Abstract
Electrochemotherapy (ECT) represents an effective local treatment for skin unresectable melanoma metastases with high overall objective response rate. ECT is based on the combination of anti-neoplastic drugs administration and cancer cells electroporation. Whether ECT can also activate the immune system is a matter of debate, however a significant recruitment of dendritic cells in melanoma treated metastases has been described. Herein we investigated immediate and late effects of ECT treatment on T cell subsets in ECT-treated lesions by fluorescent immunohistochemistry. Biopsies from melanoma patients (n = 10) were taken before ECT (t0), at d1 and d14 from treatment. At t0, CD3+CD4+ T cells were the most represented T cells, well detected in the perilesional dermis, particularly at tumour margin, while CD3+CD8+ T cells were less represented. CD4+FOXP3+ T regulatory (Treg) cells were present in the perilesional dermis and within the lesion. ECT induced a significant decrease of CD4+FOXP3+ Treg cells percentage in the perilesional dermis, observed at d1 and at d14 (p < 0.001). CD3+CD8+ T cells frequency significantly increased at d14 from treatment in the perilesional dermis (p < 0.001). Furthermore calreticulin translocation to the plasma membrane, a hallmark of immunogenic cell death, was observed in metastatic cells after ECT. The data reported here confirm that ECT induces a local response, with a lymphoid infiltrate characterized by CD4+FOXP3+ Treg cells decrease and CD3+CD8+ T cells recruitment in the treated lesions. These results might contribute to design novel combinational therapeutic approaches with ECT and immunotherapy in order to generate a systemic long-lasting anti-melanoma immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few years electrochemotherapy (ECT) has become a valid and effective technique for local treatment of skin metastasis [1–3]. This novel therapy is based on the administration of anti-neoplastic drugs, such as bleomycin or cisplatin, followed by skin metastasis electroporation [4]. The electric currents, increasing cell membrane permeability, promote the intracellular concentration of the drug thus augmenting its cytotoxicity [3, 5]. Among human cancers, ECT is particularly used for recurrent or in-transit unresectable melanoma metastases [1, 2, 6–8]. Although ECT induces a massive cancer cell death and a clinical response, with 80–90 % overall objective response rate [1, 8, 9], it is generally believed that ECT has only local effects, therefore nowadays it is used as a local palliative treatment.
Beside ECT clinical efficacy, the biological and immunological changes induced by this novel therapy on skin metastases are still to be elucidated. Preliminary investigations in mouse models have described numerous mononuclear cells in mouse ECT-treated metastatic lesions [10], characterized by CD11c+ DCs and CD8+ T cells increase, with no variation of CD4+ T cells [11]. Recently, it has been reported that in murine colon cancer cells ECT is able to induce the immunogenic cell death (ICD) in vitro [12]. ICD is a type of apoptosis characterized by the presence of endoplasmic reticulum chaperonine calreticulin (CRT) in the plasma membrane; extracellular ATP release and high mobility group box 1 (HMGB1) protein 1 liberation [13]. ICD then elicits immune responses against cancer dead-cell antigens, by promoting DCs recruitment and activation, and dead cell-associated antigens presentation to CD8+ T cells, along with the secretion of IL-1b [13, 14].
In humans, different DCs subsets are present in the cellular infiltrate of ECT-treated melanoma metastasis [15], supporting the hypothesis that ECT might induce an increase of melanoma tumour associated antigens (TAA) release, which could become available for immunity activation [16]. Although DCs recruitment in ECT-treated lesions, regression of untreated distant metastases have never been reported, suggesting that perilesional DCs might be defective or that other cells might be involved in the maintenance of a tolerogenic milieu, promoting melanoma survival and escape [17, 18]. For these reasons, the full characterization of the lymphoid infiltrate and the possible presence of ICD, before and after ECT treatment, will allow to deeply understand the biological mechanisms underlying ECT local effects and give the opportunity to potentiate this promising treatment, in order to achieve a systemic anti-tumour response [16].
In line with previous reports in mouse models [10, 11], we observed numerous cells in the inflammatory infiltrate of treated lesions, detected by the nuclear blue fluorescence, which did not express HLA-DR antigen, therefore not belonging to the antigen presenting cells family [15]. Intriguingly, due to the peculiar round morphology of the nucleus, these cells resembled T cells, but their characterization has not been performed yet. Among T cells, T regulatory (Treg) cells, responsible of peripheral tolerance to self-antigens and characterized by FOXP3 transcription factor expression [19–21], have recently been reported to be involved also in tumour tolerance [22].
Since DCs, observed in the cellular infiltrate within the local response of ECT-treated skin melanoma metastases [15], are able to initiate primary immune responses, their presence suggests a possible involvement of other immune cells, particularly T cells [16]. In this study, we aimed to investigate the main T cells subsets (CD3+CD4+ and CD3+CD8+ T cells, and CD4+FOXP3+ Treg cells) and the surface expression of CRT, as hallmark of ICD [13, 23], at the site of the lesion, before and after ECT, in order to explore the nature of the local immune response following ECT treatment.
