Abstract
The assembly of pericellular matrix containing hyaluronan (HA) and versican has been shown to be a pre-requisite for proliferation and migration of mesenchymal cells. In this study, we investigated whether treatment with recombinant versican could induce the formation of a pericellular matrix by ovarian cancer cells (OVCAR-3, OVCAR-5, and SKOV-3) and promote their motility, invasion, and adhesion to peritoneal cells in vitro. We also determined whether versican-induced pericellular matrix formation and metastatic cancer cell behavior could be blocked by small HA oligosaccharides. Only combined treatment with recombinant versican and HA resulted in pericellular matrix formation by OVCAR-5 and SKOV-3 but not by OVCAR-3 cells, which lack the HA receptor, CD44. The motility of OVCAR-5 and SKOV-3 cells was significantly increased in scratch wound and chemotaxis assays following treatment with recombinant versican and HA. Versican and HA also promoted invasion of SKOV-3 and OVCAR-5 cells but had no effect on OVCAR-3 cells. We have demonstrated that exogenous HA significantly increased OVCAR-5 and SKOV-3 adhesion to peritoneal cells but adhesion was not further increased by versican treatment. Small HA oligomers (6–10 disaccharides) were able to significantly block formation of pericellular matrix by OVCAR-5 cells, as well as the increased motility and invasion induced by recombinant versican. HA oligomers also significantly blocked OVCAR-5 adhesion to peritoneal cells both in the presence and absence of exogenous HA. The dependence of CD44 for the versican and HA mediated effects were demonstrated by the inhibition of pericellular matrix formation as well as motility and invasion of OVCAR-5 cells following treatment with CD44 neutralizing antibody in the presence of versican and HA. We conclude that the acquisition of a HA/versican pericellular matrix by ovarian cancer cells increases their metastatic potential. HA oligomers can block this mechanism and are promising inhibitors of ovarian cancer dissemination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the most lethal gynecological cancer and ranks as the fifth most common cause of cancer-related death in women in the Western world [1, 2]. It has been estimated that there will be 21,550 new cases of ovarian cancer and 14,600 deaths due to ovarian cancer in the United States in 2010 [2]. Furthermore, the mortality rate due to ovarian cancer has been reduced by only 4% between 1991 and 2005 [2]. Significant improvements in ovarian cancer survival will therefore require the development of more effective molecularly targeted diagnostics and/or therapeutics.
Ovarian cancer is believed to spread by detaching from the surface of the ovary and attaching to and invading the peritoneum which lines the organs of the abdominal cavity [3]. Once ovarian cancer cells adhere to the peritoneum, they can migrate through the peritoneal cell layer and invade local organs. There is increasing evidence to suggest that extracellular matrix (ECM) components play an active role in tumor progression and are an important determinant for the growth and progression of solid tumors [4, 5]. Tumor cells are known to interfere with the normal programming of ECM biosynthesis and can extensively modify the structure and composition of the matrix [6]. Alterations in the extracellular environment are critical for tumor initiation and progression and intra-peritoneal dissemination [7–16].
Hyaluronan (HA) is a large polysaccharide which is assembled into pericellular and ECM matrices in many tissues [17]. HA plays a role in various cell functions such as adhesion, motility, and differentiation and has also been implicated to play a key role in cancer metastasis [17, 18]. Many human tumors, including ovarian cancer, are surrounded by a connective tissue matrix enriched with HA [11, 19, 20]. Increased HA has been shown to be an independent predictor of ovarian cancer survival. Its levels significantly correlate with the degree of invasiveness and metastatic potential in ovarian cancer tumors [11, 21] and it promotes attachment of cancer cells to peritoneal cells via interactions with the HA receptor, CD44 [22–24].
Increased expression of the HA-binding proteoglycan, versican, has been identified to be significantly involved in ovarian cancer metastasis [25, 26]. Versican is also a candidate marker for the early detection of ovarian cancer [27]. Versican is a member of the lectican family of proteoglycans also known as large aggregating proteoglycans and hyalectans which also includes aggrecan, neurocan, and brevican [28, 29]. Elevated versican levels are associated with cancer relapse and poor patient outcome in breast, prostate, and many other cancer types including ovarian cancer [12, 29–38]. Elevated levels of versican have been observed in primary ovarian tumors and secondary metastases when compared with normal ovaries [39] and increased by three fold in ovarian cancer tissues when compared with normal ovarian surface epithelial cells [40]. In ovarian cancer, increased versican stroma staining was associated with advanced FIGO (International Federation of Gynaecologists and Obstetricians) stage, large residual tumor, serous histologic type, and reduced patient survival [12].
The assembly of pericellular matrix rich in HA and versican is a prerequisite for proliferation and migration of mesenchymal cells [41] and has recently been shown to promote the motility of prostate cancer cells [42]. In this study, we investigated whether treatment with recombinant versican and HA could induce the formation of a pericellular matrix around ovarian cancer cells (OVCAR-3, OVCAR-5, and SKOV-3) and promote their motility, invasion and adhesion to peritoneal cells, key processes in ovarian cancer metastasis. Furthermore, we investigated whether small HA oligomers, previously shown to inhibit tumor growth [43–45], could be used block pericellular matrix formation and versican effects on ovarian cancer cell metastatic behavior.
Materials and methods
Cell culture
The human ovarian cancer cell lines OVCAR-3 and SKOV-3 were obtained from American Type Culture Collection (ATCC, Manassas, VA). OVCAR-5 cells were obtained from Dr. Stephen Williams (Fox Chase Cancer Center, Philadelphia, PA, USA). All ovarian cancer cell lines were maintained in RPMI 1640 medium supplemented with 4 mM L-glutamine, antibiotics (100 U penicillin G, 100 μg/ml streptomycin sulfate and 0.25 μg/ml amphotericin B, Sigma–Aldrich, St Louis, MO, USA). OVCAR-3 and SKOV-3 cells were supplemented with 5% FBS (Sigma–Aldrich) whilst OVCAR-5 cells were supplemented with 10% FBS and 7.5 μg/ml insulin. All cell lines were maintained at 37°C in an environment of 5% CO2.
