Abstract
Preterm birth is associated with an increased risk for autism spectrum disorder, with various factors proposed to underlie this relationship. The aim of this systematic review was to provide a narrative synthesis of the literature regarding the prenatal, perinatal and postnatal factors associated with autism spectrum disorder in children born preterm. Medline, Embase and PsycINFO databases were searched via Ovid to identify studies published from January 1990 to December 2019. Original studies in which a standardized diagnostic tool and/or clinical assessment was used to diagnose autism, along with a risk factor analysis to identify associated predictors, were included. A total of 11 eligible studies were identified. Male sex, being born small for gestational age and general cognitive impairment were the most robust findings, with each reported as a significant factor in at least two studies. Comparisons across studies were limited by variation in risk factor measurement and gestational age ranges investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is defined by deficits in social communication and interaction as well as restrictive and repetitive behaviors, interests and activities [1]. The prevalence of ASD is higher in children born preterm compared to those born at term [2, 3]; with decreasing gestational age associated with an increased risk of an ASD diagnosis [4,5,6]. Various risk factors from the prenatal period to later childhood have been investigated in an attempt to understand the relationship between autism and preterm birth [3, 7, 8].
The developmental sociobiological vulnerability model, first proposed by Healy et al. [9], attempts to integrate both the biological and environmental factors that underlie the association between preterm birth and various psychiatric and neurodevelopmental disorders such as autism spectrum disorder [9, 10]. According to this theory, very preterm birth (caused by a combination of genetic factors, obstetric issues and other variables) leads to alterations in typical neurodevelopment as a result of perinatal brain injury and/or neonatal pain and stress. This leads to both structural and functional changes in specific brain networks and presents as deficits in cognition and social-emotional functioning. These deficits increase the preterm born child’s social-emotional vulnerability, which may be further mediated by parent stress and mental health. As a result, children born preterm are more vulnerable and at greater risk of negative social experiences, such as bullying and exclusion, which have been associated with increased stress-induced striatal dopamine release and dopamine sensitization in mesolimbic areas. The authors propose that this interplay of biological vulnerabilities and environmental influences are associated with increased risk for the later development of neurodevelopmental disorders such as ASD in children born preterm [9, 10].
Research findings add support to the developmental sociobiological vulnerability model in relation to the factors associated with autism in children born preterm. One study reported that in a sample of children born preterm, being born small for gestational age, internalizing behaviors and maternal depression were significantly associated with a positive screen for symptoms associated with ASD at 2 years of age [11]. It should be noted that while studies show an association between ASD and certain environmental factors, further research is needed to determine whether such factors have a causal link to ASD. In adolescence, increased autism spectrum symptoms have been associated with a low agar score at birth [7], and another study found bronchopulmonary dysplasia to be the only significant neonatal risk factor for autism symptoms at eight years in an extremely low birth weight cohort (mean gestational age 26 weeks) [12]. The authors suggest this may be associated with recurrent oxygen desaturation, which is linked to neonatal encephalopathy [12].
Variation exists in the literature in relation to the risk factors investigated and the tools used to assess for autism in preterm populations. Some studies have adopted standardized diagnostic tools or clinical assessment [6, 13, 14] while others have used parent screening questionnaires [7, 11, 12, 15]. Johnson et al. [3] investigated the utility of the Social Communication Questionnaire, a parent report autism screener, in identifying autism in extremely preterm (EP) born children at 11 years of age. Using the established cut-off score of 15 or greater, the authors found 16% of their EP children screened positive for autism, whereas on diagnostic assessment only 8% met the criteria for autism. The authors suggested a higher proportion of EP survivors are affected by difficulties associated with the autism spectrum than meet the diagnostic criteria.
A high rate of positive screens using parent questionnaire measures have been reported in other studies [15,16,17]. However using such screening measures in young children born preterm, such as toddlers, can be problematic as those with comorbid neurodevelopmental disabilities such as vision, hearing and motor impairments may screen positive on these measures due to their sensory or motor impairments rather than due to true autistic traits, resulting in false-positive cases [16, 17]. Specificity is likely to be compromised when using screening tools in young preterm populations and at risk cases should be monitored and followed-up with formal diagnostic assessment [16, 18].
Autism screening tools are less time intensive compared to diagnostic assessment procedures and so are appealing for research purposes with large population cohorts [18]. Numerous studies have utilized these tools when investigating antecedent risk factors for autism in young preterm populations [11, 15, 16]. However given the previous findings of false-positive screens in young children born preterm with neurodevelopmental disabilities, these studies may be confounded and not accurately reflect the risk factors specific to preterm children with autism confirmed through the more robust methods of diagnostic assessment.
The aim of this systematic review was to provide a narrative synthesis of the literature in relation to prenatal, perinatal and postnatal risk factors associated with ASD in children born preterm, diagnosed either through assessment by a clinician using internationally recognized diagnostic criteria or a standardized diagnostic tool. A synthesis of the literature on this topic was needed to help identify commonalities across study findings, potential areas of future investigation, and interventions which could be targeted.
