Abstract
Supernumerary B chromosomes (Bs) are nonessential chromosomes that are considered genetically inert. However, the maize B carries control elements that direct its behavior, such as that of nondisjunction, during the second pollen mitosis, and affects normal A chromosomes during cell division. Recently, the maize B has been found to contain transcriptionally active sequences and to affect the transcription of genes on A chromosomes. To better understand the regulatory mechanisms underlying the maize B, we constructed two small RNA libraries from maize B73 inbred lines with and without Bs. The sequencing results revealed that 18 known microRNAs (miRNAs) were significantly differentially expressed in response to the presence of the B, and most target mRNAs were characterized as transcription factors. Moreover, three novel B-derived miRNAs were identified via stem-loop reverse transcriptase-polymerase chain reaction (RT-PCR)-based analysis, and all showed consistent B-specific expression in almost all analyzed inbred lines and in all tissue types, including leaves, roots, and pollen grains. By the use of B-10L translocations, the three B-derived miRNAs were mapped to specific B regions. The results from this study suggest that the maize B can express miRNAs and affect the expression of A-derived miRNAs, which could regulate the expression of A-located genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Supernumerary B chromosomes (Bs) are nonessential chromosomes of the genome and are present in approximately 15% of eukaryotic organisms, and it is widely believed that Bs are derived from standard A chromosomes from the same species or different species (reviewed in Marques et al. 2018; Ahmad and Martins 2019). In plants, the first B was identified in maize (Kuwada 1915), and its cytological nature and genetic behaviors have since been well characterized. However, the detailed molecular organization, expression, and evolution of this chromosome are still unclear. For a long time, Bs were thought to be genetically inert (Jones et al. 2008). However, the maize B is not entirely without genetic activity. For instance, the maize B can increase the recombination frequencies of A chromosomes (Rhoades 1968), elicit breakages in A chromosomes at the second pollen mitosis (Rhoades and Dempsey 1972), and reduce fertility (Randolph 1941) and cause leaf striping (Staub 1987) when present at multiple copies.
Because the maize B is not essential for plant development, it has evolved several mechanisms to ensure its survival. There are three mechanisms that allow it to be maintained and increase in number: nondisjunction during the second pollen mitosis (Longley 1927; Roman 1947), preferential fertilization of the egg by the sperm carrying the B (Roman 1948), and prevention of lost through meiosis as a univalent (Carlson 1986). Factors that control the accumulation mechanisms are located in specific chromosomal regions. Nondisjunction requires trans-acting factors located in the proximal and distal euchromatic regions of the B long arm (Ward 1973; Lin 1978). The centromeric knob (CK) and short arm of the B are associated with nondisjunction in a cis-acting manner (Carlson 1973; Lin 1979). The element that controls preferential fertilization is a single gene located on an A chromosome (Chiavarino et al. 1998, 2001; Gonzalez-Sanchez et al. 2003). The third heterochromatic region on the B long arm is required to prevent the loss of univalent Bs during meiosis (Carlson and Roseman 1992). Although the factors required for B survival have been mapped, no related genes have been identified, and the molecular mechanism involved is still unclear.
Understanding the molecular nature of the maize B is restrained by the extremely high levels of similarity between the DNA sequences of B and A chromosomes. For several decades, various approaches have been applied to overcome this hindrance, and numerous B-derived sequences have been identified. Almost all of these sequences are highly homologous to their A-derived homologs (Alfenito and Birchler 1993; Stark et al. 1996; Cheng and Lin 2003; Lamb et al. 2005; Peng et al. 2005; Lo et al. 2009; Kao et al. 2015). This homology suggests that a high degree of similarity should also exist between the B and A chromosomes at the RNA level. However, relatively few studies have investigated the transcriptional activity of the maize B, and the current evidence suggests that the maize B is transcriptionally active and that the expression of A-located genes is affected by the presence of this chromosome (Lamb et al. 2007; Lin et al. 2014; Kao et al. 2015; Huang et al. 2016). A comparison of the complementary DNA-amplified fragment length polymorphism (cDNA-AFLP) profiles of various maize inbred lines with and without Bs revealed that the maize B has a negative impact on A-located gene transcription (Lin et al. 2014). Moreover, RNA sequencing analysis was applied to analyze the transcriptome of maize B73 lines carrying various copies of Bs, and 115 upregulated and 15 downregulated A-located genes were detected in response to the presence of the B (Huang et al. 2016). The maize B is therefore obviously capable of regulating gene expression at the RNA level.
