Abstract
Sleep-wake patterns are rarely examined in adolescents with borderline personality disorder (BPD) or bipolar disorder (BD). Within a developmental perspective, this study explores the sleep-wake cycle of adolescents aged 12–17 years with BPD or BD and healthy controls (HC) during periods with and without entrainment by school/work schedules. Eighteen euthymic BPD, six euthymic BD, and 20 HC adolescents wore wrist actigraphy during nine consecutive days to assess sleep-wake patterns. During school/work days, BPD adolescents spent more time awake when they were in bed compared to HC and BD adolescents (p = 0.039). On schedule-free days, BPD and BD youths spent more time in bed compared to HC adolescents (p = 0.015). BPD adolescents woke up over 1 h later compared to HC (p = 0.003). Total sleep time was more variable between nights in BPD adolescents compared to the HC group (p = 0.031). Future research should explore if sleep-wake pattern disruptions are a cause or a consequence of BPD symptomatology in adolescents. Addressing sleep-wake pattern during clinical assessment and treatment of BPD adolescents may potentially reduce their symptoms; this therapeutic effect still needs to be evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Context
The issue of sleep in psychiatric disorders characterised by emotional dysregulation, such as borderline personality disorder (BPD) and bipolar disorder (BD), has generated considerable attention in adult patients over the last decades. As such, questionnaire studies show that BPD patients have more sleep complaints, use more hypnotics, and report more nightmares and negative toned dreams [1–4]. Reviews of polysomnography studies highlight the presence of longer sleep latencies, a higher number of night awakenings, reduced rapid eye movement (REM) sleep latencies, longer REM sleep duration, and higher REM density in BPD adult patients compared to healthy controls (HCs) [5, 6]. Since BPD patients show comparable sleep disturbances to patients with major depressive disorder, such as delayed sleep onset and reduced total sleep time [7–10], studies must examine sleep parameters when patients are free of mood symptoms in order to control for depression as a confounding factor [5]. Assessing BPD patients without depressive disorders allows determining what sleep disturbances are more specific to this personality disorder, therefore improving knowledge about its aetiology. Although the rate of a cormobid DSM-IV mood disorder is high (55.3–58.7 %) in BPD adolescents [11, 12], they do not all have major depressive disorder or dysthymia. Even if BPD and major depressive disorder show overlap of symptoms, they are two distinct conditions which do not respond to antidepressant medication in a similar way [13]. Depressive symptoms in BPD are qualitatively different from major depression: they are more transient and stress-related, can disappear rapidly when the conflict is resolved, serve to express distress and are characterised by feelings of loneliness, emptiness and boredom, by clinging dependency, by unstable negative affect and by a deep sense of badness [13]. Affective instability in BPD usually involves fluctuating states including anger, anxiety and even euthymia, and not only depression [14]. Therefore, studies need to be conducted without the presence of its main confounder, i.e. depressive disorders, to better understand aetiology, course and treatment specific to BPD.
Sleep disturbances are also commonly observed in BD patients, particularly during acute mood episodes. Reduction of sleep need during mania and presence of insomnia/hypersomnia during depressive states are recognized as diagnostic features of BD according to DSM-IV and ICD-10 criteria [15, 16]. A systematic literature review has calculated the median prevalence of early symptoms in BD and reported that sleep disturbance is the most important prodrome for a maniac episode and it is the sixth most frequently observed prodrome for a depressive episode [17]. Furthermore, 23.6 % of adult BD patients still suffer from insomnia during euthymia (between mood episodes), and more than half are receiving sedating medication (sedating antipsychotics, benzodiazepines, sleeping aids) [18]. Actigraphy results show that euthymic BD adults also exhibit a longer sleep latency and higher interdaily variability of the sleep-wake rhythm than healthy adults [19, 20].
Many studies have highlighted the importance of adopting a developmental perspective when studying sleep, since adolescence is characterized by specific sleep features: sleep need during adolescence is about 10 h per night, slow wave sleep time decreases by 40 % during that age period, and level of daytime sleep tendency increases during midpuberty [21]. Furthermore, adolescents present a biological phase delay of their circadian rhythms (i.e. compared to children and adults, they have later bedtimes and rise times, melatonin secretion occurs later in the evening, etc.) [22–24]. In humans, this shift is strengthened by social obligations: part-time jobs, more homework, extracurricular activities, friendships and love, etc. These different biological, social and psychological factors create a common phenomenon which is being reported across the globe: adolescents are not getting enough sleep [25]. The deleterious effect of sleep disturbances on cognition and emotion in adolescents, especially those with psychiatric disorders, has been recognised over the past decades [26–30].
Sleep researches in adolescent psychiatry have focused on depression, attention deficit hyperactivity disorder (ADHD), autistic spectrum disorders, substance abuse and anxiety disorders. However, there are few studies on BD and BPD during adolescence, even though there is a solid scientific body of knowledge on the topic in adults. Studying specifically BD and BPD is relevant since both disorders are characterised by emotional dysregulation and impulsive behaviours which may increase and be increased by sleep disturbances [31, 32]. Also, both BD and BPD youths present a high-risk for suicidality [33, 34] as sleep disturbances, especially insomnia, aggravate suicide risk in BPD patients [35]. Sleep research in these two psychopathologies can shed a light on their respective aetiology. If BD and BPD symptoms are strongly linked to sleep disturbances, improving sleep in these patients may reducing symptomatology and lowering suicide risk.