Versican purification
Versican was isolated from the CM from CHO cells transfected to overexpress V1 versican (CHO V1) kindly provided by Prof R LeBaron (University of Texas at San Antonio) [46] using a combination of anion exchange and gel filtration chromatography as described previously [42]. Versican-containing fractions detected by dot blot using the 12C5 mouse monoclonal versican antibody (Developed by Dr Richard Asher and obtained from by the Developmental Studies Hybridoma Bank, NICHD, University of Iowa, USA) were then pooled and concentrated 10–30 fold using Centriprep centrifugal filters (Amicon Bioseparations, Bedford, MA, USA) and NanosepTM microconcentrators (Pall Gelman Laboratory, Ann Arbor, MI, USA) with Mr 50,000 and 300,000 cut-offs, respectively. The molecular integrity of the purified versican samples was determined by immunoblotting with 12C5 versican antibody using non-reducing conditions. Visualization was achieved by anti-mouse IgG peroxidase-conjugated secondary antibodies (Chemicon Australia, Sydney, Australia) with enhanced chemiluminescence (ECL, Amersham Biosciences, Little Chalfont, Buckinghamshire, UK) (Supplemental Fig 1a). Identical gels were stained using silver stain (BioRad, Hercules, CA, USA) to assess the purity of the versican fractions (Supplemental Fig 1b). Versican concentration was determined using an in-house ELISA assay as described previously [42].
Motility and invasion assay
Ovarian cancer cells (OVCAR-5, OVCAR-3, and SKOV-3) were trypsinized, washed, and resuspended at a concentration of 1 × 106 cells/ml in RPMI media containing 0.1% BSA and antibiotics, and labeled with calcein-AM (1 μg/ml, Invitrogen, Carlsbad, CA, USA) as described previously [47]. Cells were centrifuged and washed three times with 0.1% BSA in RPMI and subsequently resuspended at a concentration of 1 × 106 cells/ml with 0.1% BSA RPMI or rV1 versican (0–10 U/ml) and 20 μg/ml HA for 3 h at room temperature on a Nutator. Additional experiments were performed in the presence or absence of HA oligomers (HYA-OLIGO6-10, 250 μg/ml, North Star Bioproducts, Associates of Cape Cod, Inc, East Falmouth, MA, USA) or rat anti-CD44 (20 μg/ml, clone A020, Calbiochem, Merck, Darmsyadt, Germany) or control IgG immunoglobulins (Sigma–Aldrich). An aliquot (10 μl) of these cells (3 × 103 cells) from each treatment were mixed with 200 μl of a suspension of human red blood cells (107 cells/ml, 0.1% BSA in PBS) and allowed to attach in 48-well plates for 2 h at 37°C for assessment of pericellular matrix formation. For motility and invasion assays the cell suspension (50,000 cells/well in 50 μl) was added to either the top of uncoated 12-μm filter inserts (96-well plate, ChemoTx, Neuro Probe Inc, Gaitherburg, MD, USA) or to 12-μm filters coated with Geltrex (ECM matrix derived from Engelbroth-Holm-Swarm (EHS) tumors similar to Matrigel, 0.6 μl/well, 9 mg/ml, Invitrogen) containing 30 μl of 10% FBS RPMI as a chemoattractant in the lower chamber. Cells were allowed to migrate for 6 h at 37°C in an environment of 5% CO2 in air. Non-migratory cells on the top side of the filter were gently removed with a moistened cloth and the fluorescence was measured as described previously [47].
Red blood cell exclusion assay
OVCAR-3, OVCAR-5, or SKOV-3 cells were plated into 48-well plates at low density (3000 cells/well) in RPMI medium containing 0.1% BSA (serum-free RPMI) and treated with CHO V1 CM, CHO K1 CM or purified rV1 versican (0–10 U/ml) and HA (20 μg/ml, high MW from human umbilical cord, Sigma Ulrich) for 3–24 h. To demonstrate the necessity for CD44 for pericellular matrix formation, cells were additionally treated with rat anti-CD44 antibody (20 μg/ml, clone A020, Calbiochem) or control IgG (20 μg/ml Sigma–Aldrich). Pericellular matrix formation was visualised by a red blood cell exclusion assay [42] using an inverted microscope with Hoffman interference optics (BX50, Olympus Australia, Sydney, Australia). The proportions of cells with a surrounding zone excluded by red blood cells were counted in 10 random fields. To demonstrate the dependence on HA for the formation of the pericellular matrix, cells were additionally treated with Streptomyces hyaluronidase (Hase, 10 U/ml, Sigma–Aldrich) for 30 min at room temperature.
Wound migration assays and time-lapse photography
Wound migration assays were performed using OVCAR-5 and SKOV-3 cells (2 × 104 cells/well) cultured in 8-well chamber slides (Nuclon™ Lab-Tek II Chamber slide, RS Glass Slide, Roskilde, Denmark) in 500 μl of 5% FBS RPMI for 3–4 days. The resulting confluent cell monolayers were wounded and washed to remove floating cells prior to treating with CHO K1 CM (control medium) or CHO V1 CM (~5 U/ml versican). Cell migration was monitored over a 24 h treatment period using an IX 81 microscope (Olympus, Australia) equipped with a 37°C incubator (Solent Scientific, Segensworth, UK) and aerated with 5% CO2 in oxygen. Pericellular matrix formation and directional movement were assessed by particle exclusion assays (107 red blood cells/ml) and time-lapse photography over a 3 h period, respectively, using AnalysisTM software (Soft Imaging System, Munster, Germany). The number of migrating cells, the direction of motion and the proportion of cells forming a pericellular matrix were estimated. CD44 and HA abundance and localization in motile ovarian cancer cells were determined as described previously after 24 h treatment with CHO V1 or CHO K1 CM [42].