Methods
The Preferred Reporting for Systematic Reviews and Meta-analyses guidelines (PRISMA) [19] and the Center for Reviews and Dissemination [20] guidance document on systematic reviews in health care were followed in the design and reporting of this systematic review. The protocol outlining the proposed methodology for this systematic review was submitted for registration on PROSPERO prior to commencement. One revision was made to the protocol with the addition of two further exclusion criteria. Conference abstracts were excluded as the limited data available made extraction difficult and the majority of abstracts were later published as peer reviewed articles. Studies in which highly specific preterm subgroups comprised the study sample were also excluded, as these results would not be generalizable to the preterm population on the whole. Examples include studies whose samples consisted exclusively of preterm participants with cerebellar injury [21], abnormal brain imaging [22] or cognitive impairment [23].
Inclusion Criteria
Articles were included in the review if they meet the following criteria:
-
1.
Research article with original data
-
2.
Study population was born after January 1st, 1990, to reflect changes in medical practice which led to increased survival of preterm infants at that time [24, 25]
-
3.
Study population born less than 37 weeks gestation or with low birth weight (≤ 2500 g)
-
4.
Studies in which a diagnostic test was used to identify participants with ASD, such as the ADOS-2 [28], ADI-R [27] or Development and Well-Being Assessment (DAWBA) [29]
-
5.
Include a risk prediction analysis (for example logistic regression or relative risk analysis)
-
6.
English language only
-
7.
Published in peer reviewed journals
Exclusion Criteria
-
1.
Screening tool used to assess for symptoms of ASD, rather than standardized diagnostic tools or clinical assessment
-
2.
Age of participants greater than 18 years at assessment for ASD
-
3.
Specific preterm subgroup
-
4.
Animal studies
-
5.
Conference abstracts
-
6.
Study population born before the 1990′s
-
7.
Study population born after 37 weeks gestation or with birth weight > 2500 g
Search Strategy
MEDLINE, EMBASE and PsycINFO databases were searched via Ovid in December 2019. Three separate search strategies were developed using free text terms and controlled vocabulary associated with each database (for example MeSH in Medline), as well as consulting search strategies from systematic reviews on similar topics [2, 25]. These previous systematic reviews also guided the selection of the search databases. The search strategies are outlined in “Appendix”. Searches were limited to publications between the 1st of January 1990 and the 16th of December 2019. An English language restriction was applied. The literature search was completed by one author (H.O.).
Study Screening and Selection
Using Covidence systematic review online software (www.covidence.org), two researchers (H.O. and C.C.) independently undertook screening of the study titles and abstracts without conferring. The reviewers individually decided which papers to consider for full-text screening, and any conflicts regarding the eligibility of specific articles were subsequently resolved through discussion. The full texts of all articles selected through the initial screening process were then examined independently by the same researchers to confirm they met the inclusion criteria. Following this, the researchers discussed and agreed upon which papers would be included in the review.
Data Extraction
One reviewer (H.O.) extracted the data from the selected studies using a standardized form. The following data was recorded from each study: author and location, year published, year of birth for study cohort, gestational age or weight at birth, the number of participants, study description, age at assessment for ASD, diagnostic methods used in diagnosis of ASD, study exclusions, factors investigated, and results. The references cited in all articles included for data extraction were manually searched for further eligible articles that were missed through the screening process.
Risk of Bias Assessment
As the inclusion of methodologically weak studies can jeopardize the internal consistency of a systematic review, it is important that the methodologic quality of studies is thoroughly appraised with regards to their design, analysis, conduct and interpretation, in order to reduce the opportunity for bias [41]. The modified version of the Quality in Prognosis Studies tool (QUIPS) [26] was used to assess bias in the studies retrieved. This tool focuses on six domains of bias in prognostic studies: study participation, study attrition, prognostic factor measurement, outcome measurement, confounding measurement and account, and statistical analysis. Each domain is rated as having low, moderate or high risk, with prompting questions covered in each domain to inform the reviewer’s judgment on the risk of bias. In addition, an overall risk of bias rating can be assigned to each study based on the ratings achieved across the six domains [26]. The risk of bias assessment was completed by one author (H.O.).
Data Synthesis
Due to variability between studies in the risk factors investigated and the gestational ages of participants, a narrative synthesis approach was used in order to give authors the flexibility to bring coherence to the data [42]. The Center for Reviews and Dissemination [20] guidance document was followed in conducting the narrative synthesis. In studies using logistic regression, the results of the final adjusted model were extracted. A description of the study characteristics is provided in Table 1, followed by a summary of significant factors categorized by pre-, peri- and post-natal periods. Similarities and inconsistencies across studies in the risk factors analyzed were also explored.
Results
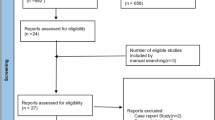
A total of 4359 studies were retrieved from the initial literature search and were screened on title and abstract. Of these 4300 were excluded as they did not meet the eligibility criteria based on the information provided. The full texts of the remaining 59 articles were reviewed for inclusion in the study. Eleven studies were identified that met the full set of eligibility criteria (see Fig. 1 for flow diagram of study screening and selection). No further studies were identified through the manual search of the reference lists of the 11 studies.