Until recently, there was a lack of research on BPD conducted among adolescent populations, since there was a controversy surrounding the diagnosis of BPD in youths [36]. Mental health clinicians were reluctant to diagnose BPD in adolescents because it was believed that personality is in flux during that age period and that this diagnosis stigmatised the patients [37, 38]. Attitudes in research BPD have changed recently and this diagnosis is being recognised as a valid mental disorder in adolescence [38, 39]. To our knowledge, sleep in BPD adolescents has only been studied by our team. We have demonstrated the relevance of assessing sleep using actigraphy in a case report of a BPD adolescent girl [40] and shown an association between poor sleep and impulsive and aggressive behaviours [41]. Literature describing sleep in BD children and adolescents is more extensive and it has been produced mainly over the last decade. In BD youths, questionnaire studies have shown a decreased need for sleep during mania, and presence of insomnia during depressive states [42–47]. Euthymic and non-euthymic BD adolescents have more difficulties falling asleep and have more frequent and longer night awakenings than healthy adolescents [48]. Moreover, sleep disturbances often precede the diagnosis of BD in childhood and adolescence [49]. Reports of objective findings are rare and the few polysomnography studies show that depressed adolescents who later developed BD had initially more stage 1 and less stage 4, increased REM latency, lower REM density, and less REM sleep than the unipolar group who never developed BD. A recent comparative study observed longer sleep duration and less wakefulness after sleep onset in euthymic BD adolescents (11–17 years old) compared to ADHD and healthy youths using actigraphy. However, BD adolescents self-reported a higher number of awakenings than their ADHD counterparts [50]. All these results obtained from BD youths must be interpreted with caution since some studies included both prepubertal children and postpubertal adolescents or did not apply all the diagnostic criteria for DSM-IV BD while others had not controlled for current mood state.
Aim of Current Study
This study explores the sleep-wake cycle of euthymic adolescents with BPD or BD. Diagnoses are defined by DSM-IV criteria as assessed by standardised interviews, and sleep-wake patterns are objectively measured using actigraphy during periods with and without entrainment by school/work schedules. Healthy adolescents are recruited as a comparison group to disentangle what is specific to this age span and what is associated with psychopathology.
Hypotheses
We hypothesise that both BPD and BD adolescents will present higher sleep disturbances, such as night awakenings and longer sleep latencies, compared to HC adolescents. We also expect that sleep-wake patterns disruptions will be more characteristic of the BD group. Hypotheses derive mainly from adult scientific literature and, therefore, predictions are limited considering that there are few studies concerning only BPD or BD adolescents. Developmental features specific to adolescence may explain differences found between data obtained among adolescents and those obtained among adults.
Methods
Participants
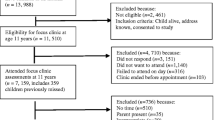
Eighteen BPD participants (16.0 ± 1.1 years old; 3 boys and 15 girls) and 6 BD (16.7 ± 1.0 years old; 2 boys and 4 girls) were recruited at Rivière-des-Prairies Hospital (Montréal, Canada), a university-affiliated psychiatric hospital for children and adolescents, and at Institute Philippe-Pinel de Montréal, a university-affiliated hospital specialised in forensic psychiatry. They were referred to this study by their psychiatrist. Among eligible participants, written consent was only obtained for 25 BPD and 10 BD adolescents. Actigraphy data of 2 BPD patients and 3 BD patients were not analyzable because of technical difficulties. Also, 6 BPD adolescents and 1 BD adolescent drop out of the study during data collection.
Concerning participants who completed the study, one BD and 3 BPD youths were not attending school or working during data collection. One BD and five BPD youths dropped out of school, and 2 BD and 5 BPD adolescents were working part time. One BD girl and one BPD girl were living in a youth centre.
For the two clinical groups, psychiatric diagnoses were established using the best-estimate method: clinical diagnoses were made by the treating psychiatrist using all available sources of information. Results from the K-SADS-PL (Schedule of Affective Disorders and Schizophrenia for School-Age Children—Present and Longitudinal Version, parent informant) and from the DIB-R (Diagnostic Interview for Borderlines Revised, adolescent informant) were included.
In this research protocol, eligible patients were included if they: (1) were euthymic at the time of data collection (defined as no current depressive symptoms), (2) had a current clinical diagnosis of BPD confirmed by the treating psychiatrist and by a DIB-R score ≥8 for the BPD group; (3) had a current clinical diagnosis of BD confirmed by the treating psychiatrist and by the K-SADS-PL semi-structured interview for the BD group: 3) were currently in treatment. As explained in the introduction, euthymia was necessary to control current depressive symptoms as a potential cofounding factor. Being in treatment was considered as an inclusion criteria to facilitate mental health care in case of emergency. Exclusion criteria were: (1) mental retardation or pervasive development disorders, (2) sleep disorder of organic origin as reported in the patient’s medical file by his/her treating physician, (3) current psychotic features, (4) current mania, (5) BD diagnosis (for the BPD group) or BPD diagnosis (for the BD group).
Clinical characteristics of the clinical groups are detailed in Table 1.
Twenty healthy adolescents (HC; 7 boys and 13 girls; 14.7 ± 1.0 years old) were recruited by public advertisement sent by email to all employees of the Rivière-des-Prairies Hospital and posted on message boards throughout the hospital. One of the authors (CH) also visited a regional high school during school hours to explain the project to three classes of about 35 students each. Parents of adolescents who were interested were contacted for the selection procedure. Exclusion criteria were psychopathological problems as assessed by the Child Behaviour Checklist (CBCL) filled by the parent and the Youth Self-Report (YSR) filled by the adolescent using a T-score ≤70 as the threshold for all scales and subscales of the CBCL and the YSR as recommended by the authors of these questionnaires [51]. BPD symptomatology was also ruled out in HC adolescents by using the Abbreviated Diagnostic Interview for Borderlines (Ab-DIB), with a threshold ≤12) to be included in the study.
Measures
Diagnoses
The K-SADS-PL comprises 35 Axis I psychiatric disorders according to DSM-IV criteria. It is one of the most commonly used instruments in child and adolescent psychiatry studies [52]. In this study, we used the K-SADS-PL to compile Axis I comorbidities. The original version in English showed high test–retest reliability kappa coefficients (0.77–1.00) for major depression, BD, generalised anxiety disorder, conduct disorder and oppositional defiant disorder [52]. We chose to use the parent as the only informant for the K-SADS-PL and not to obtain data from the adolescent in order to avoid overloading an already long data collection period for the patients with an additional 90 min of interview.
The CBCL and the YSR are questionnaires assessing general psychopathology in children and adolescents commonly used in epidemiological and clinical studies [53]. These questionnaires investigate the presence of externalised and internalised symptoms and syndromes in the last 6 months. Normative data exists in general populations from North America and others cultures [51].