Ovarian cancer cell adhesion assays
LP-9 peritoneal cells were plated at 16,000 cells/well in 96-well plates for 48 h. Confluent monolayers were washed with RPMI media containing 0.1% BSA and antibiotics for 30 min prior to the adhesion assays. Ovarian cancer cell lines (OVCAR-5 and SKOV-3) were trypsinized, washed, and resuspended at a concentration of 1 × 106 cells/ml in RPMI medium containing 0.1% BSA and antibiotics, and labeled with calcein-AM (1 μg/ml) as described previously [47]. Cells were washed and mixed at a concentration of 100,000 cells/ml with PBS or HA (20 μg/ml) in the presence (CHO-V1) or absence (CHO-K1) of versican containing CM for 3 h at room temperature on a Nutator. Additional experiments were performed in the presence of HAse (10 units/ml) or increasing concentrations of HA oligomers (0–250 μg/ml). The cell suspension (80 μl) was added to each well of LP-9 cell monolayers, and cells were allowed to adhere for 8 min. LP-9 cell monolayers were subsequently washed three times with RPMI medium containing 0.1% BSA and antibiotics. Fluorescence of adhering cells was measured with excitation and emission filters at 485 and 520 nm, respectively using a fluorescent plate reader (Fluostar Galaxy, BMG Labtech Offenburg, Germany) as described previously [47].
Flow cytometry
Ovarian cancer cells (OVCAR-3, SKOV-3, and OVCAR-5) were trypsinized and blocked with 10% normal donkey serum for 30 min prior to incubating with rat anti-CD44 antibody (0.75 μg/ 5 × 105 cells, clone A020, Calbiochem) in 500 μl of 0.1% BSA RPMI for 1 h on a Nutator. Cells were washed 3 times with 0.1% BSA RPMI and subsequently incubated with 5 μg of donkey anti-rat-FITC in 500 μl 0.1% BSA RPMI for 30 min. Following extensive washes with 0.1% BSA PBS, cells were resuspended in 500 μl of 0.1% BSA PBS and 10,000 events were analyzed using a FACScan (BioRad) and FACSDiva v6.0 software (BioRad).
Statistical analyses
All analyses were performed using the SPSS 15.0 for Windows Software (SPSS Inc., Chicago, IL, USA). The one way ANOVA test, Student’s t-test, and the Dunnett C post-hoc test were used to determine statistical significance between control and treatment groups. Statistical significance was accepted at P < 0.05.
Results
Versican induces pericellular matrix formation by ovarian cancer cells
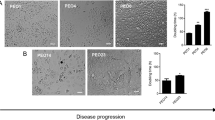
Negligible pericellular matrix formation was detected around ovarian cancer cells (OVCAR-3, OVCAR-5, and SKOV-3) under control conditions (CHO K1, control media) in the absence of versican (Fig. 1a, b, c). Treatment with either conditioned medium (CM) containing versican, CHO V1 (Fig. 1a, b) or purified recombinant versican (rV1) (Fig. 1c) in the presence of exogenous HA resulted in increased pericellular matrix formation by OVCAR-5 and SKOV-3 cells but not OVCAR-3 cells. A 3 h treatment with increasing concentrations of purified rV1 versican and exogenous HA resulted in pericellular matrix formation by OVCAR-5 in a dose-dependent manner (Fig. 1c). Pericellular matrix formation was, however, not induced in the ovarian cancer cells following treatment with HA or versican alone (Fig. 1c). Maximal pericellular matrix was observed (18% of OVCAR-5 cells) following treatment with 5 U/ml rV1 versican. Treatment with 5 U/ml rV1 versican resulted in pericellular matrix formation in 17% of SKOV-3 ovarian cancer cells (Fig. 1c). Pericellular matrix formation was dependent on the presence of HA as treatment with hyaluronidase (Hase 10 U/ml) almost completely removed the pericellular matrix around OVCAR-5 and SKOV-3 cells (Figs. 1b and 2a). The ability of ovarian cancer cells to form a pericellular matrix correlated with expression of the HA receptor, CD44. Membranous CD44 was detected in 27% of OVCAR-5 and 13.4% SKOV-3 but undetectable in OVCAR-3 cells by immunocytochemistry when plated at low density (Fig. 2b). By FACS analysis, up to 92% of OVCAR-5 and 82% of SKOV-3 cells were positively labeled with the CD44 antibody, whilst only 3.6% of OVCAR-3 cells were CD44 positive (Supplementary Fig. 2).
Versican promotes the formation of a pericellular matrix by ovarian cancer cells. a Pericellular matrix formation by ovarian cancer cells following 24 h treatment with versican was assessed using a particle exclusion assay. Ovarian cancer cells (3 × 103) were plated in 48-well tissue, cultured and treated with versican containing CHO V1 CM (~5 U/ml) or CHO K1 from parental cells not expressing versican. Red blood cell diameter = 7 μm. The white arrows illustrate OVCAR-5 cells and SKOV-3 cells with a prominent polar pericellular matrix. b Pericellular matrix formation by ovarian cancer cells following treatment with CHO V1 and CHO K1 CM. Structural necessity for HA within the pericellular matrix was confirmed by treatment with hyaluronidase (Hase) (30 min at room temperature, 10 U/ml). Data represent percentage (mean ± SD) of cells (n = 100) with pericellular matrix from triplicate determinations in three separate experiments. c Effect of purified rV1 versican on pericellular matrix formation by ovarian cancer cells. Ovarian cancer cells (OVCAR-5, OVCAR-3, and SKOV-3, 5 × 105 cells/ml) were treated with recombinant versican (0.1–10.0 U/ml) + HA (20 μg/ml) or PBS + HA (20 μg/ml) in 0.1% BSA RPMI on a rotating platform for 3 h at room temperature and plated in 48-well plates (3 × 103 cells) with red blood cells for 2 h prior to assessing pericellular matrix formation. Data represent percentage (mean ± SD) of cells (n = 100) with pericellular matrix from at least three determinations in three independent experiments. *Significantly different from control at P < 0.05 (Student t test)
Pericellular matrix formation by ovarian cancer cells is HA dependent and parallels CD44 expression. a Versican induced pericellular matrix formation was reduced following treatment with hyaluronidase (0–30 min at room temperature, 10 U/ml). b CD44 expression by ovarian cancer cell lines. Ovarian cancer cells (OVCAR-3, OVCAR-5, and SKOV-3) were plated at a low density in 8-well chamber slides. Cells were fixed and incubated with CD44 monoclonal antibody. Immunostaining was detected by immunofluorescence and nuclei are counterstained with Hoescht dye. Magnification bar = 20 μm
Versican promotes ovarian cancer cell motility and invasion
We investigated the effects of rV1 versican on ovarian cancer motility and invasion using a modified chemotaxis assay. rV1 versican treatment for 9 h significantly increased the motility of ovarian cancer OVCAR-5 and SKOV-3 but not OVCAR-3 cells. rV1 versican treatment significantly increased OVCAR-5 and SKOV-3 motility (Fig. 3a). Maximal effects of rV1 on OVCAR-5 cell motility were observed at 1 U/ml rV1 versican (125% of control) (Fig. 3a). rV1 versican also significantly increased invasion of OVCAR-5 and SKOV-3 cells through EHS basement membrane proteins (Geltrex) by up to 20% (Fig. 3b, P < 0.0001). Maximal effect on OVCAR-5 cell invasion (119% of control) was observed with 5 U/ml rV1 versican. rV1 versican treatment did not, however, affect OVCAR-3 cell motility (Fig. 3a) or invasion (Fig. 3b).
