maschinski 1996).
References
Abbot P, Withgott JH (2004) Phylogenetic and molecular evidence for allochronic speciation in gall-forming aphids (Pemphigus). Evolution 58:539–553
Allendorf FW, Leary RF, Spruell P, Wenburg JK (2001) The problems with hybrids: setting conservation guidelines. Trends Ecol Evol 16:613–622
Amrine JW, Stasny TA (1994) Catalog of the Eriophyoidea (Acarina: Prostigmata) of the world. Indira Publishing House, West Bloomfield
Amrine JW, Duncan GH, Jones AT, Gordon SC, Roberts IM (1994) Cecidophyopsis mites (Acari: Eriophyidae) on Ribes spp. (Grossulariaceae). Int J Acarol 20:139–168
Anderson B, Olivieri I, Lourmas M, Stewart BA (2004) Comparative population genetic structure and local adaptation of two mutualists. Evolution 58:1730–1747
Bailey JK, Schweitzer JA, Úbeda F, Koricheva J, LeRoy CJ, Madritch MD, Rehill BJ, Bangert RK, Fischer DG, Allan GJ, Whitham TG (2009) From genes to ecosystems: a synthesis of the effects of plant genetic factors across levels of organization. Philos Trans R Soc B 364:1607–1616
Baker EO, Kono T, Amrine JW, Delfinado-Baker M, Stasny TA (eds) (1996) Eriophyoid mites of the United States. Indira Publishing House, West Bloomfield
Bangert RK, Turek RJ, Martinsen GD, Wimp GM, Bailey JK, Whitham TG (2005) Benefits of conservation of plant genetic diversity on arthropod diversity. Conserv Biol 19:379–390
Bangert RK, Turek RJ, Rehill B, Allan GJ, Wimp GM, Schweitzer JA, Bailey JK, Martinsen GD, Keim P, Lindroth RL, Whitham TG (2006) A genetic similarity rule determines arthropod community structure. Mol Ecol 15:1379–1392
Bergh JC (2001) Ecology and aerobiology of dispersing citrus rust mites (Acari: Eriophyidae) in central Florida. Environ Entomol 30:318–326
Blair CP, Abrahamson WG, Jackman JA, Tyrrell L (2005) Cryptic speciation and host-race formation in a purportedly generalist tumbling flower beetle. Evolution 59:304–316
Buckler ES, Ippolito A, Holtsford TP (1997) The evolution of ribosomal DNA: divergent paralogues and phylogenetic implications. Genetics 145:821–832
Burnham KP, Anderson DR (1998) Model selection and multimodel inference: a practical information-theoretic approach. Springer-Verlag, New York
Carew ME, Goodisman MAD, Hoffman AA (2004) Species status and population structure of grapevine eriophyoid mites. Entomol Exp Appl 111:87–96
Carmona D, Lajeunesse MJ, Johnson MTJ (2011) Plant traits that predict resistance to herbivores. Funct Ecol 25:358–367
Cheng D, Vrieling K, Klinkhamer PGL (2011) The effect of hybridization on secondary metabolites and herbivore resistance: implications for the evolution of chemical diversity in plants. Phytochem Rev 10:107–117
Cruickshank RH (2002) Molecular markers for the phylogenetics of mites and ticks. Syst Appl Acarol 7:3–14
Dickey AM, Medina RF (2010) Testing host associated genetic differentiation in a quasi-endophage, and a parthenogen on native trees. J Evol Biol 23:945–956
Dres M, Mallet J (2002) Host races in plant-feeding insects and their importance in sympatric speciation. Philos Trans R Soc Lond B Biol Sci 357:471–492
Drouin JA, Langor DW (1992) Poplar bud gall mite. Forestry Leaflet 15, Forestry Canada, Northwestern Region, Northern Forest Centre, Edmonton, Alberta, Canada
Dungey HS, Potts BM, Whitham TG, Li HF (2000) Plant genetics affects arthropod community richness and composition: evidence from a synthetic eucalypt hybrid population. Evolution 54:1938–1946
Eckenwalder JE (1977) North American cottonwoods (Populus, Salicaceae) of sections Abaso and Aigeiros. J Arnold Arboretum 58:193–208
Eckenwalder JE (1984) Natural intersectional hybridization between North American species of Populus (Salicaceae) in sections Aigeiros and Tacamahaca. II. Taxonomy. Can J Bot 62:325–335
Evans LM, Allan GJ, Shuster SM, Woolbright SA, Whitham TG (2008) Tree hybridization and genotypic variation drive cryptic speciation of a specialist mite herbivore. Evolution 62:3027–3040
Evans LM, Clark JS, Whipple AV, Whitham TG (2012) The relative influences of host plant genotype and yearly abiotic variability in determining herbivore abundance. Oecologia 168:483–489
Excoffier L, Laval LG, Schneider S (2005) Arlequin version 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Fenton B, Malloch G, Brennan RM, Jones AT, Gordon SC, McGavin WJ, Birch ANE (1993) Taxonomic evaluation of three reputed species of Cecidophyopsis mite on Ribes. Acta Hortic 352:535–538