Small RNA (sRNA)-guided posttranscriptional regulatory mechanisms have been shown to play important roles in many aspects of plant biology, including metabolism, hormone responses, and epigenetic control of transposons (Liu and Chen 2009; Nag and Jack 2010; Lisch 2013). In plants, sRNAs can be categorized into several major classes, including microRNAs (miRNAs), heterochromatic small interfering RNAs (hc-siRNAs), phased small interfering RNAs (phased siRNAs), and natural antisense transcript small interfering RNAs (NAT-siRNAs), all of which regulate gene expression at the posttranscriptional level (Lu et al. 2005; Axtell 2013). Typically, miRNAs are approximately 22- to 24-nucleotide-long siRNA sequences that are generated from precursor miRNAs with a hairpin structure and then further processed into miRNA/miRNA* duplexes by Dicer-like (DCL) enzymes in the nucleus (Mallory and Vaucheret 2006). Mature miRNAs are then exported to the cytoplasm and incorporated into an RNA-induced silencing complex (RISC), which controls how complementary mRNAs undergo cleavage or translational repression (Li and Mao 2007). To date, the regulatory role of plant miRNAs has been exemplified by the critical regulatory behavior of miRNAs at key positions in a variety of pathways, such as root (Wang et al. 2005), shoot (Goiz 2006), leaf (Kidner and Martienssen 2004), and flower development (Mallory et al. 2004) and cell fate (Carraro et al. 2006). Heterochromatin has long been considered inert, but it is now believed to give rise to sRNAs, which, via RNA interference, direct the modification of proteins, DNA in heterochromatic repeats and transposable elements (Lippman and Martienssen 2004). Considering that the maize B contains a massive amount of heterochromatin and that the expression of some A-located genes is affected by the presence of the B, this chromosome should generate miRNAs or affect the expression of A-located miRNA genes.
The aims of this study were to verify whether the presence of the maize B can affect the expression of A-located miRNA genes and whether the B can generate miRNAs. We achieved these aims by constructing two sRNA libraries of maize B73 inbred lines with and without Bs, followed by sRNA sequencing. Comparison of known miRNAs in the two libraries revealed that the presence of the B affected the expression level of several classes of miRNAs. Using stem-loop reverse transcriptase-polymerase chain reaction (RT-PCR) analysis, we identified three novel B-derived miRNAs, and all showed consistent B-specific expression in different tissue types, including leaves, roots, and pollen grains. Furthermore, the B-specific expression of the three novel B-derived miRNAs was further confirmed in other inbred lines. By the use of B-10L translocations, the three B-derived miRNAs were mapped to specific B regions. The results from this study suggest that the maize B can generate miRNAs and affect the expression of A-located miRNA-encoding genes.
Materials and methods
Plant materials
Maize inbred lines carrying Bs were generated from a B-containing L289 plant via continuous backcrossing to four inbred lines, B73, W22, W23, and A619, for at least 15 generations. Four B-10L translocations (TB-10L18, TB-10L7, TB-10L26, and TB-10L36) in the W23 background were used to generate heterozygous translocation, tertiary trisomic and hypoploid plants. The procedure for producing the three plants of each translocation was performed as previously described (Lin et al. 2014). Chromosomal constitutions were determined in Feulgen-stained root tip cells (Lin 1977). Each translocation has a breakpoint at various positions along the B and a second breakpoint within the long arm of chromosome 10 (Lin 1979; Chien et al. 2014).