Study Characteristics
The majority of the studies were carried out in the USA (n = 6). Each of the following countries reported one study each: Australia, Israel, Japan, Taiwan and the UK/Ireland. Eight of the studies were population based or multi-center and three studies used a single center hospital site. Six of the studies prospectively followed a cohort of preterm born infants, and one study adopted both prospective follow-ups with retrospective linking to maternal and neonatal data. The remaining four studies were retrospective and extracted all data from medical records. In terms of diagnostic methods, the six prospective studies used one or more standardized diagnostic tool as part of the assessment (ADI-R [27], ADOS [28] or DAWBA [29]), and diagnostic evaluations were performed when the children were between 7 and 11 years of age in these studies. The other five studies reported that cases were diagnosed by qualified professionals using internationally recognized diagnostic criteria such as the ICD-10 [30] or DSM-V [1]; two studies did not specify the age at diagnosis, while the age varied from 2 to 11 years in the other three studies.
Four of the studies came from the prospective multi-center cohort of extremely low gestational age newborns study (ELGAN study) [8, 13, 31, 32] and investigated infants born extremely preterm (EP; < 28 weeks gestation). The EPICure study followed a population based cohort of EP born infants but only included those born at less than 26 weeks gestation [3]. This lower gestational age, defined as birth at 25 weeks or less, is considered to be of borderline viability, and is associated with a high prevalence of disability and mortality [33]. Ure et al.’s [14] prospective single center study investigated brain abnormalities in infants born at less than 30 weeks gestation, classified as very preterm. Two studies used birth weight to identify preterm births [34, 35], both cohorts were comprised of infants born with very low birth weight (VLBW; ≤ 1500 g). Ikejiri et al. [34] additionally specified that all infants were born at less than 33 weeks gestation; however the gestational age range was not detailed in Davidovitch et al. [35]. Two studies investigated infants born preterm (< 37 weeks gestation) and so included infants with gestational ages from across the different preterm categories [6, 36]. Kuzniewicz et al.’ [37] study retrospectively examined outcomes for all infants born preterm but conducted a risk analysis only on those infants born at less than 34 weeks gestation; this cut-off was chosen as all children born before 34 weeks were admitted to intensive care and thus had detailed neonatal records.
Prenatal Risk Factors
One study found that VLBW infants born to mothers with diabetes mellitus were at increased risk of autism [35]. However Bakian et al. [36] found no association between maternal diabetes and autism in the descriptive analysis of their preterm born cohort. Joseph et al. [8] reported cervical-vaginal infections to be associated with a later autism diagnosis in their EP participants with a comorbid cognitive impairment. No other study investigated cervical-vaginal infections as part of their analyses. Other non-significant prenatal factors investigated either through univariate descriptive analyses or multivariate modelling were maternal hypertensive disorders, prenatal steroid therapy, antepartum hemorrhage, chorioamnionitis, cervical insufficiency, smoking in pregnancy, medication use, urinary tract infection, fever and periodontal infection [3, 8, 35].
Perinatal Risk Factors
Although several studies investigated various perinatal characteristics including rupture of membranes, delivery mode (vaginal versus caesarean section), delivery room resuscitation, breech delivery, birth asphyxia and duration of labor [3, 6, 8, 34, 35, 37], these variables were not entered into their prediction models as they were non-significant on initial univariate exploration or did not come out as significant in these models.
Postnatal Risk Factors
Neonatal
Johnson et al. [3] investigated the association between 23 neonatal and demographic variables against a diagnosis of autism at 11 years in their EP born cohort. Only male sex was reported to be a significant risk factor for autism. This significant association between male sex and autism was also reported by Davidovitch et al. [35], Bakian et al. [36] and Hwang et al. [6] in their multivariate regression models exploring a broader range of preterm gestational ages. Joseph et al. [8] found male sex to be a significant risk factor for ASD but only in EP children with comorbid cognitive impairment. No other study investigated sex as a predictor variable for ASD.
Davidovitch et al. [35] reported that postnatal steroid therapy for the prevention or treatment of bronchopulmonary dysplasia was a significant predictor of ASD in their VLBW sample. However, this association between postnatal steroid treatment and autism was not found in another study of VLBW infants [34] or in an EP born cohort [3]. Davidovitch et al. also reported that being born small for gestational age (SGA; birth weight below the 10th percentile for gestational age) was a significant risk factor for an autism diagnosis; a finding consistent with those of Joseph et al. [8], who reported that EP children without cognitive impairment that suffered fetal growth restriction were at increased risk. Kuzniewicz et al. [37] also found a significant association between SGA and autism, which they entered as a covariate in their analyses. In the exploration of their sample characteristics, Ikejiri et al. [34] did not find an association between autism and SGA although their sample size was small (N = 59).