Fenton B, Birch ANE, Malloch G, Lanham PG, Brennan RM (2000) Gall mite molecular phylogeny and its relationship to the evolution of plant host specificity. Exp Appl Acarol 24:831–861
Finch DM, Ruggiero LF (1993) Wildlife habitats and biological diversity in the Rocky Mountains and northern Great Plains. Nat Areas J 13:191–203
Floate KD (2004) Extent and patterns of hybridization among the three species of Populus that constitute the riparian forest of southern Alberta, Canada. Can J Bot 82:253–264
Floate KD, Whitham TG (1993) The “hybrid bridge” hypothesis: host shifting via plant hybrid swarms. Am Nat 141:651–662
Floate KD, Whitham TG (1995) Insects as traits in plant systematics: their use in discriminating between hybrid cottonwoods. Can J Bot 73:1–13
Ford MEM (2004) A study of the evolutionary history of the Genus, Populus L. (Salicaceae). Dissertation, University of Colorado
Fritz RS (1999) Resistance of hybrid plants to herbivores: genes, environment, or both? Ecology 80:382–391
Fritz RS, Moulia C, Newcombe G (1999) Resistance of hybrid plants and animals to herbivores, pathogens, and parasites. Annu Rev Ecol Syst 30:565–591
Gange AC (1995) Aphid performance in an alder (Alnus) hybrid zone. Ecology 76:2074–2083
Grant V (1971) Plant speciation. Columbia University Press, New York
Haig SM, Allendorf FW (2006) Hybrids and policy. In: Scott JM, Goble DD, Davis FW (eds) The endangered species act at thirty: conserving biodiversity in human-dominated landscapes volume 2. Island Press, Washington, pp 150–163
Halverson K, Heard SB, Nason JD, Stireman JO III (2008) Origins, distribution, and local co-occurrence of polyploidy cytotypes in Solidago altissima (Asteraceae). Am J Bot 95:50–58
Helle W, Wysoki M (1996) Arrhenotokous parthenogenesis. In: Lindquist EE, Sabelis MW, Bruin J (eds) Eriophyoid mites: their biology, natural enemies, and control. Elsevier Science, Amsterdam, pp 169–172
Hochwender CG, Fritz RS (2004) Plant genetic differences influence herbivore community structure: evidence from a hybrid willow system. Oecologia 138:547–557
Hochwender CG, Janson EM, Cha DH, Fritz RS (2005) Community structure of insect herbivores in a hybrid system: examining the effects of browsing damage and plant genetic variation. Ecol Entomol 30:170–175
Kalischuk AR, Gom LA, Floate KD, Rood SB (1997) Intersectional cottonwood hybrids are particularly susceptible to the poplar bud gall mite. Can J Bot 75:1349–1355
Keim P, Paige KN, Whitham TG, Lark KG (1989) Genetic analysis of an interspecific hybrid swarm of Populus: occurrence of unidirectional introgression. Genetics 123:557–565
Kiefer HH (1940) Eriophyid studies VII. Bull Calif Dep Agric 29:21–46
Lamit LJ, Wojtowicz T, Kovacs Z, Wooley SC, Zinkgraf M, Whitham TG, Lindroth RL, Gehring CA (2011) Hybridization among foundation tree species influences the structure of associated understory plant communities. Botany 89:165–174
LeRoy CJ, Whitham TG, Keim P, Marks JC (2006) Plant genes link forests and streams. Ecology 87:255–261
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Mallet J (2007) Hybrid speciation. Nature 446:279–283
Martinsen GD, Whitham TG, Turek RJ, Keim P (2001) Hybrid populations selectively filter gene introgression between species. Evolution 55:1325–1335
McIntyre PM, Whitham TG (2003) Plant genotype affects long-term herbivore population dynamics and extinction: conservation implications. Ecology 84:311–322
Moran NA, Whitham TG (1988) Evolutionary reduction of complex life cycles: loss of host alternation in Pemphigus (Homoptera: Aphididae). Evolution 42:717–728
Nason JD, Heard SB, Williams FR (2002) Host-associated genetic differentiation in the goldenrod elliptical-gall moth, Gnorimoschema gallaesolidaginis (Lepidoptera: Gelechiidae). Evolution 56:1475–1488
Navia D, de Moraes GJ, Roderick G, Navajas M (2005) The invasive coconut mite Aceria guerreronis (Acari: Eriophyidae): origin and invasion sources inferred from mitochondrial (16S) and nuclear (ITS) sequences. Bull Entomol Res 95:505–516
Nosil P, Crespi BJ, Sandoval CP (2002) Host-plant adaptation drives the parallel evolution of reproductive isolation. Nature 417:440–443
Noss RF, LaRoe ET, Scott JM (1995) Endangered ecosystems of the United States: a preliminary assessment of loss and degradation. Biological Report 28. U.S. National Biological Service, Washington, DC
O’Brien SJ, Mayr E (1991) Bureaucratic mischief: recognizing endangered species and subspecies. Science 251:1187–1188