Versican promotes ovarian cancer motility and invasion. Ovarian cancer cells (OVCAR-5, OVCAR-3, and SKOV-3) were treated as in Fig. 1c. A cell suspension (50,000 cells/well in 50 μl) was added to the top of uncoated 12-μm filter inserts for motility assays (a) or to 12-μm filters coated with Geltrex (0.6 μl/well, 9 mg/ml) for invasion assays (b). Cells were allowed to migrate for 6 h at 37°C in an environment of 5% CO2 in air using 10% FBS in RPMI as a chemoattractant in the lower chamber. The fluorescence of migratory cells was measured at 485–520 nm. Data are expressed as percentage of control ± SD of three determinations from three independent experiments. *Significantly different from control at P < 0.05 (Student t test)
Pericellular matrix formation in migrating ovarian cancer cells
A wound migration assay was used to examine effects on directional ovarian cancer cell motility following versican treatment. A 24 h treatment with CHO-V1 CM (5 U/ml versican) and HA (20 μg/ml) significantly increased the number of SKOV-3 and OVCAR-5 cells entering the wounded area by two fold compared with treatment with CHO K1 CM and HA (20 μg/ml) in the absence of any versican (Fig. 4a, b). Time lapse photography combined with a particle exclusion assay enabled simultaneous observation of directional cell movement and pericellular matrix formation. A polar pericellular matrix was consistently observed by motile SKOV-3 cells migrating into the scratch wound over a 2 h treatment period (Fig. 5a, white asterisks and Supplementary material 3). No pericellular matrix was observed in non-motile cells (Fig. 5a, black asterisks and Supplementary material 3). Interestingly, immunohistochemical localization of CD44 demonstrated polar expression of CD44 in SKOV-3 and OVCAR-5 cells following treatment with versican-containing media (Fig. 5b,c). Polarized CD44 was not observed in SKOV-3 cells treated with control media (Fig. 5b). Polarized CD44 and HA staining is illustrated in a motile OVCAR-5 cell following treatment with versican containing media but was not present in non-motile OVCAR-5 cells (Fig. 5c).
Versican promotes ovarian cancer cell motility in a wound migration assay. Confluent monolayers of SKOV-3 cells (a) and OVCAR-5 (b) were wounded and treated with CHO K1 CM or CHO V1 CM for 24 h in presence of HA (20 μg/ml). Data represent number of cells which migrated into the wounded area (mean ± SD, eight determinations from two independent experiments). *Significantly different from CHO K1 at P < 0.05 (Student t test)
Versican promotes formation of a polarized pericellular matrix by OVCAR-5 cells. a The confluent SKOV-3 monolayer was wounded and treated with CHO V1 CM (5 U/ml) for 16 h in the presence of HA (20 μg/ml). The white asterisks indicate motile SKOV-3 cell with a polar pericellular matrix observed over a 2 h time period. The black asterisks indicate non-motile SKOV-3 cell lacking a pericellular matrix. The white arrows indicate the direction of cell movement. Red blood cells diameter = 7 μm. b CD44 expression in SKOV-3 cells following 24 h treatment with HA (20 μg/ml) and CHO K1 or CHO V1 CM (~5 U/ml). White dashed line indicates edge of wound. White arrows indicate polar CD44 expression. c Co-localization of polar CD44 and HA in a motile OVCAR-5 cell following treatment with CHO V1 CM (~5 U/ml) in the presence of HA (20 μg/ml). White dashed line indicates the edge of the wound. White arrows indicate polar CD44 and HA expression
HA-oligomers and anti-CD44 can block pericellular matrix formation by OVCAR-5 and their motility and invasion induced by versican treatment
We investigated whether small HA oligomers (6–10 disaccharides), previously shown to block pericellular matrix formation around chondrocytes [48, 49], could inhibit pericellular matrix formation by OVCAR-5 cells and their motility and invasion induced by versican. Treatment with HA oligomers (250 μg/ml) significantly inhibited pericellular matrix formation by OVCAR-5 cells (Fig. 6a, P < 0.0001) and significantly reversed OVCAR-5 motility (Fig. 6b, P = 0.007) and invasion (Fig. 6c, P = 0.013) induced by versican treatment.
HA oligosaccharides block the formation of pericellular matrix and OVCAR-5 cell motility, invasion induced by versican and adhesion to peritoneal cells. OVCAR-5 cells were treated as in Fig. 1b in the presence or absence of HA oligosaccharides (250 μg/ml). a Pericellular matrix formation was assessed as described in Fig. 1. Data represents percentage (mean ± SD) of cells (n = 100). *Significantly different from control at P < 0.05, one-way ANOVA. Motility (b) and invasion (c) of OVCAR-5 were performed as described for Fig. 3. The fluorescence of migratory cells was measured at 485–520 nm. Data are expressed as percentage of control media, mean ± SD from at least three independent experiments performed in triplicate. *Significantly different from control at P < 0.05, one-way ANOVA. d Adhesion assays were performed as described in “Material and methods” section. Ovarian cancer cells (OVCAR-5 & SKOV-3) were incubated with control media (0.1% BSA in RPMI), HA (20 μg/ml) alone or in the presence of CM containing versican (CHO V1) or no versican containing (CHO K1) for 3 h. Additional cells were also treated with Hase (10 U/ml). *Significantly different from control at P < 0.05, one-way ANOVA. e Ovarian cancer cells (OVCAR-5) were treated with HA (20 μg/ml) or PBS with increasing concentrations of HA oligosaccharides (5–250 μg/ml) for 3 h. *Significant differences from PBS control at P < 0.05, one-way ANOVA
Additionally, we investigated whether direct blocking of CD44 with a neutralizing antibody would inhibit the formation of pericellular matrix formation as well as the motility and invasion induced by versican and HA treatment. We observed that the addition of 20 μg/ml of the neutralizing CD44 antibody completely blocked the formation of pericellular matrix by OVCAR-5 cells compared with control antibody treatment (Fig. 7a, P < 0.0001). The increased motility and invasion observed following treatment of OVCAR-5 cells with versican and HA was also completely abrogated by the addition of 20 μg/ml of CD44 antibody (Fig. 7b; P < 0.0001 and 7c; P = 0.029) compared with treatment with control antibody.