The DIB-R is a semi-structured interview divided in four sections corresponding to key elements of BPD symptomatology (emotions, cognitions, impulsivity, and interpersonal relationships) and follows a dimensional approach [54, 55]. In this study, the clinical threshold was 8 out of a maximum of 10, which constitutes the best compromise between specificity (0.80), sensitivity (0.82), positive predictive value (0.74), and negative predictive value (0.87) [55]. We used a version we adapted for adolescents, with the timeframe reduced to 6 months instead of 2 years, and certain items adapted to behaviours and life situations specific to this age group.
The Ab-DIB allows the assessment of BPD symptoms through 26 yes–no items. The French adolescent version was validated in suicidal adolescents: internal consistency varies between 0.80 and 0.86, inter-rater agreement is high (0.77–0.95), and sensitivity and specificity of the instrument are good to excellent (0.88 and 0.73–0.82 respectively) [56].
Mood Assessment
The beck depression inventory (BDI-II) is the most frequently used depression scale in studies on adolescents. It evaluates cognitive, behavioural, affective and somatic aspects of depression. Many studies have shown its validity and reliability [57–59]. A score of 19 or lower on the BDI-II was required to be included in the study, since BPD patients tend to overestimate their depressive symptoms [60].
The Young Mania Rating Scale (YMRS) is filled by a trained clinician after a short interview in order to assess mania [61] and can be used with children and adolescents [62, 63]. The French version shows a very good inter-rater reliability (ICC > 0.89) and a high concurrent validity (rs > 0.96) [64]. A total score of 11 or lower was required to be included in this study.
Actigraphy
Actigraph (AW-64 Model, MiniMitter, Bend, OR) is a piezzo-electric device, worn as a wristwatch on the non-dominant hand that continuously monitors movements. More precisely, acceleration is measured and generates a voltage value [65]. This electric data is filtered and amplified inside the device, and then transferred offline to a computer. Activity counts are numerical values obtained from the digital integration, which takes into account the surrounding epochs through a weighting algorithm [65]. Actigraphic data was analysed by a commercially available software provided by the actigraph manufacturer (Actiware V. 5.0), using 1-min epochs. An activity count of 40 was used as a threshold to distinguish wake from sleep since it has a high sensitivity/specificity ratio [66]. Table 2 defines the variables and the terms used in the present paper.
We also calculated a Coefficient of Variation (CV = standard deviation (SD)/mean) to obtain a value for variability of total sleep time as previously described by BC Mullin, AG Harvey and SP Hinshaw [50]. Bed time and rise time variability was estimated by averaging individual SDs.
Protocol
This investigation was carried out in accordance with the Declaration of Helsinky (2008). The protocol and the consent form were approved by the research ethic board of Rivière-des-Prairies Hospital. Written consent was obtained from both the adolescent and one of his/her parents. All the participants were enrolled on a voluntary basis and were offered a $25 music store voucher for their participation.
After obtaining the written consent, parents of the clinical groups had to complete the K-SADS-PL if it was not already done in the past 12 months and parents of the HC group filled the CBCL. BPD symptomatology was assessed with the adolescent using the appropriate instrument (DIB-R for the clinical groups, Ab-DIB for the HC group). All adolescents in the three groups filled out the BDI-II and completed the YMRS with one of the researchers, making sure that all participants were currently euthymic.
All participants were asked to wear the wrist actigraph on their non-dominant hand for at least 9 days including 2 weekends, therefore allowing for recorded sleep-wake patterns under different schedule constraints. Participants were also given a sleep diary to be filled every morning upon waking up, recollecting characteristics of the previous night. Of note, the sleep diary did not examined nightmares. Since actigraphy only assessed sleep-wake patterns and could not determine presence of nightmares, exploration of this topic was considered beyond the scope of this current study. Participants identified the rest interval onset and offset (i.e. from lights out to final rise up) by using the event marker button. If the patient did not press the button, sleep diary data was used if time corresponded to a decline in activity as shown by actigraphy data. If sleep diary data was missing on this particular date, rest interval was determined by identifying periods of low activity levels on the graphs. Details concerning protocol feasibility are given elsewhere [67].
For each patient, actigraphy data was broken down into school/work days and schedule-free days. A school/work day was defined as having stable sleeping and rising hours because of school or work. A schedule-free day was defined as days where the patient did not have any time-related obligations. There was no contact with participants while they were wearing the actigraph.
Statistical Analyses
Statistical analyses were performed with Statistica 6.1 (StatSoft, Tulsa, OK). Means and SD were obtained for all descriptive data.
Since the sample sizes were small, non parametric Kruskal–Wallis ANOVA tests were used to compare actigraphy data between the three groups. Upon significant results, post hoc Mann–Whitney U-Tests were performed to detect which group was different from the others. Alpha level was set at 0.05.
Since this study was exploratory and the sample size was small, Bonferroni’s corrections became too conservative, and they increased the risk of Type II error [68]. In this article, Benjamini-Hochberg’s procedure was applied to reduce the false discovery rate, although it is possible that this correction may be too conservative considering the exploratory nature of our results. Therefore, both corrected and non-corrected results will be presented. Cohen’s d for size effects will also be given.
Results
On school/work days (Table 3), BPD adolescents spent a higher percentage of their time awake during their time in bed compared to euthymic BD youths (BPD: 21.5 ± 6.6 %; BD: 13.6 ± 7.2 %; p = 0.04), and consequently a lower percentage of time asleep (BPD: 78.5 ± 6.6 %; BD: 86.6 ± 7.3 %; p = 0.04). Comparing these results, BPD patients were awake about three quarters of an hour more than those with BD. Though these three results became not statistically significant after Benjamini–Hochberg’s correction, their effect size was large. Time awake during the time in bed in the BPD and HC groups were not significantly different. Although the null hypothesis was not rejected between the three groups, BPD and HC adolescents seem to show more interdaily total sleep time variability than BD youths (large effect size).