Methods
Patients and human tissues
Melanoma patients (n = 10) with histological confirmed skin metastasis undergoing ECT with bleomycin were enrolled in the study after obtaining informed written consents. The study was conducted according to the 1964 Helsinki declaration, and its later amendments or comparable ethical standards, and Local Institutional Ethics Committee approval. Enrolled patients had cutaneous and/or subcutaneous melanoma metastases located in the limbs and were not eligible for other traditional therapies. Tumor assessment was performed according to ESOPE study, after 14 days (d14) and 1–3–6–12 months after treatment. According to a recent report [24], number and size of lesions were also collected to correlate with clinical response.
Skin melanoma metastases were treated using Cliniporator™ device (IGEA Ltd, Modena, Italy), as previously reported [15]. Electric pulses were applied with the appropriate type of electrodes (IGEA Ltd), based on metastasis dimensions: by needle electrodes (Type II and III) to deeper seated metastasis and by plate electrodes (Type I) to superficial metastasis. Bleomycin was administered intravenously (15,000 UI/m2 body surface area) in a time frame of 60 s and electrical pulses (variable amplitude with 1–5000 Hz delivery frequencies) were applied between 8 and 28 min after bleomycin infusion, according to previous standardized procedure [1, 5]. Skin biopsies were obtained from different lesions and at different time points: before ECT, indicated as “t0 pre ECT”; and after treatment, at 1 and 14 days post ECT, indicated as “d1 and d14 post ECT”. Skin biopsies, performed at skin metastasis margin to include fragments of lesion and apparently healthy perilesional tissue, were embedded in OCT-like medium (Killik, Bio-Optika), snap-frozen in liquid nitrogen and stored at −80 °C; cryostat section (10 μm) were fixed for 10 min in cold acetone (Sigma-Aldrich) at 4 °C, air dried at room temperature and stored at −20 °C, as previously described [15]. Skin biopsies were also fixed in 10 % formalin and paraffin embedded for routine histology and stained with haematoxylin/eosin (HE) and immunohistochemistry (Ventana automated systems).
Antibodies
The following anti human antibodies (Abs) were used for immunohistochemistry both on acetone fixed-cryostat sections, revealed with specific fluorochromes conjugated secondary Abs (see below), and on formalin fixed-paraffin embedded sections, revealed with horseradish peroxidase-DAB substrate (Ventana automated systems): CD3 (clone 2GV6, rabbit IgG), CD4 (clone SP35, rabbit), CD8 (clone SP57, rabbit), MART-1/Melan A (melanoma antigen recognized by T-cells; clone A103, mouse IgG1), melanosome (clone HMB45, mouse IgG1), from Ventana Medical System, Inc.; CD3-FITC conjugated (clone HIT3a) and FOXP3 (236A/E7, mouse IgG1), from eBioscience, Inc.; Calreticulin (CRT; ab39897, rabbit) and ERp57 (ab11421, rabbit; used as internal control of CRT translocation) were from Abcam. The following secondary goat Abs, all from Life Technology (Thermo Fisher Scientific Inc.), were used: anti mouse and anti rabbit, either conjugated with Alexa Fluor (AF) 488 (green fluorescence) and with AF594 (red fluorescence); fluorescein signal was amplified with anti FITC-AF488 conjugated Abs. Isotype-matched Abs were used as negative controls.
Fluorescent immunohistochemistry
To investigate the main T cells subsets (helper, cytotoxic and regulatory T cells), double fluorescent immunohistochemistry analyses were performed at room temperature unless otherwise indicated. Before primary Abs incubations, sections were treated 2 h with 20 mg/ml BSA (Sigma-Aldrich) in PBS (EuroClone), to block Abs unspecific binding. Section were then stained with primary Abs for 2 or 1 h at 37 °C, according to manufacturer’s instruction, revealed with specific secondary antibodies (either conjugated with AF488 or AF594), for 1 h. Fluorescein signal of FITC-conjugated Abs was amplified with anti FITC-AF488 conjugated Abs for 90 min. For FOXP3 labelling, section were pre-treated for 10 min with 0.2 % Triton X-100 (Sigma-Fluka) in PBS, and after treatment with 20 mg/ml BSA (see above), anti human FOXP3 Abs was incubated overnight at 4 °C. As substitute of HE staining, anatomical compartments were identified by Hoechst nuclear blue fluorescent dye (20 μg/ml, Sigma-Aldrich) [15]. Slides were mounted with Prolong antiFade (Life Technology) and observed with Leica DMLB microscope equipped for epifluorescence (Leica Microsystems GmbH).