CD44 neutralizing antibody blocks formation of pericellular matrix and OVCAR-5 cell motility, invasion induced by versican and HA. OVCAR-5 cells were treated as in Fig. 1b with control medium or versican + HA in the presence of CD44 antibody (20 μg/ml) or control IgG (20 μg/ml). a Pericellular matrix formation was assessed as described in Fig. 1. Data represents percentage (mean ± SD) of cells (n = 100) from three independent experiments. Motility (b) and invasion (c) of OVCAR-5 were performed as described for Fig. 3. The fluorescence of migratory cells was measured at 485–520 nm. Data are expressed as percentage of control media, mean ± SD from at least three independent experiments performed in triplicate. *Significantly different from control media at P < 0.05, Student t test. **Significantly different from IgG control at P < 0.05, Student t test
HA oligomers inhibit ovarian cancer adhesion to peritoneal cells
Cell adhesion experiments were conducted to determine whether versican treatment could augment ovarian cancer cell adhesion to peritoneal cells (LP-9). Whilst HA significantly increased adhesion of OVCAR-5 and SKOV-3 cell to LP-9 cells (Fig. 6d, P < 0.0001), treatment with versican containing media (CHO V1) in the presence of HA did not have any additional effect on ovarian cancer cell adhesion (Fig. 6d). Treatment with Hase (10 U/ml) and HA oligomers (250 μg/ml) significantly reduced OVCAR-5 cell adhesion to LP-9 cells to below that of the control level in the presence and absence of exogenous HA (Fig. 6e, P < 0.0001).
Discussion
Our study demonstrates for the first time that ovarian cancer cells can assemble a pericellular matrix utilizing the ECM components HA and versican which promotes their motility and invasion in vitro. These findings indicate a role for versican and HA in ovarian cancer metastasis. Furthermore, we show that small HA oligomers (6–10 disaccharides) inhibit versican/HA pericellular matrix formation around CD44-expressing ovarian cancer cells. They also block the motility and invasion induced by versican and ovarian cancer adhesion to peritoneal cells. Our results therefore encourage further investigations into utilizing HA oligomers as a therapeutic to block ovarian cancer metastasis.
The formation of a HA pericellular matrix has been shown to be essential for breast and prostate carcinoma cells for specific adhesion to bone marrow endothelial cells and contributes to the common bone metastasis by these malignancies [50, 51]. We demonstrate the formation of a polar HA/versican pericellular matrix in motile ovarian cancer cells in the presence of both HA and versican but not HA or versican alone. These findings indicate that both versican and HA are required for pericellular matrix formation. Pericellular matrix formation is only observed in CD44 positive ovarian cancer cells and is particularly visible at the trailing edge of motile ovarian cancer cells. These findings are in agreement with our previous work demonstrating the formation of a polar HA/versican matrix in motile prostate cancer cells [42]. Furthermore, we demonstrate that versican treatment can induce ovarian cancer cell invasion through an ECM barrier. These findings are also supported by a recent study demonstrating that versican treated ovarian cancer cells have increased invasion potential [52]. Our results suggest that formation of a CD44/HA/versican macromolecular complex promotes the motility and invasion of ovarian cancer cells. The dependence of CD44 for the versican and HA mediated effects was confirmed by the inhibition of pericellular matrix formation as well as motility and invasion of OVCAR-5 cells following treatment with CD44 neutralizing antibody in the presence of versican and HA.
Our study verifies the observations of previous work which demonstrated that HA plays an important role in promoting cell attachment to peritoneal cells [22–24, 53]. However, versican treatment in the presence of HA did not further increase ovarian cancer cell adhesion to peritoneal cells. Our findings support the notion that adhesion of ovarian cancer cells to peritoneal cells is mediated by HA binding CD44 found on both cell types which allows a strong anchoring and interaction between ovarian cancer and peritoneal cells. Our working model proposes that versican from the peritumoral stroma binds HA in the ECM (Fig. 8). The formation of a stabilized HA/versican pericellular matrix surrounding ovarian cancer cells, protects the ovarian cancer cells against the mechanical forces in the peritoneal cavity and enables strong ovarian cancer cell adhesion to CD44 expressed by peritoneal cells. This provides the basis for subsequent ovarian cancer dissemination throughout the abdominal cavity.
Proposed model of HA, CD44 and versican interactions between ovarian cancer and peritoneal cells. The formation of a stabilized HA/versican pericellular matrix surrounding ovarian cancer cells increases motility and protects the ovarian cancer cells against the mechanical forces in the peritoneal cavity and enable ovarian cancer cells to strongly adhere to CD44 expressed on peritoneal cells. This allows subsequent ovarian cancer invasion and peritoneal dissemination
The molecular mechanisms whereby a HA/versican pericellular matrix promotes the motility and invasion of cancer cells have not been elucidated in this study but are likely to involve signaling pathways activated by HA-CD44 interactions. HA-CD44 interactions have been shown to regulate several oncogenic pathways important for mediating motility and invasion of ovarian cancer cells [54–61]. The assembly of HA pericellular matrices by articular chondrocytes during joint formation has been shown to involve the activation of the MEK-ERK cascade [62]. More recently, p38 MAPK inhibitors have been shown to block HA pericellular matrix assembly in embryonic chicken joints [63]. It is conceivable that activation of p38 MAPK and ERK leading to the accumulation of HA-rich ECM may also play a role in cancer cell metastatic behaviour.