On schedule-free days (Table 4), BD and BPD youths both spent more time in bed than HC adolescents (non significant after Benjamini–Hochberg’s correction, but the effect size was large). All groups of patients went to bed at the same time, however the clinical groups rose up one hour later, which meant they spent more time in bed and slept longer. This result remained statistically significant after correction and the effect size was moderate (total sleep time: BPD vs. HC) to large (time in bed: BPD vs. HC; time in bed: BD vs. HC; total sleep time: BD vs. HC). Although the null hypothesis was not rejected between the three groups, BPD adolescents seem to show more interdaily total sleep time variability than BD and HC youths (large effect size). The BD group show less variability than the HC group (large effect size). Also, even though the result was not statistically significant, a large effect size was observed in the time that the clinical groups got out of bed compared to HC.
When data from school/work and schedule free days were combined, rise time in the two patient groups was about an hour later than HC adolescents (Table 5). Post hoc analyses showed that this difference was actually significant between the BPD and the HC group. The two clinical groups had more total sleep time than the HC group; the difference was found to be statistically significant only between BPD and HC adolescents. Variability analyses showed that total sleep time was more variable from one night to the other in BPD adolescents compared to the HC group. There was more variability in rising time in BPD adolescents compared to HC youths. All the differences found when data from school/work and schedule free days were combined were no longer significant after Benjamini–Hochberg’s correction, but their effect size remained large.
For school/work days, schedule-free days and both types of days combined, there were no significant differences for sleep onset latency, sleep efficiency and final awakening.
Discussion
The following trends emerged from this explorative study on sleep-wake patterns in BPD and BD adolescents: (1) stabilised euthymic BD adolescents sleep longer and better than BPD and HC adolescents on school/work days, (2) BD and BPD patients woke up later than the HC adolescents on schedule-free days and (3) BPD adolescents have a higher variability in total sleep time and in rise time compared to BD and HC youths. Of note, when differences were calculated, a number of results were significant when correction was not performed, but they were no longer statistically significant after applying Benjamini–Hochberg’s procedure. However, the calculated effect size was large for all these results, suggesting that they were not due to chance. Considering the small sample size and the exploratory nature of this research, correction might be too conservative [68, 69]. To determine if this is the case, replication through confirmation studies is needed with a larger sample size to increase statistical power and to determine clinical significance. Meanwhile, this section will discuss our exploratory data before statistical correction. Observations reported in this study should be viewed as interesting research questions that need further investigation, rather than definitive conclusive results.
Each of the two clinical groups showed selective differences compared to the HC group. We found that stabilised euthymic BD adolescents sleep longer and better than BPD and HC adolescents on school/work days. This result is in agreement with a previous report showing that euthymic BD youths (n = 13; all on mood stabilisers and/or antidepressants and none taking stimulants or hypnotics during data collection) slept longer than ADHD (n = 14; three patients with antidepressants and none with stimulants or hypnotics during data collection) and healthy youths (n = 21) according to actigraphic recordings [50]. Longer sleep duration has also been reported in previous actigraphy studies using euthymic BD adults [19, 20, 70, 71].
It could be argued that long sleep durations in our study could be due to the fact that more BD patients (67 %) than BPD adolescents (22 %) were being prescribed quetiapine, which has sedating qualities and which is effective to improve sleep quality and quantity [72]. However, other sedating medications were also prescribed to the BPD group (see Table 1). Pharmacotherapy cannot fully explain this finding. Another possible explanation could be that therapeutic interventions, including education about sleep, may be involved. In the present study, all BD adolescents, but none of the BPD youths, received psychoeducation about their disorder. Addressing the issue of sleep-wake disturbances during treatment seems to, therefore, be beneficial in euthymic BD adolescents. A similar approach may also help BPD youths to have better sleep hygiene. Presently, only Dialectical Behaviour Therapy addresses sleep problems in BPD [35]. Cognitive Behavioural Therapy can also be considered as a potential intervention to reduce dysfunctional sleep cognitions which maintain sleep disturbances [73] which are associated with non-recovery in BPD adults [74]. Luminotherapy constitutes another therapeutic strategy that can help improving certain aspects of BPD [75, 76].
On schedule-free days, we found that both BD and BPD patients woke up later than the HC group. Without any obligations during these schedule-free days, BD patients may have felt less pressured to comply with principles of sleep education, and optimal and stable sleep schedules. This may be interpreted as BD adolescents having a greater need to sleep during euthymic phases. We speculate that both clinical groups do not participate as much in activities requiring an early rising time during weekends (seeing friends for social activities, sport training, family gatherings) than the HC adolescents. Other studies report that BPD youths have more difficulties concerning peer relationships and family interactions compared to children and adolescents with other personality disorders or with other Axis I disorders [12, 77]. It can be hypothesized that this poor social functioning can lead to low participation in social activities. To the best of our knowledge, no study has specifically investigated social activities engagement in BD and BPD adolescents. Research in this area will shed light on this hypothesis.
Our results also show that BPD adolescents have a higher variability in total sleep time and in rise time compared to BD and HC youths. This observation is in agreement with a previous study by Verkes et al. [78] who examined 24 h periodicity to assess circadian variability using actigraphy. In actigraphy analysis, a 24 h periodicity refers to the presence of a peak of activity counts which is repeated every 24 h. Presence of 24 h periodicity can be translated as having a regular schedule for everyday life activities such as eating, sleeping, and physical activity. Non-24 periodicity defines the presence of more than one peak of motor activity or the presence of a single peak occurring in a time period smaller or larger than 24 h. Verkes et al. [78] found that among suicidal adults, in whom 69.5 % had a diagnosis of BPD, patients with a non-24 h periodicity had significantly more BPD symptoms than those with 24 h periodicity. Furthermore, non-24 h periodicity was significantly correlated with depression and hopelessness and an improvement of mood was associated with more regular bed times within subjects. This non-24 h periodicity reflected a diminished entrainment of the activity rhythm to zeitgebers in suicidal BPD adults.