Orians CM (2000) The effects of hybridization in plants on secondary chemistry: implications for the ecology and evolution of plant-herbivore interactions. Am J Bot 87:1749–1756
Rehill B, Clauss A, Wieczorek L, Whitham T, Lindroth R (2005) Foliar phenolic glycosides from Populus fremontii, Populus angustifolia, and their hybrids. Biochem Syst Ecol 33:125–131
Rieseberg LH, Sinervo B, Linder CR, Ungerer MC, Arias DM (1996) Role of gene interactions in hybrid speciation: evidence from ancient and experimental hybrids. Science 272:741–745
Rundle HD, Schluter D (2004) Natural selection and ecological speciation in sticklebacks. In: Dieckmann U, Doebeli M, Metz JAJ, Tautz D (eds) Adaptive speciation. Cambridge University Press, Cambridge, pp 192–209
Rundle HD, Nagel L, Boughman JW, Schluter D (2000) Natural selection and parallel speciation in sympatric sticklebacks. Science 287:306–308
Scheffer SJ, Hawthorne DJ (2007) Molecular evidence of host-associated genetic divergence in the holly leafminer Phytomyza glabricola (Diptera: Agromyzidae): apparent discordance among marker systems. Mol Ecol 16:2627–2637
Schweitzer JA, Bailey JK, Rehill BJ, Martinsen GD, Hart SC, Lindroth RL, Keim P, Whitham TG (2004) Genetically based trait in a dominant tree affects ecosystem processes. Ecol Lett 7:127–134
Soltis PS, Soltis DE (2009) The role of hybridization in plant speciation. Annu Rev Plant Biol 60:561–588
Stace CA (1987) Hybridization and the plant species. In: Urbanska KM (ed) Differentiation patterns in higher plants. Academic Press, London, pp 115–127
Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989
Stireman JO III, Nason JD, Heard SB (2005) Host-associated genetic differentiation in phytophagous insects: general phenomenon or isolated exceptions? Evidence from a goldenrod-insect community. Evolution 59:2573–2587
Tovar-Sanchez E, Oyama K (2006) Effect of hybridization of the Quercus crassifolia x Quercus crassipes complex on the community structure of endophagous insects. Oecologia 147:702–713
Travis SE, Baggs JE, Maschinski J (2008) Disentangling the role of hybridization in the evolution of the endangered Arizona cliffrose (Purshia subintegra; Rosacae): a molecular and morphological analysis. Conserv Genet 9:1183–1194
Whitham TG (1989) Plant hybrid zones as sinks for pests. Science 244:1490–1493
Whitham TG, Maschinski J (1996) Current hybrid policy and the importance of hybrid plants in conservation. In: Maschinski J, Hammond DH, Holter L (eds) Southwestern Rare and Endangered Plants: Proceedings of the Second Conference; 1995 September 11–14; Flagstaff, AZ. USDA Forest Service, Rocky Mountain Forest and Range Expt. Station, Ft. Collins, CO. RM- GTR-283, pp 103–112
Whitham TG, Morrow PA, Potts BM (1991) Conservation of hybrid plants. Science 254:779–780
Whitham TG, Morrow PA, Potts BM (1994) Plant hybrid zones as centers of biodiversity: the herbivore community of two endemic Tasmanian eucalypts. Oecologia 97:481–490
Whitham TG, Martinsen GD, Floate KD, Dungey HS, Potts BM, Keim P (1999) Plant hybrid zones affect biodiversity: tools for a genetic-based understanding of community structure. Ecology 80:416–428
Wimp GM, Martinsen GD, Floate KD, Bangert RK, Whitham TG (2005) Plant genetic determinants of arthropod community structure and diversity. Evolution 59:61–69
Yuan M-L, Wei D-D, Wang B-J, Dou W, Wang J-J (2010) The complete mitochondrial genome of the red mite Panonychus citri (Acari: Tetranychidae): high genome rearrangement and extremely truncated tRNAs. BMC Genomics 11:597
Zhao S, Amrine JW (1997) A new method for studying aerial dispersal behavior of eriophyoid mites (Acari: Eriophyoidea). Syst Appl Acarol 2:107–111
Acknowledgments
We thank the Cottonwood Ecology Group, Amy Whipple, Tamara Max, Randy Bangert, Suzanne Hagell, Katie Mayer, and Alyssa Bennett for helpful discussion and laboratory assistance. This work was supported by National Science Foundation—FIBR DEB-0425908 to TGW and GJA, Science Foundation Arizona Graduate Student Fellowship and NSF IGERT and Doctoral Dissertation Improvement Grant to LME, and Northern Arizona University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evans, L.M., Allan, G.J. & Whitham, T.G. Populus hybrid hosts drive divergence in the herbivorous mite, Aceria parapopuli: implications for conservation of plant hybrid zones as essential habitat. Conserv Genet 13, 1601–1609 (2012). https://doi.org/10.1007/s10592-012-0409-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-012-0409-z