It is known that HA oligomers compete with larger HA polymers for CD44 binding [44, 64]. Whilst HA fragments in the 30–50 disaccharide range have been shown to be angiogenic [65], smaller HA fragments have been shown to inhibit tumor growth [43–45] and block the formation of a pericellular matrix formation around chondrocytes [48, 49]. Small HA oligomers in the 6–10 disaccharides range appear to have unique biological properties and can also inhibit a variety of tumors in vitro and in vivo [43, 44, 66–69]. Ghatak et al. [44] have demonstrated that HA oligomers can inhibit PI 3-kinase activity and phosphorylation of Akt and stimulate the expression of PTEN, a phosphatase which degrades the major signaling product of PI 3-kinase action, phosphoinositide 3,4,5-trisphosphate. It is therefore likely that HA oligomers inhibit tumor growth by suppressing the PI 3-kinase/Akt cell survival pathway [44, 66]. In our study, we have demonstrated that small HA oligomers (6–10 disaccharides) can inhibit the formation of HA-versican pericellular matrix around CD44 expressing ovarian cancer cells and the motility and invasion induced by versican treatment. Furthermore, these small HA oligomers also inhibited the increase in peritoneal ovarian cancer adhesion induced by HA treatment and peritoneal ovarian cancer adhesion in the absence of HA. These findings suggest that HA oligomers act by blocking HA binding to CD44 on the peritoneal cells. A recent study demonstrated that HA octasaccharides inhibited the formation of pericellular matrix by osteosarcoma cells and reduced HA accumulation in local tumors, tumor growth, motility, invasion, and the formation of distant lung metastases [67].
More recently HA oligomers have also been shown to reverse chemotherapy resistance of myeloid leukemia [70] and lymphoma cell lines [71] and to increase chemotherapy sensitivity in multi-drug resistant malignant peripheral nerve matrix tumor [68] and ovarian cancer cells [72]. Our results support further investigations into the use of HA oligomers either alone or in conjunction with chemotherapeutic agents to block adhesion and metastasis of CD44 positive ovarian cancers. A weekly intra-peritoneal administration of HA oligomers (500 μg) appears to have been well tolerated by SCID mice xenografted with ovarian cancer cells [72]. Intra peritoneal administration of a bioconjugate of HA and paclitaxel was well tolerated and significantly decreased growth human ovarian cancer xenografts in SCID mice compared with paclitaxel alone [73, 74]. More recently phase I and phase II clinical trials have demonstrated that HA and the chemotherapy drug irinotecan can be safely administered together to patients with metastatic colon cancer and improve progression free survival [75, 76]. We envisage that HA oligomers could be administered intra-peritoneally together with chemotherapy drugs to patients following debulking surgery to inhibit residual CD44 positive ovarian cancer cells from repopulating and invading the peritoneal surfaces.
In conclusion, our results show that ovarian cancer cells have the ability to recruit stromal ECM components to form a pericellular matrix which in turn promotes ovarian cancer cell motility and invasion. HA oligomers can block this mechanism and are therefore a potential therapeutic against ovarian cancer progression.
Abbreviations
- BSA:
-
Bovine serum albumin
- CM:
-
Conditioned media
- ECM:
-
Extracellular matrix
- EHS:
-
Engelbreth-Holm-Swarm
- FBS:
-
Fetal bovine serum
- FIGO:
-
Federation of Gynecologist and Obstetricians
- ERK:
-
Extracellular signal-regulated kinase
- HA:
-
Hyaluronan
- Hase:
-
Hyaluronidase
- MAPK:
-
Mitogen-activated protein kinase
- PI 3:
-
Phosphatidylinositol 3
References
Stewart BW (2003) WHO world cancer report. Lyon, France
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics, 2009. CA Cancer J Clin 59(4):225–249
Doig T, Monaghan H (2006) Sampling the omentum in ovarian neoplasia: when one block is enough. Int J Gynecol Cancer 16(1):36–40
Liotta LA, Kohn EC (2001) The microenvironment of the tumour-host interface. Nature 411(6835):375–379
Zigrino P, Loffek S, Mauch C (2005) Tumor-stroma interactions: their role in the control of tumor cell invasion. Biochimie 87(3–4):321–328
Ricciardelli C, Rodgers RJ (2006) Extracellular matrix of ovarian tumors. Semin Reprod Med 24(4):270–282
Gardner MJ, Jones LM, Catterall JB et al (1995) Expression of cell adhesion molecules on ovarian tumour cell lines and mesothelial cells, in relation to ovarian cancer metastasis. Cancer Lett 91(2):229–234
Gardner MJ, Catterall JB, Jones LM et al (1996) Human ovarian tumour cells can bind hyaluronic acid via membrane CD44: a possible step in peritoneal metastasis. Clin Exp Metastasis 14(4):325–334
Wilson KE, Bartlett JM, Miller EP et al (1999) Regulation and function of the extracellular matrix protein tenascin-C in ovarian cancer cell lines. Br J Cancer 80(5–6):685–692
Strobel T, Cannistra SA (1999) Beta1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol Oncol 73(3):362–367
Anttila MA, Tammi RH, Tammi MI et al (2000) High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res 60(1):150–155
Voutilainen K, Anttila M, Sillanpaa S et al (2003) Versican in epithelial ovarian cancer: relation to hyaluronan, clinicopathologic factors and prognosis. Int J Cancer 107(3):359–364
Freedman RS, Deavers M, Liu J et al (2004) Peritoneal inflammation––a microenvironment for epithelial ovarian cancer (EOC). J Transl Med 2(1):23
Salani R, Neuberger I, Kurman RJ et al (2007) Expression of extracellular matrix proteins in ovarian serous tumors. Int J Gynecol Pathol 26(2):141–146
Kenny HA, Kaur S, Coussens LM et al (2008) The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest 118(4):1367–1379