Thus, irregular sleep pattern is a phenomenon observed in both adult and adolescent BPD. What is the impact of this sleep disturbance on mood and behaviour in adolescents, especially those with BPD symptomatology? In young adolescents without psychopathology, longer sleep duration during weekends and larger differences in total sleep time between weekdays and weekends increased the association between perceptions of conflict between parents (reported by the mother and the adolescent) and the adolescent’s level of aggressiveness [79]. It is proposed that irregular sleep duration and timing between weekdays and weekends cause a jetlag-like syndrome and increases a state of pre-existing vulnerability (emotional regulation difficulties, fatigue, irritability, and impulsiveness) weakening the adolescent’s capacities to cope with psychosocial stress [80] and exacerbating BPD symptoms. This is confirmed by our own observation according to which total sleep time and aggressiveness/impulsiveness are inversely related [41]. This supports the view that sleep disturbances actually BPD symptomatology.
Furthermore, better sleep quality seems to be associated with BPD recovery in adults [74]. This supports the view that sleep disturbances are actually associated with worse BPD symptomatology. The precise mechanisms underlying the association between sleep disturbances and symptoms in BD or BPD are currently unknown. There are a few hypotheses that have received preliminary support, however. For example, it was proposed that sleep loss may increase impulsivity and aggressiveness observed in BD and in BPD by decreasing serotonin activity [81, 82]. Low serotonin levels have been observed in both disorders [83, 84]. Sleep disturbances could also explain the reduced connectivity between the frontal cortex and the limbic system observed in these disorders [85–87], so the frontal cortex could not efficiently regulate the expression of intense emotions. Conversely, emotional arousal caused by BD or BPD symptoms can disrupt sleep. In the BPD literature, it has been suggested that emotional cascades during the day, which are defined as self-amplifying loops between rumination and negative emotions leading to emotional dysregulation behaviours, increases arousal during sleep, which can lead to night awakenings and nightmares [88]. Moreover, maladaptive sleep-related cognitions can explain why sleep disturbances are more prevalent in non-recovered BPD patients compared to recovered BPD patients [73, 74]. Further research is needed to uncover the mechanisms that link sleep and emotions and behaviours in BD and BPD. This will hopefully allow for a better understanding of the nature and phenomenology of sleep disturbances in these two psychiatric disorders and eventually improve assessment and treatment.
This study has methodological limitations that need to be considered. First, the sample size was small, particularly in the BD group, so that the present results need to be confirmed with more participants. Power calculations were performed to estimate the sample size required (44 participants per group). Based on prevalence of BPD and of BD at Rivière-des-Prairies Hospital in the last 2 years (2004–2005) before the beginning of data collection, the calculated targeted number of participants seemed realistic. During the data collection period (August 2006–December 2011), 100 adolescents registered at Rivière-des-Prairies Hospital received an official diagnosis of BPD and 33 other adolescents were diagnosed with BD. However, only 58 BPD and 23 TB patients were eligible based on our inclusion and exclusion criteria. This low eligibility rate was explained mainly by the fact that many patients had a current mood episode or still had residual depressive symptoms during our recruitment period. In other words, euthymia was the limiting criterion. Also, consent rate was low and not all participants who consented completed the study. Therefore, the small sample size could be explained by a low number of eligible euthymic participants who consented and completed the study. Second, we could not control for factors which may influence sleep patterns, such as sex, socio-economical status, race, pubertal development, BMI, and drug abuse, by analyzing subgroups. Third, a number of patients used illicit drugs and/or were prescribed medication during data collection. Although these factors have an impact on sleep, they are extremely common in BPD and BD patients seen in clinical settings. To perform a pharmacological washout of many days with adolescents suffering from severe psychopathology would be unethical and would be unrepresentative of these clinical populations. Our results may possibly include the effects of medication and/or illicit drugs use. Because of the sample size, it was not possible to control for this important confounding factor. A replication study taking this factor into account is thus needed. Finally, although this study attempted to control depression as a cofounding factor by excluding BPD patients with current depressive disorders, it is acknowledged that this sample is therefore selective and can be atypical from BPD adolescents seen in clinical settings [89].
Beyond these limitations, this study presents methodological strengths. Unlike previous studies, especially those concerning paediatric BD, our groups did not include prepubertal children. Moreover, we used strict diagnostic criteria. All patients had to present all DSM-IV criteria for their respective disorder, including the required duration and number of symptoms. In the case of BD, patients had to have a mania episode as defined by the DSM-IV, including cardinal symptoms such as euphoric mood and grandiosity for at least 4 days (or less if hospitalisation was needed). By doing so, patients who presented severe mood dysregulation and not BD [90] were not included and all patients were euthymic. Finally, using actigraphy as an ambulatory assessment, we were able to examine sleep-wake patterns as they occur in naturalistic environments.
Although this study is exploratory, it is among the first to examine sleep-wake patterns in BPD adolescents using actigraphy. This research also brings further evidence that sleep remains a crucial issue that needs to be addressed during adolescence, both in the presence and in the absence of psychopathology [26]. The presence of sleep disturbances in adults with BD is acknowledged in clinical practice and these are often assessed and addressed during therapy. For example, interventions targeting sleep disturbances, such as education about sleep and stabilisation of social rhythms, exist for BD adult patients to prevent relapse and maintain euthymia [91]. In the present research, we found that such interventions were also beneficial for euthymic BD adolescents: they actually sleep longer and better that the two other groups, probably because sleep disturbances were targeted during treatment. In BPD adolescents, on the contrary, sleep problems and sleep-wake patterns variability were not systematically addressed during assessment and therapy. Targeting sleep at the beginning of the disorder when the BPD diagnosis is made could contribute improving cognition and behaviour and introduce a positive asset in the course of their disorder.
Education about sleep should prove to be beneficial for youths with psychopathology. Healthy adolescents naturally experience a phase delay and many suffer from sleep restriction due to disturbing external factors. Unfortunately, they are not made aware about simple sleep hygiene rules that can improve sleep duration and quality [25]. More generally, we know that over 40 % of adolescents have sleep problems, with negative consequences on school performance and mood of students [25, 92]. As it is the case for drug use and sexuality, education about sleep should thus also be implemented in schools so that teachers, school psychologists and nurses as well as administrators are informed and involved. Such a strategy could prevent the worsening of mood and behavior problems in adolescents, with or without psychopathological problems.