In addition to male sex, Hwang et al. [6] found low birth weight and neonatal cerebral dysfunction to be predictive of autism in their regression model. An association between low birth weight and autism was also reported by Davidovitch et al. [35] and Bakian et al. [36] on initial univariate exploration, however Davidovitch et al. excluded birth weight from subsequent analyses due to its high correlation with gestational age, and Bakian et al. reported that birth weight was not a significant covariate in their multivariable regression model. Johnson et al. [3] and Ikejiri et al. [34] also found no relationship between ASD and birth weight in their univariate analyses. There was variability between these studies in how they investigated birth weight, either maintaining the variable as continuous and examining significant group differences or adopting various categorical approaches. Hwang et al. [6] identified significant cerebral dysfunction from medical records if medical codes 779.0–779.2 from the International Classification of Diseases, 9th Revision, Clinical Modified (ICD-9-CM) [38] were specified. ICD-9-CM codes 779.0–779.2 encompass convulsions in newborns; other and unspecified cerebral irritability in newborns; and cerebral depression, coma and other abnormal cerebral signs, but excludes intraventricular hemorrhage, intrauterine cerebral ischemia, and cerebral ischemia due to birth trauma [38]. None of the other studies reviewed investigated these specific issues in their analyses.
Ikejiri et al. [34] found attenuated growth in weight during early infancy to be the only significant risk factor for an autism diagnosis. No other study reported weight-related growth factors in their analyses. Gestational age was not a significant predictor in their model, in keeping with the findings of Bakian et al. [36], Davidovitch et al.[35], and Johnson et al. [3]. In contrast, Joseph et al. [8] found gestational age to be a significant predictor of ASD in EP children with and without cognitive impairment. Kuzniewicz et al. [37] also found a significant association between gestational age and autism diagnosis which they entered as a covariate in their analyses. Differences in how gestational age was categorized between studies and the ranges of gestational ages investigated may explain the variation in findings.
Logan et al. [32] investigated whether a neonatal measure examining physiological functioning, the revised Score for Neonatal Acute Physiology (SNAP-II), was predictive of a diagnosis of autism at 10 years. High scores indicate worse physiological functioning and are associated with neonatal mortality. A moderate but not a high SNAP-II score was a significant risk factor. Johnson et al. [3] did not find a significant association between an autism diagnosis and the clinical risk index for babies (CRIB score), a similar measure of physiological functioning to the SNAP-II [39].
High frequency ventilation and intracranial hemorrhage were the only significant predictor variables for ASD reported by Kuzniewicz et al. [37]. Hwang et al. [6] found no association between intraventricular hemorrhage, an intracranial hemorrhage subtype, and autism in their model. Ure et al. [14] found that children without autism were more likely to have an abnormal cranial ultrasound result, which included intraventricular hemorrhage, while Johnson et al. [3] did not find a significant association between autism diagnosis and abnormal cranial ultrasound in their study. Ikejiri et al. [34] did not find a significant association between ASD and intraventricular hemorrhage or duration of ventilation on univariate investigation.
Ure et al. [14] was the only study that used magnetic resonance imaging (MRI) methods. The authors reported that neonatal brain abnormalities on MRI, specifically cystic lesions in cortical white matter, were associated with an increased risk of ASD. As the authors point out, a limitation of the study was the small number of preterm children in their sample subsequently diagnosed with ASD (n = 6). Accordingly, the results need replication.
No association was found between bacteremia [31] or neonatal magnesium levels [36] and a diagnosis of ASD. Kuzniewicz et al. [37], Davidovitch et al. [35], and Ikejiri et al. [34] also investigated neonatal infections as part of their analyses but did not report a significant association to ASD. No other study explored neonatal magnesium levels as a predictor.
Early Childhood
Two included studies investigated factors in childhood associated with a diagnosis of autism. Johnson et al. [3] conducted a stepwise multivariate logistic regression examining antecedent variables at three time points—discharge from hospital, 2.5 years and 6 years. At 2.5 years the only behavioral problem predictive of a later diagnosis of autism was withdrawn behavior (as measured by the Child Behavior Checklist). No neonatal factors were significant. At 6 years, pervasive peer problems as measured by the Strengths and Difficulties Questionnaire, and cognitive impairment were independently associated with a diagnosis of autism at 11 years; neonatal factors and behavioral outcomes at 2.5 years were not significant in this model.
In keeping with the above findings, Hirschberger et al. [13] found that at 10 years of age, children with cognitive impairment had seven times the risk of a diagnosis of autism relative to other extreme preterm children. In a separate risk analysis, a history of epilepsy was also associated with an increased risk of autism in this study.