Heyman L, Kellouche S, Fernandes J et al (2008) Vitronectin and its receptors partly mediate adhesion of ovarian cancer cells to peritoneal mesothelium in vitro. Tumour Biol 29(4):231–244
Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4(7):528–539
Tammi MI, Day AJ, Turley EA (2002) Hyaluronan and homeostasis: a balancing act. J Biol Chem 277(7):4581–4584
Hiltunen EL, Anttila M, Kultti A et al (2002) Elevated hyaluronan concentration without hyaluronidase activation in malignant epithelial ovarian tumors. Cancer Res 62(22):6410–6413
Boregowda RK, Appaiah HN, Siddaiah M et al (2006) Expression of hyaluronan in human tumor progression. J Carcinog 5:2
Jojovic M, Delpech B, Prehm P et al (2002) Expression of hyaluronate and hyaluronate synthase in human primary tumours and their metastases in scid mice. Cancer Lett 188(1–2):181–189
Yeo TK, Nagy JA, Yeo KT et al (1996) Increased hyaluronan at sites of attachment to mesentery by CD44-positive mouse ovarian and breast tumor cells. Am J Pathol 148(6):1733–1740
Catterall JB, Jones LM, Turner GA (1999) Membrane protein glycosylation and CD44 content in the adhesion of human ovarian cancer cells to hyaluronan. Clin Exp Metastasis 17(7):583–591
Casey RC, Skubitz AP (2000) CD44 and beta1 integrins mediate ovarian carcinoma cell migration toward extracellular matrix proteins. Clin Exp Metastasis 18(1):67–75
Lancaster JM, Dressman HK, Clarke JP et al (2006) Identification of genes associated with ovarian cancer metastasis using microarray expression analysis. Int J Gynecol Cancer 16(5):1733–1745
Bignotti E, Tassi RA, Calza S et al (2007) Gene expression profile of ovarian serous papillary carcinomas: identification of metastasis-associated genes. Am J Obstet Gynecol 196(3):245 e1–245 e11
Bast RC (2004) Early detection of ovarian cancer: new technologies in pursuit of a disease that is neither common nor rare. Trans Am Clin Climatol Assoc 115:233–248
Wight TN (2002) Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol 14(5):617–623
Ricciardelli C, Sakko AJ, Ween MP et al (2009) The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev 28:233–245
Ricciardelli C, Mayne K, Sykes PJ et al (1998) Elevated levels of versican but not decorin predict disease progression in early-stage prostate cancer. Clin Cancer Res 4(4):963–971
Ricciardelli C, Brooks JH, Suwiwat S et al (2002) Regulation of stromal versican expression by breast cancer cells and importance to relapse-free survival in patients with node-negative primary breast cancer. Clin Cancer Res 8(4):1054–1060
Hanekamp EE, Gielen SC, Smid-Koopman E et al (2003) Consequences of loss of progesterone receptor expression in development of invasive endometrial cancer. Clin Cancer Res 9(11):4190–4199
Suwiwat S, Ricciardelli C, Tammi R et al (2004) Expression of extracellular matrix components versican, chondroitin sulfate, tenascin, and hyaluronan, and their association with disease outcome in node-negative breast cancer. Clin Cancer Res 10(7):2491–2498
Pukkila MJ, Kosunen AS, Virtaniemi JA et al (2004) Versican expression in pharyngeal squamous cell carcinoma: an immunohistochemical study. J Clin Pathol 57(7):735–739
Pirinen R, Leinonen T, Bohm J et al (2005) Versican in nonsmall cell lung cancer: relation to hyaluronan, clinicopathologic factors, and prognosis. Hum Pathol 36(1):44–50
Kodama J, Hasengaowa, Kusumoto T et al (2007) Versican expression in human cervical cancer. Eur J Cancer 43(9):1460–1466
Kodama J, Hasengaowa, Kusumoto T et al (2007) Prognostic significance of stromal versican expression in human endometrial cancer. Ann Oncol 18(2):269–274
Pukkila M, Kosunen A, Ropponen K et al (2007) High stromal versican expression predicts unfavourable outcome in oral squamous cell carcinoma. J Clin Pathol 60(3):267–272
Casey RC, Oegema TR Jr, Skubitz KM et al (2003) Cell membrane glycosylation mediates the adhesion, migration, and invasion of ovarian carcinoma cells. Clin Exp Metastasis 20(2):143–152
Lu KH, Patterson AP, Wang L et al (2004) Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clin Cancer Res 10(10):3291–3300
Evanko SP, Angello JC, Wight TN (1999) Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19(4):1004–1013
Ricciardelli C, Russell DL, Ween MP et al (2007) Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J Biol Chem 282(14):10814–10825
Zeng C, Toole BP, Kinney SD et al (1998) Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer 77(3):396–401
Ghatak S, Misra S, Toole BP (2002) Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem 277(41):38013–38020
Ward JA, Huang L, Guo H et al (2003) Perturbation of hyaluronan interactions inhibits malignant properties of glioma cells. Am J Pathol 162(5):1403–1409
LeBaron RG, Zimmermann DR, Ruoslahti E (1992) Hyaluronate binding properties of versican. J Biol Chem 267(14):10003–10010
Ween MP, Lokman NA, Hoffmann P et al (2010) Transforming growth factor beta-induced protein secreted by peritoneal cells increases the metastatic potential of ovarian cancer cells. Int J Cancer. doi:10.1002/ijc.25494
Knudson W, Knudson CB (1991) Assembly of a chondrocyte-like pericellular matrix on non-chondrogenic cells. Role of the cell surface hyaluronan receptors in the assembly of a pericellular matrix. J Cell Sci 99(Pt 2):227–235
Knudson W, Aguiar DJ, Hua Q et al (1996) CD44-anchored hyaluronan-rich pericellular matrices: an ultrastructural and biochemical analysis. Exp Cell Res 228(2):216–228
Simpson MA, Reiland J, Burger SR et al (2001) Hyaluronan synthase elevation in metastatic prostate carcinoma cells correlates with hyaluronan surface retention, a prerequisite for rapid adhesion to bone marrow endothelial cells. J Biol Chem 276(21):17949–17957
Draffin JE, McFarlane S, Hill A et al (2004) CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res 64(16):5702–5711