The present study also demonstrates that use of actigraphy in BD and BPD adolescents is feasible and useful in order to collect data concerning sleep-wake patterns in a natural environment: as of yet, few studies have used actigraphy in such patients [76]. Further research should combine actigraphy with additional ambulatory assessment tools (applications for tablets and smart phones) to examine more behavioral and emotional aspects of BD and BPD adolescents in real-time [93]. This should allow for a better understanding of the association between sleep-wake patterns and psychopathological manifestations.
Summary
In sum, this research suggests that sleep-wake disruptions are part of BD and BPD pathology, even in the absence of depression. We hypothesize that these problems can lead to impairment in daily functioning. This reinforces our overall key messages: (1) sleep disturbances are more than mere symptoms, they constitute factors contributing to the onset, maintenance or relapse of psychopathologies; (2) sleep should be systematically assessed and addressed while treating adolescents with BD or BPD due to the influence of sleep on quality of life, daily functioning, and symptoms [94, 95].
References
Frankenburg FR, Zanarini MC (2004) The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry 65(12):1660–1665
Semiz UB, Basoglu C, Ebrinc S, Cetin M (2008) Nightmare disorder, dream anxiety, and subjective sleep quality in patients with borderline personality disorder. Psychiatry Clin Neurosci 62(1):48–55
Schredl M, Paul F, Reinhard I, Ebner-Priemer UW, Schmahl C, Bohus M (2012) Sleep and dreaming in patients with borderline personality disorder: a polysomnographic study. Psychiatry Res 200(2–3):430–436
Harty L, Duckworth R, Thompson A, Stuewig J, Tangney JP (2010) Are inmates’ subjective sleep problems associated with borderline personality, psychopathy, and antisocial personality independent of depression and substance dependence? J Forensic Psychiatry Psychol 21(1):23–39
Huynh C, Guilé JM, Godbout R (2012) Polysomnographic studies on sleep in adult borderline personality disorder. Presse Med 41(2):e63–e75
Fleischer M, Schafer M, Coogan A, Hassler F, Thome J (2012) Sleep disturbances and circadian CLOCK genes in borderline personality disorder. J Neural Transm 119(10):1105–1110
McNamara E, Reynolds CF 3rd, Soloff PH, Mathias R, Rossi A, Spiker D et al (1984) EEG sleep evaluation of depression in borderline patients. Am J Psychiatry 141(2):182–186
Reynolds CF 3rd, Soloff PH, Kupfer DJ, Taska LS, Restifo K, Coble PA et al (1985) Depression in borderline patients: a prospective EEG sleep study. Psychiatry Res 14(1):1–15
De la Fuente JM, Bobes J, Vizuete C, Mendlewicz J (2001) Sleep-EEG in borderline patients without concomitant major depression: a comparison with major depressives and normal control subjects. Psychiatry Res 105(1–2):87–95
Lahmeyer HW, Val E, Gaviria FM, Prasad RB, Pandey GN, Rodgers P et al (1988) EEG sleep, lithium transport, dexamethasone suppression, and monoamine oxidase activity in borderline personality disorder. Psychiatry Res 25(1):19–30
Speranza M, Revah-Levy A, Cortese S, Falissard B, Pham-Scottez A, Corcos M (2011) ADHD in adolescents with borderline personality disorder. BMC Psychiatry 11:158
Chanen AM, Jovev M, Jackson HJ (2007) Adaptive functioning and psychiatric symptoms in adolescents with borderline personality disorder. J Clin Psychiatry 68(2):297–306
Beatson JA, Rao S (2013) Depression and borderline personality disorder. Med J Aust 199(6 Suppl):S24–S27
Koenigsberg HW, Harvey PD, Mitropoulou V, Schmeidler J, New AS, Goodman M et al (2002) Characterizing affective instability in borderline personality disorder. Am J Psychiatry 159(5):784–788
American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders : DSM-IV-TR, 4th edn. American Psychiatric Association, Washington, DC
World Health Organization (2008) International Statistical Classification of Diseases and Related Health Problems. 10th, revision edn. World Health Organization, Geneva
Jackson A, Cavanagh J, Scott J (2003) A systematic review of manic and depressive prodromes. J Affect Disord 74(3):209–217
Brill S, Penagaluri P, Roberts RJ, Gao Y, El-Mallakh RS (2011) Sleep disturbances in euthymic bipolar patients. Ann Clin Psychiatry 23(2):113–116
Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM (2005) Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry 162(1):50–57
Jones SH, Hare DJ, Evershed K (2005) Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord 7(2):176–186
Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC (1980) Pubertal changes in daytime sleepiness. Sleep 2(4):453–460
Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J (2001) Development of sleep patterns in early adolescence. J Sleep Res 10(1):59–67
Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R (1997) An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms 12(3):278–289
Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R (1998) Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep 21(8):871–881
Godbout R, Huynh C, Martello E (2010) Le sommeil et les adolescents. Revue québécoise de psychologie 31(2):133–148
Brand S, Kirov R (2011) Sleep and its importance in adolescence and in common adolescent somatic and psychiatric conditions. Int J Gen Med 4:425–442
Soffer-Dudek N, Sadeh A, Dahl RE, Rosenblat-Stein S (2011) Poor sleep quality predicts deficient emotion information processing over time in early adolescence. Sleep 34(11):1499–1508
Dagys N, McGlinchey EL, Talbot LS, Kaplan KA, Dahl RE, Harvey AG (2012) Double trouble? The effects of sleep deprivation and chronotype on adolescent affect. J Child Psychol Psychiatry 53(6):660–667
Roberts RE, Duong HT (2014) The prospective association between sleep deprivation and depression among adolescents. Sleep 37(2):239–244
Gregory AM, Sadeh A (2012) Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Med Rev 16(2):129–136