RNA isolation, library preparation, and sequencing
Total RNA was extracted by TRIzol® Reagent (Invitrogen, USA) according to the instruction manual. The RNA purity was quantified at OD260 nm using an ND-1000 spectrophotometer (Nanodrop Technology, USA) and purified using a Bioanalyzer 2100 (Agilent Technology, USA) with an RNA 6000 labchip kit (Agilent Technologies, USA). The sRNA library construction and deep sequencing were carried out at Biotechnology Company (Welgene, Taiwan). Samples were prepared using an Illumina sample preparation kit according to the TruSeq Small RNA Sample Preparation Guide. The 3′ and 5′ adapters were ligated to the total RNA, which was used to perform reverse transcription followed by PCR amplification. The enriched cDNAs were size fractionated on a 6% polyacrylamide gel, and the bands containing 18–40 nucleotide RNA fragments (140–155 nucleotides in length with both adapters) were purified. The libraries were sequenced on an Illumina instrument (75 cycle single read), and the resulting sequencing data were processed by Illumina software. The sRNA raw sequencing data are available from the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under accession PRJNA482490.
sRNA sequencing analysis
The sequences generated were initially filtered to obtain qualified reads. Trimmomatic was implemented to trim or remove the reads according to the quality score. After the low-quality (> 80% of Q20) data were filtered, the qualified reads were analyzed using MiRDeep2 to remove the 3′ adapter sequence and discard reads shorter than 18 nt and longer than 28 nt before the reads were aligned to the Zea mays genome from the NCBI database. Only reads that mapped perfectly to the genome five or fewer times were used for miRNA detection, since miRNAs usually map to few genomic locations. MiRDeep2 was then used to estimate the expression levels of known miRNAs and identify novel miRNAs (Friedländer et al. 2012).
Stem-loop RT-PCR analysis
Validation of potential B-derived miRNAs was carried out by stem-loop RT-PCR as described by Varkonyi-Gasic et al. (2007), with some modifications. Total RNA (0.2 μg) was treated via a Transcriptor High Fidelity cDNA Synthesis Kit (Roche Applied Science, Mannheim, Germany) with RT primers (Table 1). The reactions were incubated at 65 °C for 10 min, followed by 50 °C for 30 min. The reactions were then terminated by incubation at 85 °C for 5 min. PCR was carried out at a volume of 20 μl consisting of 1× Super Run EX-Taq buffer, 0.2 mM dNTPs, forward and reverse primers (at 0.2 μM each) (Table 1), and 5 μl of diluted cDNA under the following conditions: 94 °C for 2 min, followed by 35 cycles of 94 °C for 15 s and 58 °C for 1 min. miR156 was selected as an internal control. The PCR products were visualized on 4% agarose gels by ethidium bromide staining.
Results
Overview of sRNA libraries sequencing
To investigate sRNAs related to the maize B, we constructed two sRNA libraries from the third leaf of single B73 inbred plants containing no (B73+0B) and two Bs (B73+2B) at the four-leaf stage for deep sequencing. Total reads of 21,385,975 (B73+0B) and 22,871,438 (B73+2B) were generated by Illumina HiSeq in the two libraries. After removing the low-quality tags, null 3′-adapters, null inserts, 5′-adapter contaminants, polyA reads, and reads shorter than 18 nt and longer than 28 nt, 2,816,673 (B73+0B) and 2,806,967 (B73+2B) clean reads were obtained. Of these sRNAs, the 24 nt category was the most abundant, followed by 21 nt and 22 nt categories (Fig. 1). These results were consistent with the lengths of sRNAs in plants.
Differentially expressed known miRNA in the presence of the B
To identify known miRNAs, sRNA sequences were aligned against published maize miRNAs registered in miRBase (http://www.mirbase.org/). A total of 44 known miRNAs belonging to 18 miRNA families were identified (Suppl. Table S1). The expression of the known miRNAs in B73+0B and B73+2B was compared to identify changes in miRNA expression, and the results revealed that 18 miRNAs were significantly differentially expressed. Compared with the levels of miRNA expression between the B73+0B and B73+2B libraries, the expression levels of 9 miRNAs (miR528a/b, miR408b, miR164b/c/g, miR169m, miR171l, and miR396g) were upregulated (fold change greater than 2), whereas those of the other 9 miRNAs (miR164e, miR169a/b/o/r, and miR395a/h/j/p) were downregulated (fold change less than − 2) in the presence of the B (Fig. 2). These results suggested that the expression levels of some miRNAs changed significantly in response to the presence of the B in maize.