Risk of Bias Assessment
The majority of studies included were rated as having an overall low to moderate risk of bias using the revised QUIPS tool [26]. See Table 2 for risk of bias ratings. Studies rated as having a low risk of bias on the study participation domain covered a wide population from multiple centers. Studies rated as having a high risk of bias had a sample that was not representative of the general population of preterm infants, due to either the exclusion criteria applied, or recruitment from a single center or from centers only accessible to those with medical insurance (indicating underrepresentation of those from lower socio-economic backgrounds). On the study attrition domain, studies were rated as having high to moderate risk of bias if the attrition rate was high, no description of the demographics or characteristics of the lost participants were provided, or the participants lost to follow-up were representative of a specific subgroup (e.g. from lower socio-economic backgrounds or with poor neurodevelopmental outcomes).
Studies rated as low risk on the prognostic factor measurement had a clear description of how risk factors were measured. Those with moderate ratings had limited information on how risk factors were measured or used imputation methods that were open to bias. For outcome measurement, only studies in which ASD was diagnosed by a qualified health professional using ICD or DSM diagnostic criteria and/or through the use of standardized diagnostic tools such as the ADOS-2 [28], ADI-R [27]and DAWBA [29] were included in the review. Accordingly, no study was rated as having a high risk of bias on outcome measurement. Studies were rated as having a moderate risk of bias on outcome measurement if data were retrospectively collected from medical records with limited information on how a diagnosis was reached other than applied by a qualified professional. It is unclear in these studies if diagnostic tools in addition to clinical interview were used to inform the diagnostic decision. Prospective studies in which participants were assessed for ASD as part of the follow-up assessments using standardized tools and/or clinical interview and retrospective studies in which the details of the diagnostic procedure were thoroughly described were rated as having a low risk of bias.
Confounding measurement bias was considered low if important confounders were adjusted for in the analyses and these were clearly defined and measured. Studies were rated as having a low risk of bias on the statistical analysis and reporting domain if the statistical method chosen was appropriate to the study design and any model building strategy was clearly detailed. Studies were considered to have a moderate risk of bias if appropriate statistical adjustments were not made, such as for multiple comparisons.
Discussion
The aim of this systematic review was to provide a narrative synthesis of the literature in relation to risk factors associated with ASD, diagnosed either through assessment by a clinician using internationally recognized diagnostic criteria or using a standardised diagnostic tool, in children born preterm. A total of 11 studies were identified that met the eligibility criteria. The search was restricted to articles published from 1990 onwards, however all studies included in the review were published in the past decade, with 8/11 studies in the last four year period, highlighting the recent interest in this area. There was considerable variability across studies with regards to the design (prospective versus retrospective), sampling (population based or multicenter versus single center site), the gestational age range used, the risk factors investigated, as well as the age at which the ASD diagnostic assessments were completed. Despite this variability all studies were rated as having a low to moderate risk of bias.
To identify commonalities and summarize the findings from the individual studies, risk factors were separated into pre-, peri- and post-natal. Although a number of prenatal predictors were investigated, only maternal diabetes and cervical-vaginal infections were significantly associated with an increased risk of ASD on multivariable modelling. Joseph et al. [8] suggest that cervical-vaginal infections may lead to inflammation as part of the immune response and that this is associated with cerebral damage in the preterm infant, increasing their risk of ASD. This finding requires replication as it was the only study reviewed that investigated cervical-vaginal infections as an antecedent risk factor and was restricted to EP born infants. However the sample size from this prospective multicenter study was large (n = 857) and thorough procedures were used in the diagnostic assessment process. The significant finding in relation to maternal diabetes [35] also requires further investigation due to contradictory results found by Bakian et al. [36]. Furthermore, the authors did not propose an explanation for this association. No study reported the rupture of membranes, delivery mode (vaginal versus caesarean section), delivery room resuscitation, breech delivery, birth asphyxia or duration of labor to be significant factors for ASD.
The majority of studies focused on risk factors in the neonatal period. Five studies reported a significant association between male sex and autism [3, 6, 8, 35, 36]. This finding of a greater prevalence of ASD in males is in keeping with trends in the general population; with one proposal that autism is an extreme presentation of the typical male brain [40]. Thus this finding is unlikely related to preterm birth, but rather the higher male to female ratio of ASD in the general population. Being small for gestational age was reported as a significant risk factor in three studies [8, 35, 37]. Joseph et al. [8] suggest that both preterm birth and SGA act like a double hit of risk factors, and also indicate that inflammation or epigenetic phenomenon might underlie this relationship between SGA and autism in these infants. Two studies found low gestational age to be a significant predictor for ASD [8, 37], which Joseph et al. suggest is possibly due to the vulnerability of the immature preterm brain, lack of neuroprotective factors, inflammatory response and physiological instability. However this association was not reported by others [3, 34,35,36]. The variation in findings is likely due to differences in how this variable was categorized across studies as well as characteristics of the samples themselves, such as sample size and range of gestational ages investigated.
Mixed findings were reported in relation to postnatal steroid therapy, low birth weight, neonatal physiological functioning, ventilation and intracranial hemorrhages, with some studies finding them as significant predictors and others noting no association to ASD. Single study factors identified as significant include cerebral dysfunction [6], attenuated growth in weight during early infancy [34] and cystic lesions in cortical white matter [14]; however these findings require further investigation. No evidence was found in support of bacteremia [31] or neonatal magnesium levels [36] as antecedents for ASD.