Selby EA (2013) Chronic sleep disturbances and borderline personality disorder symptoms. J Consult Clin Psychol 81(5):941–947
Harvey AG (2009) The adverse consequences of sleep disturbance in pediatric bipolar disorder: implications for intervention. Child Adolesc Psychiatr Clin N Am 18(2):321–338
Brent DA, Johnson BA, Perper J, Connolly J, Bridge J, Bartle S et al (1994) Personality disorder, personality traits, impulsive violence, and completed suicide in adolescents. J Am Acad Child Adolesc Psychiatry 33(8):1080–1086
Halfon N, Labelle R, Cohen D, Guile JM, Breton JJ (2013) Juvenile bipolar disorder and suicidality: a review of the last 10 years of literature. Eur Child Adolesc Psychiatry 22(3):139–151
Winsper C, Tang NK (2014) Linkages between insomnia and suicidality: prospective associations, high-risk subgroups and possible psychological mechanisms. Int Rev Psychiatry 26(2):189–204
Koehne K, Hamilton B, Sands N, Humphreys C (2013) Working around a contested diagnosis: borderline personality disorder in adolescence. Health 17(1):37–56
Chanen A, McCutcheon LK (2008) Personality disorder in adolescence: the diagnosis that dare not speak its name. Personal Ment Health 2(1):35–41
Paris J (2013) Personality disorders begin in adolescence. J Can Acad Child Adolesc Psychiatry 22(3):195–196
Miller AL, Muehlenkamp JJ, Jacobson CM (2008) Fact or fiction: diagnosing borderline personality disorder in adolescents. Clin Psychol Rev 28(6):969–981
Guilé JM, Huynh C, Desrosiers L, Bouvier H, MacKay J, Chevrier É et al (2009) Exploring sleep disturbances in adolescent borderline personality disorder using actigraphy: a case report. Int J Adolesc Med Health 21(1):123–126
Huynh C, Guilé JM, Breton JJ, Godbout R (2012) Sleep and mood in adolescents with a borderline personality disorder. vol. Suppl. 35. Boston (MA): Sleep: A333
Kowatch RA, Youngstrom EA, Danielyan A, Findling RL (2005) Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord 7(6):483–496
Geller B, Zimerman B, Williams M, Delbello MP, Frazier J, Beringer L (2002) Phenomenology of prepubertal and early adolescent bipolar disorder: examples of elated mood, grandiose behaviors, decreased need for sleep, racing thoughts and hypersexuality. J Child Adolesc Psychopharmacol 12(1):3–9
Staton D (2008) The impairment of pediatric bipolar sleep: hypotheses regarding a core defect and phenotype-specific sleep disturbances. J Affect Disord 108(3):199–206
Staton D, Volness LJ, Beatty WW (2008) Diagnosis and classification of pediatric bipolar disorder. J Affect Disord 105(1–3):205–212
Baroni A, Hernandez M, Grant MC, Faedda GL (2012) Sleep disturbances in pediatric bipolar disorder: a comparison between bipolar I and bipolar NOS. Front Psychiatry, 3(22)
Faedda GL, Baldessarini RJ, Glovinsky IP, Austin NB (2004) Pediatric bipolar disorder: phenomenology and course of illness. Bipolar Disord 6(4):305–313
Roybal DJ, Chang KD, Chen MC, Howe ME, Gotlib IH, Singh MK (2011) Characterization and factors associated with sleep quality in adolescents with bipolar I disorder. Child Psychiatry Hum Dev 42(6):724–740
Ritter PS, Marx C, Bauer M, Leopold K, Pfennig A (2011) The role of disturbed sleep in the early recognition of bipolar disorder: a systematic review. Bipolar Disord 13(3):227–237
Mullin BC, Harvey AG, Hinshaw SP (2011) A preliminary study of sleep in adolescents with bipolar disorder, ADHD, and non-patient controls. Bipolar Disord 13(4):425–432
Achenbach TM, Rescorla LA (2001) Manual for the ASEBA School-Age Forms & Profilesedition. University of Vermont, Research Center for Children, Youth & Families, Burlington, Vermont
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al (1997) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36(7):980–988
Ivanova MY, Dobrean A, Dopfner M, Erol N, Fombonne E, Fonseca AC et al (2007) Testing the 8-syndrome structure of the child behavior checklist in 30 societies. J Clin Child Adolesc Psychol 36(3):405–417
Gunderson JG, Kolb JE, Austin V (1981) The diagnostic interview for borderline patients. Am J Psychiatry 138(7):896–903
Zanarini M, Gunderson J, Frankenburg FR, Chauncey D (1989) The revised diagonstic interview for borderlines: discriminating borderline from other axis II disorders. J Pers Disord 3(1):10–18
Guilé JM, Greenfield B, Berthiaume C, Chapdelaine C, Bergeron L (2009) Reliability and diagnostic efficiency of the abbreviated-diagnostic interview for borderlines in an adolescent clinical population. Eur Child Adolesc Psychiatry 18(9):575–581
Beck AT, Steer RA, Ball R, Ranieri W (1996) Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess 67(3):588–597
Steer RA, Ball R, Ranieri WF, Beck AT (1997) Further evidence for the construct validity of the Beck Depression Inventory-II with psychiatric outpatients. Psychol Rep 80(2):443–446
Myers K, Winters NC (2002) Ten-year review of rating scales. II: scales for internalizing disorders. J Am Acad Child Adolesc Psychiatry 41(6):634–659
Stanley B, Wilson ST (2006) Heightened subjective experience of depression in borderline personality disorder. J Pers Disord 20(4):307–318
Young RC, Biggs JT, Ziegler VE, Meyer DA (1978) A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133(5):429–435
Fristad MA, Weller EB, Weller RA (1992) The mania rating scale: can it be used in children? A preliminary report. J Am Acad Child Adolesc Psychiatry 31(2):252–257
Youngstrom EA, Danielson CK, Findling RL, Gracious BL, Calabrese JR (2002) Factor structure of the young mania rating scale for use with youths ages 5 to 17 years. J Clin Child Adolesc Psychol 31(4):567–572
Favre S, Aubry JM, Gex-Fabry M, Ragama-Pardos E, McQuillan A, Bertschy G (2003) Translation and validation of a French version of the young mania rating scale (YMRS). Encephale 29(6):499–505
Chen KY, Bassett DR Jr (2005) The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc 37(11 Suppl):S490–S500