Image acquisition and analysis
Images were acquired at 4.25, 1.20, 0.529 and 0.265 µm per pixel resolution, corresponding to ×25, 100, 200 and 400 original magnifications respectively, using Leica DFC200310 FX microscope digital colour camera and LAS software with overlay module (Leica Microsystems GmbH). For each specimen, n = 5 microscopic non-consecutive fields were acquired at ×200, examined for quantitative analyses by two independent observers (P.D.G. and G.G.), and the average number of positive cells was determined [15]. Then the mean number of positive cells from all the melanoma samples (n = 10), at different time-points (t0, d1, d14), was calculated. Quantitative analyses were first performed by counting T cells as CD3+ cells. Then, T cells subsets quantitative analyses were performed by using specific monoclonal antibodies and double immuno-labelling strategies: helper T cells were analysed as CD3+CD4+ cells; cytotoxic T cells, as CD3+CD8+ cells; and regulatory T cells (indicated as Treg), as CD4+FOXP3+ cells. Adobe Photoshop CS2 software (Adobe Systems Incorporated, WA) was used for image processing and figure creating.
Statistical analysis
The values reported throughout the text are expressed as mean ± SD and represent the mean number of positive cells from all the patients (n = 10), at different time-points (t0, d1, d14). Statistical evaluation was performed using two-sided Student’s T test with p < 0.05 adopted as significance level.
Results
We investigated ECT response by fluorescent immunohistochemistry analyses in skin biopsies from ten patients (n = 10; 5 men and 5 women; median age 79 years; range 49–100) with skin melanoma metastases located in the limbs (see Table 1 for details). The number of lesions varied among patients, being less than 10 in six patients and more than 10 in four patients (ranges 3–9 and 12–34, respectively). Lesion size diameter was less than 2 cm (even <1 cm) in four patients and between 1 and 2 cm or more than 2 cm in six patients. All patients enrolled in this study underwent radical surgery; one patient underwent isolated limb infusion while two of them underwent isolated limb perfusion and even ECT (of note, for patients already treated with ECT, skin biopsies were always taken from new lesions).
Despite clinical differences among patients, a clinical response was observed in all treated lesions at least at 12 months. As reported in Table 1, six patients displayed a complete response (CR) of treated lesions and four patients displayed partial response (PR). However, four patients exhibited new lesions beside the CR/PR of the treated lesions.
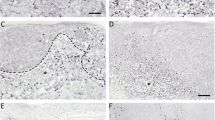
Before ECT treatment, metastatic melanoma lesions appeared as well-delimited nodules, localized immediately below the epidermis (Fig. 1a) or in the subcutaneous dermis, identified by the expression of MART-1 (Fig. 1c), as previously reported [15]. Notably we observed the presence of small cells, with a round morphology, which expressed CD3 by enzymatic and fluorescent immunohistochemistry, therefore confirming the presence of resident CD3+ T cells before ECT, with a predominant focal distribution in the tissue (Fig. 1a, c). Few days after ECT treatment, all treated lesions were characterized by the well-known clinical signs of inflammation, i.e., rubor, tumor, calor and dolor, as already shown [15], which augmented until d14 after treatment. In parallel with the regression of the lesions, we observed the increase of small cells infiltrate (Fig. 1b, d). At d14 after ECT treatment, the cellular infiltrate was characterized by CD3+ T cells, which displayed a diffuse distribution, particularly in the perilesional dermis and within the lesion burden (Fig. 1b, d). Of note, the lesion, highly reduced in size after 14 days, did not appear as a well circumscribed mass but it was composed by fragmented nodules or scattered cells (Fig. 1b, d). Quantitative analyses showed that CD3+ T cells frequency in the perilesional dermis at d1 post treatment was similar to the number observed before ECT (t0) (65.33 ± 9.78 and 63.91 ± 10.47, respectively; p = 0.74), while it significantly increased at d14 (84.38 ± 15.76), as compared both to t0 and d1 (p < 0.001 and p < 0.01, respectively) (Fig. 1e).