Two studies of EP births reported that cognitive impairment in childhood was significantly associated with an autism diagnosis [3, 13]. Johnson et al. [3] suggest that preterm birth leads to alterations in normal brain development, resulting in cognitive impairment and social and communication difficulties. In addition the preterm infant may experience an abnormal psychosocial environment which may further impact on the development of the social brain.
The developmental sociobiological vulnerability model of preterm birth [9, 10] proposes that structural and functional brain changes are an important factor in the manifestation of autism in children born preterm in combination with psychosocial variables. The majority of the antecedents reviewed in this paper were linked to ASD due to the alterations they incite in the developing brain and thus add support to this model. The model also suggests that cognitive impairment results from damage to the developing brain which is linked to greater socioemotional vulnerability in the preterm born child. Accordingly, cognitive impairment was found to be significantly associated with an autism diagnosis in two studies [3, 13]. Lastly the developmental sociobiological vulnerability model suggests that impaired social competence also increases socioemotional vulnerability and leads to negative social experiences, which may increase the preterm child’s risk of neurodevelopmental disorders such as autism. In line with this proposal Johnson et al. [3] found withdrawn behavior at 2.5 years of age and pervasive peers problems at 6 years were associated with a diagnosis of autism at 11 years.
Limitations
A limitation of this review was the inability to combine findings across studies through meta-analysis or draw greater conclusions due to the variation in methodologies used and how predictor variables were measured. For example, while group differences (ASD vs Non-ASD) in birth weight were explored across a number of the studies, some measured the variable as continuous and others treated it as categorical, with varying categorizations used. Additionally birth weight was identified as a predictor in some studies, but as a covariate in others. Differences in risk factor measurement as well as the gestational age ranges investigated demonstrate how findings can easily differ across studies, and this presented a further challenge in drawing comparisons or combining findings. It should also be highlighted that different diagnostic criteria and diagnostic approaches were used across the studies which may have resulted in variation between studies in case detection. This difference in diagnostic criteria and diagnostic approaches may be another factor underlying the variation in findings between studies.
Clinical implications
Children born preterm are at an increased risk of ASD [2,3,4,5,6] and this highlights the need for clinicians to thoroughly investigate pregnancy and birth complications in their initial assessments with clients, whether through self-report or collateral interview with a parent. In addition to ascertaining information on prematurity, attention should be paid to the risk factors highlighted above, particularly maternal infections during pregnancy, size for gestational age, cognitive development and any early behavioral or socialization concerns.
It should be highlighted that a number of other risk factors identified through this systematic review may be important to investigate during clinical assessment, such as maternal diabetes, intracranial hemorrhage or reduced growth in infancy, however due to the mixed findings reported or limited evidence in support of them, their underlying role in the development or presentation of ASD in preterm born children is unclear and the results need to be interpreted with caution.
A further implication is the need to screen preterm born children for ASD periodically throughout childhood, particularly those in the lowest gestational age category (< 28 weeks gestation), given the increased risk of autism in these children. This would help to identify children with social difficulties who warrant further clinical investigation and to ensure timely intervention and supports are made available. However these screening tools have limitations in young children born preterm in which high false-positive rates have been found [16, 17], highlighting the need to screen beyond early childhood when it may be easier to differentiate between neurosensory impairments and symptoms of autism.
Future Directions
A number of identified risk factors require further investigation, such as cervical-vaginal infections, brain abnormalities on imaging, and early childhood behavioral indicators. Larger birth cohorts covering a greater range of preterm gestational ages should be used, to allow sub-analyses by gestational age groupings.
Numerous studies were excluded from this review, as screening measures were used to identify autistic symptoms rather than standardized diagnostic tools or clinical assessment. However Johnson et al. [3] suggest that a greater proportion of preterm survivors are affected by subclinical social and communication difficulties associated with autism than meet diagnostic criteria. An investigation as to whether the risk factors associated with clinical versus subclinical autistic features differ may be worthwhile, and may also guide development of interventions for the different at-risk groups. Broadening the inclusion criteria to include studies that used screening measures to assess for autism would have increased the number of studies identified in this systematic review. However, screening tools have been shown to have a high false-positive rate when used in toddlers due to comorbid motor disorders and sensory impairments [16, 17]. Results may have been cofounded if studies that used screening measures had been included, as the risk factors identified may not be specific to autism but broader neurodevelopmental impairments.