Identification of novel B-derived miRNAs
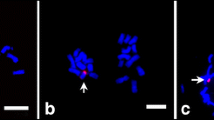
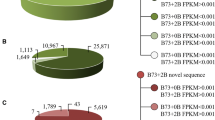
To identify the novel miRNAs derived from the maize, we mapped unannotated sequences to the genome sequences of maize and removed the reference genome reads from the sRNA sequencing results. As a result, B73+0B contained 107,665 nonreference sRNA reads, while B73+2B contained 133,354 reads. There were 76,785 common reads among the nonreference reads of B73+0B and B73+2B. Therefore, 30,880 nonreference reads were unique to B73+0B, and 56,569 nonreference reads were unique to B73+2B. The B73+2B unique nonreference reads were analyzed via MiRDeep2 to identify putative novel miRNAs, and stem-loop RT-PCR experiments further confirmed which novel miRNAs were derived from the B. We selected the top 14 B73+2B unique novel miRNAs on the basis of their expression levels and designed stem-loop RT primers to analyze the total RNA from the leaves of B73+0B, B73+1B, and B73+4B. Among these, the primers of three novel miRNAs of the maize B (miRmB1, miRmB2, and miRmB3) amplified B-specific PCR products from B73+1B and B73+4B but not from B73+0B (Table 1; Fig. 3). Moreover, the B-specific expression of the three B-derived miRNAs was consistent in all tested tissue types, including mature leaves, roots, and pollen grains (Fig. 4).
Stem-loop RT-PCR of B-derived miRNAs in different maize inbred lines. Stem-loop RT-PCR primers of three B-derived miRNAs, miRmB1, miRmB2, and miRmB3 were used to analyze the total RNA from the leaves of five maize inbred lines, B73, W22, W23, A679, and L289, with and without Bs. miR156 was used to confirm equal amounts of RNA. M 100 bp marker, NC water as a negative control
Stem-loop RT-PCR of B-derived miRNAs in different tissue types. Stem-loop RT-PCR primers of three B-derived miRNAs, miRmB1, miRmB2, and miRmB3, were used to analyze the total RNA from the mature leaves, roots, and pollen of B73+0B and B73+2B. miR156 was used to confirm equal amounts of RNA. M 100 bp marker, NC water as a negative control
We further verified the B-specificity of the three novel B-derived miRNAs in four other inbred backgrounds (W22, W23, A619, and L289; Fig. 3). The results revealed that the miRmB1 and miRmB2 primers amplified B-specific PCR products from all four inbred lines with Bs, with the exception that the miRmB2 primers amplified PCR products from L289 without Bs. The miRmB3 primers amplified B-specific PCR products from W22 and W23 but not from A619 and L289. The results suggested that these novel miRNAs are derived from the maize B but are also expressed from the A chromosomes of particular inbred lines.
Mapping of novel B-derived miRNA genes on the B
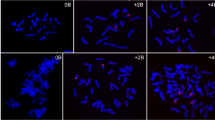
A systematic B deletion derived from four B-10L translocations (TB-10L18, TB-10L7, TB-10L26, and TB-10L36) in the W23 background was applied to map the positions of novel B-derived miRNA genes on the B. As shown in Fig. 5, the breakpoints of the four B-10L translocations are as follows: TB-10L18 is located within the short arm, TB-10L7 is located in the central region of the proximal euchromatin (PE), TB-10L26 is located near the junction of distal heterochromatin (DH) 2 and 3, and TB-10L36 is located between DH3 and DH4. The rationale of this mapping was as follows: when a B-specific RT-PCR product was amplified from the tertiary trisomic RNA of a B-10L translocation, its gene was mapped to the B portion proximal to the breakpoint. In contrast, when a B-specific RT-PCR product was amplified from the hypoploid RNA of a B-10L translocation, its gene was mapped to the B section distal to the breakpoint.
Map positions of B-derived miRNA genes (black lines) in relation to the breakpoints (arrows) of four B-10L translocations. S short arm, CK centromeric knob, PPE proximal half of the proximal euchromatin, DPE distal half of the proximal euchromatin, DH1–DH4 distal heterochromatin 1–4, DE distal euchromatin
Stem-loop RT-PCR primers of the three B-derived miRNAs were used to analyze the total RNA of B-10L translocations. As shown in Fig. 6, the B-specific PCR product of miRmB3 was present in the tertiary trisomic RNA of TB-10L18 and TB-10L7 but was absent in hypoploid RNA of both TB-10L18 and TB-10L7, suggesting that the position of miRmB3 gene was between the breakpoints of the two translocations, which contains the CK and the proximal half of PE (PPE). The map position of the miRmB1 gene could be assigned to DH3 because it could be amplified from the tertiary trisomic RNA of TB-10L36 and the hypoploid RNA of TB-10L26 but not from the tertiary trisomic RNA of TB-10L26 and the hypoploid RNA of TB-10L36. The B-specific PCR product of miRmB2 was present in the hypoploid RNA of TB-10L36 but was absent in the tertiary trisomic RNA of TB-10L36, suggesting that miRmB2 gene located in the region containing both DH4 and distal euchromatin (DE). The map positions of the three novel B-derived miRNA genes in relation to the B breakpoints of the four B-10L translocations are summarized in Fig. 5.