CD3+ T cells in metastatic melanoma lesions before and after ECT. Enzymatic and double fluorescent immunohistochemistry analyses of skin melanoma metastases (n = 10) were performed before ECT (t0) and after 14 days from treatment (d14 post) with the indicated antibodies. Details from representative images are shown. a, b CD3+ T cells, analysed by immunohistochemistry on paraffin-embedded sections and revealed with horseradish peroxidase-DAB substrate (brown), were observed before ECT (a), with predominantly a focal distribution, and they significantly increased after d14 from ECT (b), displaying also a diffuse distribution (Original magnification ×25). c, d CD3+ T cells were analysed within the MART-1+ melanoma metastases by double fluorescent immunohistochemistry on frozen sections, revealed with Alexa Fluor 594 (red) and 488 (green) respectively. Nuclei were labelled with Hoechst dye (blue). CD3+ T cells were present in the perilesional dermis before ECT (c), and sometime also in the lesion. At d14 from ECT, CD3+ T lymphocytes significantly increased (d) (Original magnification ×200). e For quantitative analyses, the number of perilesional CD3+ cells for each specimen was assessed by calculating the average cell number counted in n = 5 different fields. Values represent the mean number of positive cells from n = 10 melanoma samples. ** and *** indicate p < 0.01 and p < 0.001, respectively. CD3+ T cells showed a significant increase at d14 as compared both to t0 (dark asterisks) and d1 (gray asterisks). (Color figure online)
Different T cells subsets in metastatic melanoma lesion before ECT
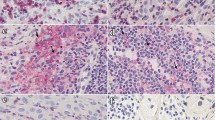
Among CD3+ T cells, we observed helper and cytotoxic T cells, identified by the co-expression of CD3 with CD4 or CD8 antigens, respectively (Fig. 2a, b). Before ECT, CD3+CD4+ T cells were well detected in the perilesional dermis (Fig. 2a, c), but less numerous within the lesion (Fig. 2e), although their frequency was higher at tumour border than deeply in the tumour mass. Among CD3+CD4+ T cells, we observed the presence of Treg cells, characterized by the nuclear expression of the transcription factor FOXP3; Treg cells were always observed both at tumour border and within the melanoma mass (Fig. 2c, e). CD3+CD8+ T cells were detected in the perilesional dermis (Fig. 2b, d), but they were rare at tumour border and within the lesion (Fig. 2f). Of note, we did not observe CD8+FOXP3+ Treg cells in the samples analysed.
T cells subsets before ECT treatment (t0). Double fluorescent immunohistochemistry analyses on frozen sections of melanoma metastases before ECT (n = 10) were performed with the indicated antibodies as described in Fig. 1 legend. Details from representative images are shown. a, b CD3+CD4+ (a) and CD3+CD8+ (b) T cells were found in the perilesional dermis before ECT, indicated by CD3 (green) and CD4/CD8 (red) co-expression (yellow/orange). Of note, the frequency of CD3+CD4+ T cells was higher than CD3+CD8+ T cells number, especially at tumour burden (Original magnification ×200). c, d Among perilesional CD4+ T cells (green), FOXP3+ cells (red) were also observed (c), with nuclear FOXP3 staining, representing Treg cells; no CD8+ FOXP3+ cells were observed (d) (Original magnification ×200). e, f In the lesion, CD4+ T cells (green) were present (e) and a high number of CD4+ FOXP3+ Treg cells (green and red) were observed with nuclear FOXP3 staining; cells with cytoplasmic FOXP3 staining were rare. Within the lesion, only few CD8+ T cells (green) were observed (f) (Original magnification ×400). (Color figure online)
Treg decrease in the perilesional dermis after ECT
After ECT treatment, a marked reduction of the nuclear FOXP3 expression was observed among CD4+ Treg cells, particularly in the perilesional dermis and in the lesion (Fig. 3a, b, d). CD4+FOXP3+ Treg cells significantly decreased at d1 from treatment, as compared to the number observed before ECT in the perilesional dermis (2.50 ± 2.38 and 12.07 ± 3.93, respectively; p < 0.001) (Fig. 3a, b). Treg cells further decreased in the perilesional dermis after d14 from treatment, as compared to the number observed before ECT (1.73 ± 1.96, p < 0.001) (Fig. 3b, d).
Treg cells decreased in the perilesional dermis after ECT, while CD3+CD4+ T cells number did not differ. Double fluorescent immunohistochemistry analyses on frozen sections of melanoma metastases (n = 10), after 1 and 14 days from treatment (d1 and d14), were performed with the indicated antibodies as described in Fig. 1 legend. Details from representative images are shown. a, d In the perilesional dermis, FOXP3 expression (red) significantly decreased at d1 and d14, while the number of CD4+ T cells (green) did not vary (Original magnification ×200). b, c Quantitative analyses were performed as reported in Fig. 1 legend. Values represent the mean number of Treg cells (b) and CD3+CD4+ T cells (d) from n = 10 melanoma samples per digital image; asterisks (***) indicate p < 0.001. e At d14 after treatment, beside CD3+CD4+ T cells (yellow-orange), numerous CD3+CD4− cells (green) were observed with different size and morphology, where the small and round ones likely represented CD3+CD8+ T cells. (Original magnification ×200). (Color figure online)
Perilesional CD3+CD4+ T cells did not vary after ECT
CD3+CD4+ T cells frequency in the perilesional dermis after treatment, both at d1 and d14 after ECT (Fig. 3a, c–e), was similar to that observed before treatment (t0: 36.07 ± 6.57; d1: 35.98 ± 6.85; and d14: 41.65 ± 8.86; p = 0.97 and p = 0.07, respectively).