Summary
The prevalence of ASD is significantly higher in preterm compared to term born children [2, 3]. Across studies, male sex, being small for gestational age, and cognitive impairment are the most consistent risk factors proposed to underlie this relationship. A number of other significant antecedents from the prenatal and postnatal period were identified, but these findings require replication in larger cohort studies. To allow comparisons across studies, care needs to be taken in how risk factors are measured.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Arlington
Agrawal S, Rao SC, Bulsara MK, Patole SK (2018) Prevalence of autism spectrum disorder in preterm infants: a meta-analysis. Pediatrics 142(3):e20180134
Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N (2010) Autism spectrum disorders in extremely preterm children. J Pediatr 156(4):525–531. https://doi.org/10.1016/j.jpeds.2009.10.041
Moster D, Lie RT, Markestad T (2008) Long-term medical and social consequences of preterm birth. N Engl J Med 359(3):262–273
Atladottir HO, Schendel DE, Henriksen TB, Hjort L, Parner ET (2016) Gestational age and autism spectrum disorder: trends in risk over time. Autism Res 9(2):224–231
Hwang YS, Weng SF, Cho CY, Tsai W (2013) Higher prevalence of autism in Taiwanese children born prematurely: a nationwide population-based study. Res Dev Disabil 34(9):2462–2468
Indredavik MS, Vik T, Evensen KA, Skranes J, Taraldsen G, Brubakk AM (2010) Perinatal risk and psychiatric outcome in adolescents born preterm with very low birth weight or term small for gestational age. J Dev BehavPediatr 31:286–294
Joseph RM, Korzeniewski SJ, Allred EN, O’Shea TM, Heeren T, Frazier JA, Ware J, Hirtz D, Leviton A, Kuban K, Coster T (2017) Extremely low gestational age and very low birthweight for gestational age are risk factors for autism spectrum disorder in a large cohort study of 10-year-old children born at 23–27 weeks gestation. Am J Obstet Gynecol 216(3):304.e1–304.e16
Healy E, Reichenberg A, Nam KW, Allin MP, Walshe M, Rifkin L, Murray RM, Nosarti C (2013) Preterm birth and adolescent social functioning-alterations in emotion-processing brain areas. J Pediatr 163(6):1596–1604. https://doi.org/10.1016/j.jpeds.2013.08.011
Montagna A, Nosarti C (2016) Socio-emotional development following very preterm birth: pathways to psychopathology. Front Psychol 12:80
Gray PH, Edwards DM, O'Callaghan MJ, Gibbons K (2015) Early human development screening for autism spectrum disorder in very preterm infants during early childhood. Early Hum Dev 91(4):271–276. https://doi.org/10.1016/j.earlhumdev.2015.02.007
Hack M, Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N (2009) Behavioral outcomes of extremely low birth weight children at age 8 years. J Dev Behav Pediatr 30(2):122–130
Hirschberger RG, Kuban KC, O'Shea TM, Joseph RM, Heeren T, Douglass LM, Stafstrom CE, Jara H, Frazier JA, Hirtz D, Rollins JV (2018) Co-occurrence and severity of neurodevelopmental burden (cognitive impairment, cerebral palsy, autism spectrum disorder, and epilepsy) at age ten years in children born extremely preterm. Pediatr Neurol 79:45–52
Ure AM, Treyvaud K, Thompson DK, Pascoe L, Roberts G, Lee KJ, Seal ML, Northam E, Cheong JL, Hunt RW, Inder T (2016) Neonatal brain abnormalities associated with autism spectrum disorder in children born very preterm. Autism Res 9(5):543–552
Limperopoulos C, Bassan H, Sullivan NR, Soul JS, Robertson RL, Moore M, Ringer SA, Volpe JJ, du Plessis AJ (2008) Positive screening for autism in ex-preterm infants : prevalence and risk factors. Pediatrics 4:758–765
Moore T, Johnson S, Hennessy E, Marlow N (2012) Screening for autism in extremely preterm infants: problems in interpretation. Dev Med Child Neurol 54(6):514–520
Kuban KCK, O’Shea TM, Allred EN, Tager-Flusberg H et al (2009) Positive screening on the Modified Checklist for Autism in Toddlers (M-CHAT) in extremely low gestational age newborns. J Pediatr 154(4):535–540.e1
Johnson S, Marlow N (2009) Positive screening results on the modified checklist for autism in toddlers: implications for very preterm populations. J Pediatr 154(4):478–480
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341
Center for Reviews and Dissemination (2009) Systematic reviews – CRDs guidance for undertaking reviews in health care. York Publishing Services, New York
Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, Du Plessis AJ (2014) Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex 24(3):728–736
Padilla N, Eklöf E, Mårtensson GE, Bölte S, Lagercrantz H, Ådén U (2017) Poor brain growth in extremely preterm neonates long before the onset of autism spectrum disorder symptoms. Cereb Cortex 27(2):1245–1252
Korzeniewski SJ, Allred EN, O’Shea TM, Leviton A, Kuban KC (2018) Elevated protein concentrations in newborn blood and the risks of autism spectrum disorder, and of social impairment, at age 10 years among infants born before the 28th week of gestation. Transl Psychiatry 8(1):115