Mapping of B-derived miRNA genes by B-10L translocations. Stem-loop RT-PCR primers of three B-derived miRNAs, miRmB1, miRmB2, and miRmB3, were used to analyze the total RNA from W23+0B, W23+1B, and four B-10L translocations (TB-10L18, TB-10L7, TB-10L26, and TB-10L36). miR156 was used to confirm equal amounts of RNA. H translocational heterozygote, T tertiary trisome, O hypoploid, M 100 bp marker, NC water as a negative control
Discussion
The presence of the B affects the expression of A-located genes via miRNAs
Although the maize B is not essential for plant growth and development, its presence affects A chromosomes in some way (Rhoades 1968; Rhoades and Dempsey 1972; Randolph 1941; Staub 1987). Current evidence has further revealed that the maize B is transcriptionally active and can affect the expression of A-located genes at the transcription level (Lin et al. 2014; Huang et al. 2016). How the B regulates the expression of A-located genes is unclear; however, it is known that sRNAs play important roles in gene regulation in plants (Jones-Rhoades et al. 2006). In this study, we identified 18 known miRNAs that were significantly differentially expressed in the presence of the B (Fig. 2). These known miRNAs were members of seven miRNA families, miR164, miR169, miR171, miR395, miR396, miR408 and miR528, and their target genes have previously been predicted in maize (Zhang et al. 2009). The members of four of these miRNA families, miR164, miR169, miR171 and miR396, target various families of transcription factors, such as no apical meristem (NAM) proteins, CCAAT-binding factors (CBFs), GRAS transcription factors, and zinc finger proteins, respectively. miR395 regulates sulfate metabolism by targeting sulfate transporter genes and ATP sulfurylase proteins. Both miR408 and miR528 target the copper-containing proteins cupredoxin and multicopper oxidase and lassase genes and thus might play a critical role in regulating physiological processes and stress responses. These results suggest that the maize B might affect the expression of A-located genes by miRNA-directed regulation in posttranscriptional regulation and transcription networks.
The maize B is capable of expressing miRNAs
Because nine known upregulated miRNAs were identified in the presence of the B, we were interested in whether any upregulated miRNAs were also expressed from the maize B. However, it is unable to discriminate between miRNA genes that are located on both the B and A chromosomes. To verify whether the B is capable of expressing miRNAs, we used MiRDeep2 to identify putative novel miRNAs in B73+2B unique nonreference reads and obtained three novel miRNAs that exhibited amplification from B73+Bs but not from B73+0B by stem-loop RT-PCR analysis (Table 1; Fig. 3). Moreover, the stem-loop RT-PCR primers of the three novel miRNAs amplified B-specific PCR products from at least three inbred lines carrying Bs (Fig. 3), and from the leaves, roots and pollen grains of B73+2B (Fig. 4). Via B-10L translocations, the three novel B-derived miRNA genes were mapped to specific B regions (Figs. 5 and 6). The results provide strong evidence that the B can express miRNAs. Those B-derived novel miRNAs might target B-located genes to direct the B’s behaviors during cell division, such as nondisjunction during the second pollen mitosis and prevention of a univalent B lost during meiosis.
In this study, we confirmed that the maize B is capable of affecting the expression of A-derived miRNAs. A total of 18 known miRNAs were differentially expressed in the presence of the B. We also identified three novel B-derived miRNAs and mapped their genes on specific B regions via B-10L translocations. Our results may improve the understanding of the molecular mechanisms that control the genetic behaviors and activities of the B in maize.