CD3+CD8+ T cells increase in the perilesional dermis after ECT
Unlike CD3+CD4+ T cells, CD3+CD8+ T cells frequency in the perilesional dermis significant increased after d14 treatment (Fig. 4a–d), as compared to t0 (42.73 ± 9.05 and 27.83 ± 4.86, respectively; p < 0.001) and to d1 (29.36 ± 3.86; p < 0.01), while no significance difference was found between t0 and d1 (p = 0.44).
CD3+CD8+ T cells increased in the perilesional dermis at d14 from ECT. Double fluorescent immunohistochemistry analyses on frozen sections of melanoma metastases (n = 10), after 1 and 14 days from treatment (d1 and d14), were performed with the indicated antibodies as described in legend for Fig. 1. Details from representative images are shown. a, c In the perilesional dermis, the number of CD8+ T cells (green) increased both at d1 (a), and at d14 (c) (Original magnification ×200). b Quantitative analyses were performed as described in legend for Fig. 1; values represented the mean frequency of CD3+CD8+ T cells from n = 10 melanoma samples per digital image. ** and *** indicate p < 0.01 and p < 0.001, respectively. CD3+CD8+ T cells showed a significant increase at d14 as compared both to t0 (dark asterisks) and d1 (gray asterisks). d At d14 after treatment, beside CD3+CD8+ T cells (yellow-orange), some CD3+CD8− cells (green) were observed, likely representing CD3+CD4+ T cells, although few of them appeared bigger than T cells (Original magnification ×200). (Color figure online)
Calreticulin translocation to the plasma membrane after ECT
In order to establish whether ECT treatment was able to induce ICD, we investigated the presence on the plasma membrane of two endoplasmic reticulum chaperonines: calreticulin (CRT; Fig. 5) and ERp57 (as internal control, data not shown). The staining protocol was performed without the permeabilization step, to detect only CRT and ERp57 out of the endoplasmic reticulum. CRT surface expression was not observed in metastatic cells before ECT (Fig. 5a), but CRT+ cells were found after treatment (d1 and d14), displaying at d1 the typical “dotted” membrane staining (Fig. 5b) [23], while at d14 CRT surface expression was weaker (Fig. 5c).
Calreticulin translocation to the plasma membrane after ECT. Fluorescent immunohistochemistry analyses on frozen sections from skin melanoma metastases (n = 4) were performed before ECT (t0) and after 1 and 14 days from treatment (d1 and d14) with the anti calreticulin (CRT) antibody, revealed with Alexa Fluor 488 (green); nuclei were labelled with Hoechst dye (blue). Details from representative images are shown. a Before ECT, CRT expression was not detected among metastatic cells. b, c After ECT treatment, few CRT+ cells were observed among metastatic cells, displaying at d1 the typical “dotted” membrane staining (b, and inset for detail), while at d14 CRT expression was weaker (c) (Original magnification ×400). (Color figure online)
Discussion
The aim of this study was to further investigate the immunobiological features of local response induced by ECT in patients with skin melanoma metastases. We described the presence of the main T cells subsets (CD3+CD4+ and CD3+CD8+ T cells and CD4+FOXP3+ Treg cells) in the perilesional dermis of melanoma metastases, in both cutaneous and subcutaneous lesions before and after ECT. Furthermore we reported the presence of CRT, an hallmark of ICD, on the plasma membrane of metastatic cells after ECT. Despite the clinical heterogeneity between patients enrolled in the study, a clinical response was observed in all treated lesions at least at 12 months, confirming therapeutic advantages induced by ECT for patients with skin melanoma metastases.
Before ECT treatment (t0), CD3+CD4+ T cells were the most represented T cells subset in the perilesional dermis, often located at the tumour border, while CD3+CD8+ T cells were less frequent. CD4+FOXP3+ Treg cells were present both in the perilesional dermis and tumour margin, and always observed within the lesion burden as scattered cells. These results are in line with previous analyses of tumour infiltrating lymphocytes (TILs) in human melanoma metastases before ECT, characterized by the presence of CD3+, CD4+, CD8+ and FOXP3+ cells [25]. Notably the Authors reported that a “brisk” pattern of CD8+ T cells within the infiltrate before ECT is associated with a higher clinical response rate [25]. Although there are still few evidences supporting a possible ECT role in eliciting systemic effects due to immune cells activation, these data suggest an involvement of the immune system and support the concept that the combination of ECT with immune modifiers could improve ECT clinical activity [21]. Indeed it has been reported that the combination of ECT with IL-2 induces anti-cancer systemic immunity with the generation of specific cytotoxic T cells responses [26, 27].