Paquet J, Kawinska A, Carrier J (2007) Wake detection capacity of actigraphy during sleep. Sleep 30(10):1362–1369
Huynh C, Guilé JM, Breton JJ, Desrosiers L, Cohen D, Godbout R (2010) Is it possible to study sleep-wake patterns in adolescent borderline personality disorder? An actigraphic feasibility study. Int J Adolesc Med Health 22(4):547–560
Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ 316(7139):1236–1238
Bender R, Lange S (1999) Multiple test procedures other than Bonferroni’s deserve wider use. BMJ 318(7183):600–601
Millar A, Espie CA, Scott J (2004) The sleep of remitted bipolar outpatients: a controlled naturalistic study using actigraphy. J Affect Disord 80(2–3):145–153
Ritter PS, Marx C, Lewtschenko N, Pfeiffer S, Leopold K, Bauer M et al (2012) The characteristics of sleep in patients with manifest bipolar disorder, subjects at high risk of developing the disease and healthy controls. J Neural Transm 119(10):1173–1184
Cohrs S, Gade K, Meier A, Jordan W, Falkai P, Ruther E et al (2010) Quetiapine improves sleep disturbance in acute bipolar disorder: a case series. Pharmacopsychiatry 43(4):154–155
Plante DT, Frankenburg FR, Fitzmaurice GM, Zanarini MC (2013) Relationship between maladaptive cognitions about sleep and recovery in patients with borderline personality disorder. Psychiatry Res 210(3):975–979
Plante DT, Frankenburg FR, Fitzmaurice GM, Zanarini MC (2013) Relationship between sleep disturbance and recovery in patients with borderline personality disorder. J Psychosom Res 74(4):278–282
Prasko J, Brunovsky M, Latalova K, Grambal A, Raszka M, Vyskocilova J et al (2010) Augmentation of antidepressants with bright light therapy in patients with comorbid depression and borderline personality disorder. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 154(4):355–361
Bromundt V, Wirz-Justice A, Kyburz S, Opwis K, Dammann G, Cajochen C (2013) Circadian sleep-wake cycles, well-being, and light therapy in borderline personality disorder. J Pers Disord 27(5):680–696
Wolke D, Schreier A, Zanarini MC, Winsper C (2012) Bullied by peers in childhood and borderline personality symptoms at 11 years of age: a prospective study. J Child Psychol Psychiatry 53(8):846–855
Verkes RJ, Kerkhof GA, Beld E, Hengeveld MW, van Kempen GM (1996) Suicidality, circadian activity rhythms and platelet serotonergic measures in patients with recurrent suicidal behaviour. Acta Psychiatry Scand 93(1):27–34
Lemola S, Schwarz B, Siffert A (2012) Interparental conflict and early adolescents’ aggression: is irregular sleep a vulnerability factor? J Adolesc 35(1):97–105
Dahl RE, Lewin DS (2002) Pathways to adolescent health sleep regulation and behavior. J Adolesc Health 31(6 Suppl):175–184
Kamphuis J, Meerlo P, Koolhaas JM, Lancel M (2012) Poor sleep as a potential causal factor in aggression and violence. Sleep Med 13(4):327–334
Monti JM (2011) Serotonin control of sleep-wake behavior. Sleep Med Rev 15(4):269–281
New AS, Goodman M, Triebwasser J, Siever LJ (2008) Recent advances in the biological study of personality disorders. Psychiatr Clin North Am 31(3):441–461
Miklowitz DJ, Johnson SL (2006) The psychopathology and treatment of bipolar disorder. Annu Rev Clin Psychol 2:199–235
McKenna BS, Eyler LT (2012) Overlapping prefrontal systems involved in cognitive and emotional processing in euthymic bipolar disorder and following sleep deprivation: a review of functional neuroimaging studies. Clin Psychol Rev 32(7):650–663
Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP (2007) The human emotional brain without sleep–a prefrontal amygdala disconnect. Curr Biol 17(20):R877–R878
Lis E, Greenfield B, Henry M, Guile JM, Dougherty G (2007) Neuroimaging and genetics of borderline personality disorder: a review. J Psychiatry Neurosci 32(3):162–173
Selby EA, Ribeiro JD, Joiner TE (2013) What dreams may come: emotional cascades and nightmares in borderline personality disorder. Dreaming 23(2):126–144
Oltmanns JR, Oltmanns TF (2015) Borderline personality pathology, polysomnography, and self-reported sleep problems: a review. Curr Sleep Med Rep 1(2):141–149
Leibenluft E (2011) Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry 168(2):129–142
Naismith SL, Hermens DF, Ip TK, Bolitho S, Scott E, Rogers NL et al (2012) Circadian profiles in young people during the early stages of affective disorder. Transl Psychiatry 2:e123
Vignau J, Bailly D, Duhamel A, Vervaecke P, Beuscart R, Collinet C (1997) Epidemiologic study of sleep quality and troubles in French secondary school adolescents. J Adolesc Health 21(5):343–350
Trull TJ, Ebner-Priemer U (2013) Ambulatory assessment. Annu Rev Clin Psychol 9:151–176
Plante DT, Winkelman JW (2008) Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry 165(7):830–843
Fava M (2004) Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry 65(Suppl 16):27–32
Acknowledgments
This research was made possible with the financial support of Opération Enfant-Soleil, Fondation des Petits Trésors of Rivière-des-Prairies Hospital, Fonds de la recherche en santé du Québec (FRSQ), Fondation Pfizer and Fondation André Dédé Fortin. The authors acknowledge the skilful technical assistance of Élyse Chevrier, the collaboration of Dr. Martin Gignac from Institute Philippe-Pinel de Montréal and of the therapists from the Mood Disorders Clinic. We also express our appreciation to the adolescents for their participation.
Conflicts of interest
The authors have no financial or other conflicts of interest to disclose, and no sources of funding to acknowledge.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huỳnh, C., Guilé, JM., Breton, JJ. et al. Sleep-Wake Patterns of Adolescents with Borderline Personality Disorder and Bipolar Disorder. Child Psychiatry Hum Dev 47, 202–214 (2016). https://doi.org/10.1007/s10578-015-0557-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10578-015-0557-8