Marlow N, Wolke D, Bracewell MA, Samara M, Group EPICure Study (2005) Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 352(1):9–19
Linsell L, Malouf R, Johnson S, Morris J, Kurinczuk JJ, Marlow N (2016) Prognostic factors for behavioral problems and psychiatric disorders in children born very preterm or very low birth weight: a systematic review. J Dev BehavPediatr 37(1):88–102
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C (2013) Assessing bias in studies of prognostic factors. Ann Intern Med 158(4):280–286
Rutter M, Le Couteur A, Lord C (2003) Autism diagnostic interview – Revised. Western Psychological Services, Los Angeles, CA
Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S (2012) Autism diagnostic observation schedule, 2nd edn. Western Psychological Services, Torrance
Goodman R, Ford T, Richards H, Gatward R, Meltzer H (2000) The development and well-being assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 41(5):645–655
World Health Organization (1993) The ICD–10 classification of mental and behavioral disorders: diagnostic criteria for research. WHO, Geneva
Babata K, Bright HR, Allred EN, Erdei C, Kuban KC, Joseph RM, O’Shea TM, Dammann O, Leviton A, ELGAN Study Investigators (2018) Socioemotional dysfunctions at age 10 years in extremely preterm newborns with late-onset bacteremia. Early Hum Dev 121:1–7
Logan JW, Dammann O, Allred EN, Dammann C, Beam K, Joseph RM, O'Shea TM, Leviton A, Kuban KC (2017) Early postnatal illness severity scores predict neurodevelopmental impairments at 10 years of age in children born extremely preterm. J Perinatol 37(5):606–614
Marlow N (2004) Outcome following extremely preterm birth. Curr Paediatr 14(4):275–283
Ikejiri K, Hosozawa M, Mitomo S, Tanaka K, Shimizu T (2016) Reduced growth during early infancy in very low birth weight children with autism spectrum disorder. Early Hum Dev 98:23–27
Davidovitch M, Kuint J, Lerner-Geva L, Zaslavsky-Paltiel I, Rotem RS, Chodick G, Shalev V, Reichman B (2019) Postnatal steroid therapy is associated with autism spectrum disorder in children and adolescents of very low birth weight infants. Pediatr Res 2:1–7
Bakian AV, Bilder DA, Korgenski EK, Bonkowsky JL (2018) Autism spectrum disorder and neonatal serum magnesium levels in preterm infants. Child Neurol Open 5:28–30
Kuzniewicz MW, Wi S, Qian Y, Walsh EM, Armstrong MA, Croen LA (2014) Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J Pediatr 164(1):20–25
Buck C (2003) 2001 ICD-9-CM (Vols. 1–3). WB Saunders, New York
Dorling JS, Field DJ, Manktelow B (2005) Neonatal disease severity scoring systems. Arch Dis Child Fetal Neonatal Ed 90(1):1–6
Baron-Cohen S (2002) The extreme male brain theory of autism. Trends Cogn Sci 6(6):248–254
Deeks JJ, Dinnes J, D'Amico R et al (2003) Evaluating non-randomised intervention studies. Health Tech Assess 7(27):3–173
Popay J, Roberts H, Sowden A, et al (2006) Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version, pp 1-92
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Medline Search Strategy (Via Ovid)
-
1.
(Preterm birth or Preterm infant).mp. or exp infant, premature, diseases/ or exp gestational age/ or exp infant, low birth weight/ or exp infant, premature/ or exp premature birth/
-
2.
autism.mp. or exp Autistic Disorder/ or exp autism spectrum disorder/ or exp Asperger Syndrome/ or exp child development disorders, pervasive/ or exp Child Development Disorders/ or pervasive developmental disorder.mp.
-
3.
1 AND 2
-
4.
limit 3 to yr = "1990 -Current"
Embase Search Strategy (via Ovid)
-
1.
exp autism/ or 'autism spectrum disorder'.mp. or 'autistic disorder'.mp. or 'child development disorders'.mp. or 'kanners syndrome'.mp. or 'pervasive developmental disorders'.mp.
-
2.
exp premature labor/ or exp prematurity/ or exp low birth weight/ or exp gestational age/ or 'preterm birth'.mp. or 'premature infant'.mp. or 'preterm infant'.mp.
-
3.
1 AND 2
-
4.
limit 3 to yr = "1990 -Current"
PsycINFO Search Strategy (Via Ovid)
-
1.
exp Premature Birth/ or exp pregnancy outcomes/ or exp birth weight/ or preterm birth.mp. or preterm infant.mp. or low birth weight.mp. or gestational age.mp. or premature infant.mp. or premature labor.mp.
-
2.
exp autism spectrum disorders/ or exp neurodevelopmental disorders/ or exp autistic traits/ or child development disorders.mp. or aspergers syndrome.mp. or pervasive developmental disorders.mp. or autistic*.mp.
-
3.
1 AND 2
-
4.
limit 3 to yr = "1990 -Current"
Rights and permissions
About this article
Cite this article
Cogley, C., O’Reilly, H., Bramham, J. et al. A Systematic Review of the Risk Factors for Autism Spectrum Disorder in Children Born Preterm. Child Psychiatry Hum Dev 52, 841–855 (2021). https://doi.org/10.1007/s10578-020-01071-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10578-020-01071-9