Interestingly, the ECT treatment induced a significant modification of the original T cells infiltrate. Treg cells were particularly affected by ECT as a significant decrease in the number of CD4+FOXP3+ T cells was detected as soon as d1 after treatment, both in the perilesional dermis and in the lesion burden. Treg cells further decreased until d14 from treatment. CD3+CD4+ T cells seemed to be unaltered by ECT, as their frequency did not vary after the treatment. On the contrary, CD3+CD8+ T cells resulted particularly influenced by ECT as they significantly increased in the perilesional dermis at d14 after treatment. Unexpectedly in some patients this increase was observed already at d1 after ECT. These results are in line with previous data observed in mouse melanoma models where no variations were observed in the number of CD4+ cells in the ECT-treated lesions, while CD8+ cells strongly increased [11]. In addiction we described the CRT translocation to the plasma membrane in metastatic cells after ECT treatment, according to a recent report in murine cancer cells in vitro [12]. All together these data suggest that ECT is able to induce a local immune response, since the presence of CRT surface expression indicates the induction of ICD [13, 14], which then explains the recruitment of different DCs subsets [15] and the increase of CD3+CD8+ T cells at d14 after ECT.
Surprisingly, we did not observe any differences in T cell subsets distribution and behaviour between treated patients, both at d1 and d14 from treatment, suggesting that at least after 2 weeks, ECT effects seem to be predominant over melanoma escape mechanisms, but not sufficient to achieve anti-melanoma immunity (some patients developed new lesions beside the treated ones). Notably, patients with less than 10 lesions and less than 2 cm diameter (n = 2) displayed CR at 12 months, without developing new lesions, as patients with more than 10 lesions but less than 2 cm diameter (n = 2). In contrast, patients with increased lesion size (more than 2 cm diameter; n = 6) displayed CR/PR at least at 12 months, but four of them developed new lesions. Our observations, although in a small group of patients, are in line with a previous report where, among treated patients, the probability to achieve a CR was significantly correlated with number of nodules less than 10 and lesion size less than 2 cm diameter [24].
Despite the local presence of DCs and T cells in ECT-treated lesions, this event is not sufficient to generate systemic anti-melanoma immunity [1, 2, 5], although the clinical efficacy of ECT is well-established towards electroporation and bleomycin administration alone, as previously reported in mouse melanoma models [10, 11]. Indeed the induction of ICD appear to be only a partial event because we found only few CRT+ cells in metastatic lesions and CRT surface expression was weaker at d14 as compared to that at d1 after treatment, suggesting that ECT alone is not able to induce a complete immune response, as already reported in the mouse [11, 12]. Probably melanoma stem cells might be involved in the generation of new nodules after ECT, confirming once again the local efficacy of this treatment. Although melanoma immune responses, achieved by melanoma-specific T cells activation, have been reported [28], ex vivo TILs analyses revealed T cells anergy particularly among the CD8+ T cells subset [29, 30]. It is worth to mention that the presence of immunosuppressive factors and tolerogenic cells in the melanoma milieu might impair effector T cells functions [31]. Notably melanoma cells are able to secrete immunosuppressive factors and cytokines, like TGF-beta and IL-10 [32], and immunosuppressive cells have been observed in the melanoma microenvironment, represented by tumour associated macrophages, myeloid derived suppressive cells, immature DCs and Treg cells [33].
Particularly Treg cells, responsible of peripheral tolerance to self-antigens [19, 34], are thought to be involved also in tumour tolerance [22], since tumour antigens are often self-antigens [35]. Recently it has been reported that melanoma cells are able to recruit Treg cells in the perilesional milieu and to stimulate their proliferation and expansion, due to the release of immunosuppressive cytokines [18]. This might explain the presence of numerous Treg in the infiltrate of melanoma metastases that we documented before ECT. Importantly, it has been reported that inflammatory cytokines are able to down-regulate FOXP3 expression, thus blocking Treg cells immunosuppressive functions and potentiating effector T cells [36]. Since a local response is induced by ECT, this could explain the early CD4+FOXP3+ T cells decrease observed in our treated lesions. Moreover, the presence of plasmacytoid DCs (pDCs) in the cellular infiltrate [15] might contribute to FOXP3 down-regulation thanks to the production of type I IFN [37, 38]. Notably, pDCs are known to stimulate cytotoxic T cells responses by priming melanoma specific CD8+ T cells [39] and in the ECT treated lesions they might also play a role in the stimulation of the CD3+CD8+ T cells increase at d14.
Although ECT treatment might modify the melanoma-induced immunosuppressive microenvironment, the presence of Treg cells and only few CD83+ DCs at d14 from treatment [15], along with the weak CRT surface expression, confirm once again the hypothesis that ECT alone is not sufficient to induce a systemic immune response [16, 25]. In order to stimulate a long-lasting anti-melanoma immunity, ECT treatment should be combined with the administration of immune-adjuvants, such as TLRs agonists (CPG-ODNs and imiquimod) or pro-inflammatory cytokines, such as GM-CSF, which are able to potentiate DCs activation [11, 40, 41]. In particular, the association of ECT and TLR9 agonists CPG-ODN is able to achieve anti-tumour effects on distant untreated lesions in two mouse melanoma models [11], probably due to increased tumour immunogenicity or at least reduced tumour immunosuppression.