Ghosh S, Albitar L, Lebaron R et al (2010) Up-regulation of stromal versican expression in advanced stage serous ovarian cancer. Gynecol Oncol 119:114–120
Tzuman YC, Sapoznik S, Granot D et al (2010) Peritoneal adhesion and angiogenesis in ovarian carcinoma are inversely regulated by hyaluronan: the role of gonadotropins. Neoplasia 12(1):51–60
Bourguignon LY, Zhu H, Chu A et al (1997) Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biol Chem 272(44):27913–27918
Bourguignon LY, Zhu H, Shao L et al (2001) CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem 276(10):7327–7336
Bourguignon LY, Singleton PA, Zhu H et al (2002) Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem 277(42):39703–39712
Turley EA, Noble PW, Bourguignon LY (2002) Signaling properties of hyaluronan receptors. J Biol Chem 277(7):4589–4592
Ghatak S, Misra S, Toole BP (2005) Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem 280(10):8875–8883
Bourguignon LY (2008) Hyaluronan-mediated CD44 activation of Rho GTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol 18(4):251–259
Misra S, Obeid LM, Hannun YA et al (2008) Hyaluronan constitutively regulates activation of COX-2-mediated cell survival activity in intestinal epithelial and colon carcinoma cells. J Biol Chem 283(21):14335–14344
Toole BP (2009) Hyaluronan-CD44 interactions in cancer: paradoxes and possibilities. Clin Cancer Res 15(24):7462–7468
Bastow ER, Lamb KJ, Lewthwaite JC et al (2005) Selective activation of the MEK-ERK pathway is regulated by mechanical stimuli in forming joints and promotes pericellular matrix formation. J Biol Chem 280(12):11749–11758
Lewthwaite JC, Bastow ER, Lamb KJ et al (2006) A specific mechanomodulatory role for p38 MAPK in embryonic joint articular surface cell MEK-ERK pathway regulation. J Biol Chem 281(16):11011–11018
Stern R, Asari AA, Sugahara KN (2006) Hyaluronan fragments: an information-rich system. Eur J Cell Biol 85(8):699–715
Lokeshwar VB, Obek C, Soloway MS et al (1997) Tumor-associated hyaluronic acid: a new sensitive and specific urine marker for bladder cancer. Cancer Res 57(4):773–777
Alaniz L, Garcia MG, Gallo-Rodriguez C et al (2006) Hyaluronan oligosaccharides induce cell death through PI3-K/Akt pathway independently of NF-kappaB transcription factor. Glycobiology 16(5):359–367
Hosono K, Nishida Y, Knudson W et al (2007) Hyaluronan oligosaccharides inhibit tumorigenicity of osteosarcoma cell lines MG-63 and LM-8 in vitro and in vivo via perturbation of hyaluronan-rich pericellular matrix of the cells. Am J Pathol 171(1):274–286
Slomiany MG, Dai L, Bomar PA et al (2009) Abrogating drug resistance in malignant peripheral nerve sheath tumors by disrupting hyaluronan-CD44 interactions with small hyaluronan oligosaccharides. Cancer Res 69(12):4992–4998
Alaniz L, Rizzo M, Malvicini M et al (2009) Low molecular weight hyaluronan inhibits colorectal carcinoma growth by decreasing tumor cell proliferation and stimulating immune response. Cancer Lett 278(1):9–16
Cui X, Zhou S, Xu H et al (2009) Reversal effects of hyaluronan oligosaccharides on adriamycin resistance of K562/A02 cells. Anticancer Drugs 20(9):800–806
Cordo Russo RI, Garcia MG, Alaniz L et al (2008) Hyaluronan oligosaccharides sensitize lymphoma resistant cell lines to vincristine by modulating P-glycoprotein activity and PI3 K/Akt pathway. Int J Cancer 122(5):1012–1018
Slomiany MG, Dai L, Tolliver LB et al (2009) Inhibition of functional hyaluronan-CD44 interactions in CD133-positive primary human ovarian carcinoma cells by small hyaluronan oligosaccharides. Clin Cancer Res 15(24):7593–7601
Auzenne E, Ghosh SC, Khodadadian M et al (2007) Hyaluronic acid-paclitaxel: antitumor efficacy against CD44(+) human ovarian carcinoma xenografts. Neoplasia 9(6):479–486
Banzato A, Bobisse S, Rondina M et al (2008) A paclitaxel-hyaluronan bioconjugate targeting ovarian cancer affords a potent in vivo therapeutic activity. Clin Cancer Res 14(11):3598–3606
Gibbs P, Brown TJ, Ng R et al (2009) A pilot human evaluation of a formulation of irinotecan and hyaluronic acid in 5-fluorouracil-refractory metastatic colorectal cancer patients. Chemotherapy 55(1):49–59
Gibbs P, Clingan PR, Ganju V et al (2010) Hyaluronan-Irinotecan improves progression-free survival in 5-fluorouracil refractory patients with metastatic colorectal cancer: a randomized phase II trial. Cancer Chemother Pharmacol. doi:10.1007/s00280-010-1303-3
Acknowledgments
This work was supported by the University of Adelaide Faculty of Health Sciences (Hilda Farmer Research Fellowship to CR) and the Ovarian Cancer Research Foundation of Australia.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplemental material 1
Versican purification. a. Fractions separated with Sephacryl 400 immunoblotted with 12C5 mouse antibody to human versican. Unconcentrated CM from CHO V1 cells cultured in MEM + 10% FBS (lane 1, 20 μl), CM after Q-Sepharose absorption (lane 2, 20 μl), 2 M NaCl elute (lane 3, 5 μl) which was then loaded onto the Sephacryl column and pooled fractions (lanes 4 and 5, 10 μl of 25× concentrate). b. Corresponding silver stained gel. No contaminating proteins were detected in fraction pools 1 or 2. (PDF 512 kb)
Supplemental material 2
FACS analysis of CD44 staining in OVCAR-5 (a), SKOV-3 (b) and OVCAR-3 (c) cells incubated with no primary antibody or CD44 antibody. (TIFF 340 kb)
Time lapse movie of SKOV-3 cells following treatment with versican containing media (CHO V1) over a 2 h time period.(AVI 4231 kb)
Rights and permissions
About this article
Cite this article
Ween, M.P., Hummitzsch, K., Rodgers, R.J. et al. Versican induces a pro-metastatic ovarian cancer cell behavior which can be inhibited by small hyaluronan oligosaccharides. Clin Exp Metastasis 28, 113–125 (2011). https://doi.org/10.1007/s10585-010-9363-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-010-9363-7