Abbreviations
- CBF:
-
CCAAT-binding factor
- cDNA-AFLP:
-
Complementary DNA-amplified fragment length polymorphism
- CK:
-
Centromeric knob
- DCL:
-
Dicer-like
- DE:
-
Distal euchromatin
- DH:
-
Distal heterochromatin
- DPE:
-
Distal portion of proximal euchromatin
- hc-siRNA:
-
Heterochromatic small interfering RNA
- miRNA:
-
MicroRNA
- NAM:
-
No apical meristem
- NAT-siRNA:
-
Natural antisense transcript small interfering RNA
- NCBI:
-
National Center for Biotechnology Information
- PE:
-
Proximal euchromatin
- Phased siRNA:
-
Phased small interfering RNA
- PPE:
-
Proximal portion of proximal euchromatin
- RISC:
-
RNA-induced silencing complex
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- sRNA:
-
Small RNA
- SRA:
-
Sequence read archive
References
Ahmad SF, Martins C (2019) The modern view of B chromosomes under the impact of high scale omics analysis. Cells 8:156
Alfenito MR, Birchler JA (1993) Molecular characterization of a maize B chromosome centric sequence. Genetics 135:589–597
Axtell MJ (2013) Classification and comparison of small RNAs from plants. Annu Rev Plant Biol 64:137–159
Carlson WR (1973) A procedure for localizing genetic factors controlling mitotic nondisjunction in the B chromosome of maize. Chromosoma 42:127–136
Carlson WR (1986) The B chromosome of maize. CRC Crit Rev Plant Sci 3:201–226
Carlson WR, Roseman RR (1992) A new property of the maize B chromosome. Genetics 131:211–223
Carraro N, Peaucelle A, Laufs P, Yraas J (2006) Cell differentiation and organ initiation at shoot apical meristem. Plant Mol Biol 60:811–826
Cheng YM, Lin BY (2003) Cloning and characterization of maize B chromosome sequences derived from microdissection. Genetics 164:299–310
Chiavarino AM, González-Sánchez M, Poggio L, Puertas MJ, Rosato M, Rosi P (2001) Is maize B chromosome preferential fertilization controlled by a single gene? Heredity 86:743–748
Chiavarino AM, Rosato M, Rosi P, Poggio L, Naranjo CA (1998) Localization of the gene controlling B chromosome transmission rate in maize (Zea mays ssp. mays, Poaceae). Am J Bot 85:1851–1585
Chien YL, Lin CY, Lo KL, Cheng YM (2014) Development and mapping of CL-repeat display markers on the maize B chromosome. Cytogenetic Genome Research 144:227–236
Friedländer MR, Mackowiak SD, Li N, Chen W, Rajewsky N (2012) miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res 40:37–52
Goiz JF (2006) Signaling between the shoot apical meristem and developing lateral organs. Plant Mol Biol 60:889–903
González-Sánchez M, González-González E, Molina F, Chiavarino AM, Rosato M, Puertas MJ (2003) One gene determines maize B chromosome accumulation by preferential fertilization; another gene(s) determines their meiotic loss. Heredity 90:122–129
Huang W, Du Y, Zhao X, Jin W (2016) B chromosome contains active genes and impacts the transcription of A chromosomes in maize (Zea mays L.). BMC Plant Biol 16:88
Jones RN, Viegas W, Houben A (2008) A century of B chromosomes in plants: So what? Ann Bot 101:767–775
Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol 57:19–53
Kao KW, Lin CY, Peng SF, Cheng YM (2015) Characterization of four B-chromosome-specific RAPDs and the development of SCAR markers on the maize B-chromosome. Mol Gen Genomics 290:431–441
Kidner CA, Martienssen RA (2004) Spatially restricted microRNA directs leaf polarity through ARGONAUTE1. Nature 428:81–84
Kuwada Y (1915) Ueber die Chromosomenzahl von Zea mays L. Bot Mag Tokoyo 29:83–89
Lamb JC, Kato A, Birchler JA (2005) Sequences associated with A chromosome centromeres are present throughout the maize B chromosome. Chromosoma 113:337–349
Lamb JC, Riddle NC, Cheng YM, Theuri J, Birchler JA (2007) Localization and transcription of a retrotransposon-derived element on the maize B chromosome. Chromosom Res 15:383–398
Li AL, Mao L (2007) Evolution of plant microRNA gene families. Cell Res 17:212–218