Recently, innovative immunological strategies have been introduced in melanoma therapy, through immune checkpoints blockade by the use of anti PD-1/PDL-1 and CTLA-4 antibodies, which “inhibits the inhibitors”, in order to potentiate patient’s immune cells [42–46]. For these reasons, the characterization of the different immune cells and their variations in the cellular infiltrate of ECT-treated melanoma metastases, and the detection of ICD, might help clinicians to choose the proper immunotherapy to be associated with ECT [47], to propose new combining therapeutic approaches in metastatic melanoma patients, in order to achieve a systemic lifelong anti-melanoma immunity.
Abbreviations
- CRT:
-
Calreticulin
- DCs:
-
Dendritic cells
- ECT:
-
Electrochemotherapy
- HE:
-
Haematoxylin/eosin
- ICD:
-
Immunogenic cell death
- MART-1:
-
Melanoma antigen recognized by T-cells
- pDCs:
-
Plasmacyotid DCs
- TAA:
-
Tumour associated antigens
- TILs:
-
Tumour infiltrating lymphocytes
- Treg cells:
-
T regulatory cells
References
Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, Billard V, Geertsen PF, Larkin JO, Miklavcic D, Pavlovic I et al (2006) Electrochemotherapy—an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer Suppl 4:3–13
Möller MG, Salwa S, Soden DM, O’Sullivan GC (2009) Electrochemotherapy as an adjunct or alternative to other treatments for unresectable or in-transit melanoma. Expert Rev Anticancer Ther 9:1611–1630
Cadossi R, Ronchetti M, Cadossi M (2014) Locally enhanced chemotherapy by electroporation: clinical experiences and perspective of use of electrochemotherapy. Future Oncol 10:877–890
Mir LM (2006) Basis and rationale of the electrochemotherapy. Eur J Cancer Suppl 4:38–44
Mir LM, Gehl J, Sersa G, Collins CG, Garbay J-R, Billard V, Geertsen PF, Rudolf Z, O’Sullivan GC, Marty M (2006) Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the cliniporator by means of invasive or non-invasive electrodes. Eur J Cancer Suppl 4:14–25
Caracò C, Mozzillo N, Marone U, Simeone E, Benedetto L, Di Monta G, Di Cecilia ML, Botti G, Ascierto PA (2013) Long-lasting response to electrochemotherapy in melanoma patients with cutaneous metastasis. BMC Cancer 13:564–568
Campana LG, Testori A, Mozzillo N, Rossi CR (2014) Treatment of metastatic melanoma with electochemotherapy. J Surg Oncol 109:301–307
Queirolo P, Marincola F, Spagnolo F (2014) Electrochemotherapy for the management of melanoma skin metastasis: a review of the literature and possible combinations with immunotherapy. Arch Dermatol Res 306:521–526
Ricotti F, Giuliodori K, Cataldi I, Campanati A, Ganzetti G, Ricotti G, Offidani A (2014) Electrochemotherapy: an effective local treatment of cutaneous and subcutaneous melanoma metastases. Dermatol Ther 27:148–152
Mekid H, Tounekti O, Spatz A, Cemazar M, El Kebir FZ, Mir LM (2003) In vivo evolution of tumour cells after the generation of double-strand DNA breaks. Br J Cancer 88:1763–1771
Roux S, Bernat C, Al-Sakere B, Ghiringhelli F, Opolon P, Carpentier AF, Zitvogel L, Mir LM, Robert C (2008) Tumour destruction using electrochemotherapy followed by CpG oligodeoxynucleotide injection induces distant tumour responses. Cancer Immunol Immunother 57:1291–1300
Calvet CY, Famin D, André FM, Mir LM (2014) Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology 3:e28131
Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N et al (2014) Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 3:e955691
Kroemer G, Galluzzi L, Kepp O, Zitvogel L (2013) Immunogenic cell death in cancer therapy. Annu Rev Immunol 31:51–72
Gerlini G, Sestini S, Di Gennaro P, Urso C, Pimpinelli N, Borgognoni L (2013) Dendritic cells recruitment in melanoma metastasis treated by electrochemotherapy. Clin Exp Metastasis 30:37–45
Gerlini G, Di Gennaro P, Borgognoni L (2012) Enhancing antimelanoma immunity by electrochemotherapy and in vivo dendritic-cell activation. Oncoimmunology 1:1655–1657
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565–1570
Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ (2012) Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncol 13:e32–e42
Whiteside TL, Schuler P, Schilling B (2012) Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther 12:1383–1397
Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R et al (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203:1693–1700
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Fazekas, Groth B et al (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203:1701–1711
Nishikawa H, Sakaguchi S (2010) Regulatory T cells in tumor immunity. Int J Cancer 127:759–767
Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, Fimia GM, Kepp O, Piacentini M, Froehlich KU, van Endert P, Zitvogel L, Madeo F, Kroemer G (2008) The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ 15:1499–1509
Solari N, Spagnolo F, Ponte E, Quaglia A, Lillini R, Battista M, Queirolo P, Cafiero F (2014) Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: a series of 39 patients treated with palliative intent. J Surg Oncol 109:270–274
Quaglino P, Osella-Abate S, Marenco F, Nardo T, Gado C, Novelli M, Savoia P, Bernengo MG (2011) FoxP3 expression on melanoma cells is related to early visceral spreading in melanoma patients treated by electrochemotherapy. Pigment Cell Melanoma Res 24:734–736
Andersen MH, Gehl J, Reker S, Pedersen LØ, Becker JC, Geertsen P, Thor Straten P (2003) Dynamic changes of specific T cell responses to melanoma correlate with IL-2 administration. Semin Cancer Biol 13:449–459
Renkvist N, Castelli C, Robbins PF, Parmiani G (2001) A listing of human tumour antigens recognized by T cells. Cancer Immunol Immunother 50:3–15
Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, Rimoldi D, Chen JL, Liénard D, Cerottini JC, Cerundolo V (1998) Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med 188:1641–1650
Guilloux Y, Viret C, Gervois N, Le Dréan E, Pandolfino MC, Diez E, Jotereau F (1994) Defective lymphokine production by most CD8+ and CD4+ tumor-specific T cell clones derived from human melanoma-infiltrating lymphocytes in response to autologous tumor cells in vitro. Eur J Immunol 24:1966–1973
Mortarini R, Piris A, Maurichi A, Molla A, Bersani I, Bono A, Bartoli C, Santinami M, Lombardo C, Ravagnani F et al (2003) Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res 63:2345–2535
Gajewski TF, Meng Y, Harlin H (2006) Immune suppression in the tumor microenvironment. J Immunother 29:233–240
Gerlini G, Tun-Kuy A, Dudli C, Burg G, Pimpinelli N, Nestle FO (2004) Metastatic melanoma secreted IL-10 down-regulates CD1 molecules on dendritic cells in metastatic tumour lesions. Am J Pathol 165:1853–1863
Stewart TJ, Abrams SI (2008) How tumours escape mass destruction. Oncogene 27:5894–5903
Wing K, Sakaguchi S (2010) Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol 11:7–13
Pardoll DM (1998) Cancer vaccines. Nat Med 4:525–531
Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA (2009) Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol 21:281–285
Colonna M, Trinchieri G, Liu YJ (2004) Plasmacytoid dendritic cells in immunity. Nat Immunol 5:1219–1226
Charles J, Chaperot L, Salameire D, Di Domizio J, Aspord C, Gressin R, Jacob MC, Richard MJ, Beani JC, Plumas J et al (2010) Plasmacytoid dendritic cells and dermatological disorders: focus on their role in autoimmunity and cancer. Eur J Dermatol 20:16–23
Salio M, Cella M, Vermi W, Facchetti F, Palmowski MJ, Smith CL, Shepherd D, Colonna M, Cerundolo V (2003) Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol 33:1052–1062
Stoitzner P, Green LK, Jung JY, Price KM, Tripp CH, Malissen B, Kissenpfennig A, Hermans IF, Ronchese F (2008) Tumour immunotherapy by epicutaneous immunization requires langerhans cells. J Immunol 180:1991–1998
Min L, Mohammad Isa SA, Shuai W, Piang CB, Nih FW, Kotaka M, Ruedl C (2010) Cutting edge: granulocyte-macrophage colony-stimulating factor is the major CD8+ T cell-derived licensing factor for dendritic cell activation. J Immunol 184:4625–4629
Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G et al (2005) Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65:1089–1096
Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jr et al (2010) Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 11:155–164
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Srivastava N, McDermott D (2014) Update on benefit of immunotherapy and targeted therapy in melanoma: the changing landscape. Cancer Manag Res 6:279–289
Calvet CY, Mir LM (2016) The promising alliance of anti-cancer electrochemotherapy with immunotherapy. Cancer Metastasis Rev 35:165–177
Acknowledgments
This work was supported by Grant (No 2014.0722) from Fondazione Cassa di Risparmio di Firenze to the FiorGen Foundation, Florence, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Di Gennaro, P., Gerlini, G., Urso, C. et al. CD4+FOXP3+ T regulatory cells decrease and CD3+CD8+ T cells recruitment in TILs from melanoma metastases after electrochemotherapy. Clin Exp Metastasis 33, 787–798 (2016). https://doi.org/10.1007/s10585-016-9814-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-016-9814-x