Lin BY (1977) A squash technique for studying the cytology of maize endosperm and other tissues. Stain Technol 52:197–201
Lin BY (1978) Regional control of nondisjunction of the B-chromosome in maize. Genetics 90:613–627
Lin BY (1979) Two new B-10L translocations involved in the control of nondisjunction of the B chromosome in maize. Genetics 92:931–945
Lin HZ, Lin WD, Lin CY, Peng SF, Cheng YM (2014) Characterization of maize B-chromosome-related transcripts isolated via cDNA-AFLP. Chromosoma 123:597–607
Lippman Z, Martienssen R (2004) The role of RNA interference in heterochromatic silencing. Nature 431:364–370
Lisch D (2013) How important are transposons for plant evolution? Nat Rev Genet 14:49–61
Liu Q, Chen YQ (2009) Insight into the mechanism of plant development: interactions of miRNA pathway with phytohormone response. Biochem Biophys Res Commun 384:1–5
Lo KL, Lin YP, Chen LJ, Lin BY (2009) Isolation and characterization of new maize B sequences from a microdissected library. Plant Mol Biol Report 27:350–354
Longley AE (1927) Supernumerary chromosomes in Zea mays. J Agric Res 35:769–784
Lu C, Tei SS, Luo S, Haudenschild CD, Meyers BC, Green PJ (2005) Elucidation of the small RNA component of the transcriptome. Science 309:1567–1569
Mallory AC, Vaucheret H (2006) Functions of microRNAs and related small RNAs in plants. Nat Genet 38:S31–S36
Mallory AC, Dugas DV, Bartel DP, Bartel B (2004) MicroRNA regulation of NAC-domain targets is required for proper formation and separation of adjacent embryonic, vegetative, and floral organs. Curr Biol 14:1035–1046
Marques A, Klemme S, Houben A (2018) Evolution of plant B chromosome enriched sequences. Genes 9:515
Nag A, Jack Y (2010) Sculpting the flower; the role of miRNAs in flower development. Curr Top Dev Biol 91:349–378
Peng SF, Lin YP, Lin BY (2005) Characterization of AFLP sequences from regions of maize B chromosome defined by 12 B-10L translocations. Genetics 169:375–388
Randolph LF (1941) Genetic characteristics of the B chromosomes in maize. Genetics 26:608–631
Rhoades MM (1968) Studies on the cytological basis of crossing over. In: Peacock WJ, Brock RD (eds) Replication and recombination of genetic material. Australian Acad Sci, Canberra, pp229–241.
Rhoades MM, Dempsey E (1972) On the mechanism of chromatin loss induced by the B chromosome of maize. Genetics 71:73–96
Roman H (1947) Mitotic nondisjunction in the case of interchanges involving the B-type chromosome in maize. Genetics 32:391–409
Roman H (1948) Directed fertilization in maize. Proc Natl Acad Sci U S A34:36–42
Stark EA, Connerton I, Bennett ST, Barnes SR, Parker JS, Forster JW (1996) Molecular analysis of the structure of the maize B-chromosome. Chromosom Res 4:15–23
Staub RW (1987) Leaf striping correlated with the presence of B chromosomes in maize. J Hered 78:71–74
Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3:12
Wang JW, Wang LJ, Mao YB, Cai WJ, Xue HW, Cen XY (2005) Control of root cap formation by MicroRNA-targeted auxin response factors in Arabidopsis. Plant Cell 17:2204–2216
Ward E (1973) Nondisjunction: localization of the controlling site in the maize B chromosome. Genetics 73:387–391
Zhang L, Chia JM, Kumari S, Stein JC, Liu Z, Narechania A, Maher CA, Guill K, McMullen MD, Ware D (2009) A genome-wide characterization of microRNA genes in maize. PLoS Genet 5:e1000716
Funding
This work was supported by grants from the Ministry of Science and Technology of Taiwan (MOST 104-2311-B-005-012-MY3 and MOST 107-2311-B-005-003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans De Jong.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Huang, YH., Peng, SF., Lin, YP. et al. The maize B chromosome is capable of expressing microRNAs and altering the expression of microRNAs derived from A chromosomes. Chromosome Res 28, 129–138 (2020). https://doi.org/10.1007/s10577-019-09620-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-019-09620-2