Abstract
Rye is an important and valuable gene resource for wheat improvement. However, due to extensive growing of cultivars with disease resistance genes from short arm of rye chromosome 1R and coevolution of pathogen virulence and host resistance, these cultivars successively lost resistance to pathogens. Identification and deployment of new resistance gene sources in rye are, therefore, of especial importance and urgency. A new wheat–rye line, designated as WR41-1, was produced through distant hybridization and chromosome engineering protocols between common wheat cultivar Xiaoyan 6 and rye cultivar German White. It was proved to be a new wheat–rye T4BL·4RL and T7AS·4RS translocation line using sequential genomic in situ hybridization (GISH), multicolor fluorescence in situ hybridization (mc-FISH), and expressed sequence tag-simple sequence repeat (EST-SSR) marker analysis. WR41-1 showed high levels of resistance to powdery mildew (Blumeria graminis f. sp. tritici, Bgt) pathogens prevalent in China at the adult growth stage and 13 of 23 Bgt isolates tested at the seedling stage. According to its resistant pattern to 23 different Bgt isolates, WR41-1 may possess new gene(s) for resistance to powdery mildew, which differed from previously identified and known powdery mildew genes from rye (Pm7, Pm8, Pm17, and Pm20). In addition, WR41-1 was cytologically stable, had a desirable fertility, and is expected to be useful in wheat improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Powdery mildew of wheat (Triticum aestivum L., 2n = 6x = 42, AABBDD) caused by Blumeria graminis f. sp. tritici (Bgt) is one of the most economically important wheat diseases in China and other parts of the world. Developing and deploying resistant cultivars is the most effective and environment friendly means to control the disease. However, as coevolution of pathogen virulence and host resistance and frequent emergence of virulent isolates, cultivars carrying a single isolate-specific resistance gene are often overcome by new virulent isolates and then become susceptible a few years after being grown for commercial production on a large scale. In recent years, especially in 2009, powdery mildew was prevalent in the main wheat-producing regions of China and threaten wheat production (He et al. 2011). Therefore, it is considered valid for prolongation of disease resistance of cultivars to exploit new durable resistance genes or pyramid different resistance genes.
Rye (Secale cereale L., 2n = 2x = 14, RR), a species closely related to wheat, has been used extensively as a valuable source for wheat in improving disease resistance, yield, and environment adaptation (Friebe et al. 1996). The wheat–rye T1BL·1RS chromosome translocation, possessing genes for resistance to diseases and pests, and for high yield and broad adaptability on the 1RS arm, has been intensively utilized in wheat breeding programs and production for many years around the world (Jiang et al. 1994). However, due to the development of virulent isolates in the pathogen populations, the resistance genes that originated from rye are no longer effective, including the powdery mildew resistance gene Pm8, along with stripe rust (Puccinia striiformis Westend. f. sp. tritici Eriks.) resistance gene Yr9, stem rust (P. graminis Pers. f. sp. tritici Eriks) resistance gene Sr31, leaf rust (P. triticina Eriks.) resistance gene Lr26 derived from 1RS of Petkus rye, and powdery mildew resistance gene Pm17 from 1RS of Insave rye and Pm7 derived from 2RL of Rosen rye (Zhuang and Li 1993; Zhuang 2003). Therefore, it is critical to develop desirable germplasm and search for novel resistance gene sources from other rye genotypes against new virulent isolates.
Winter rye cultivar German White is a valuable resistant resource for wheat improvement due to its superior resistance to various isolates of powdery mildew pathogens prevalent in China. Common winter wheat cultivar Xiaoyan 6 was developed by Prof. Zhensheng Li and his colleagues from a wheat–Thinopyrum ponticum (2n = 10x = 70) cross. Because the cultivar possesses the characters of high-yielding, good bread-making quality with high molecular weight glutenin subunits (HMW-GS) 1Bx14 and 1By15 (He et al. 2001), early maturity, stress tolerance, and wide adaptation, it has been widely grown for two decades (Zhuang 2003). Xiaoyan 6 has also been extensively used as a founder parent for developing more than 50 wheat cultivars in China since it was released in 1980 (Li et al. 2008). However, Xiaoyan 6 was susceptible to powdery mildew. To improve its powdery mildew resistance, we transferred chromosomes or chromosome segments of German White rye into Xiaoyan 6 by distant hybridization, chromosome manipulation, and self-cross for many generations since 1995, resulting in a number of wheat–rye hybrids, including alien chromosome translocation (Wang et al. 2009b), substitution (An et al. 2006), and addition lines (An et al. unpublished data). Among them, a new wheat–rye 4R chromosome translocation line, WR41-1, showed a high level of resistance to powdery mildew in wheat growing areas. The objectives of this study were to deal with the development of chromosome translocation line, determine the genomic composition of WR41-1 using molecular cytogenetic methods, characterize its resistance to powdery mildew using different isolates of the pathogens, and evaluate its agronomic performance.
Materials and methods
Plant materials
A wheat–rye line, designated as WR41-1, was produced by crossing winter wheat cultivar Xiaoyan 6 with winter rye cultivar German White. Xiaoyan 6 was developed from a cross between common wheat and T. ponticum (Li et al. 2008). Wheat cultivars Mingxian 169 and Huixianhong were used in this study as susceptible controls for testing resistance to powdery mildew. To determine the resistance gene in WR41-1, a differential set of 38 wheat genotypes carrying known powdery mildew resistance gene (Pm) or gene combinations were used as controls, including CI14189 with Pm7 derived from rye chromosome 2RL, Kavkaz with Pm8, and Amigo with Pm17 both from rye chromosome 1RS, and TAM104/Thatcher with Pm20 from rye chromosome 6RL. The differential set was tested with 23 single-pustule-derived powdery mildew virulent isolates in the same way in order to compare their disease responses.
Total DNA extracted from wheat cultivar Chinese Spring (CS, ABD genomes) was used as blocking DNA in genomic in situ hybridization (GISH) and multicolor fluorescence in situ hybridization (mc-FISH) detection. We used the following lines as controls to detect rye chromatin in WR41-1 by polymerase chain reaction (PCR) analysis: two T1BL·1RS wheat–rye chromosome translocation lines, Lovrin 10 and Lovrin 13 (Rabinovich 1998); two triticale lines, 06CT456 and 06CT461 (AABBRR); three wheat–rye lines (WR64, WR81, and WR91) of ‘Xiaoyan 6 × German White’ developed and identified by using GISH and mc-FISH; the complete set disomic addition lines (DA1R to 7R); and 4RS and 4RL ditelosomic addition lines of ‘CS × Imperial’ kindly provided by Dr S. Reader (John Innes Centre, Norwich, UK).
Production of wheat–rye chromosome translocation line
Crosses between wheat cultivar Xiaoyan 6 and rye cultivar German White were performed in 1995. Sixteen to 18 days after pollination, inflorescences were collected and stored at 4 °C for 48–72 h. Hybrid embryos were dissected from inflorescences and 17 plants were obtained by embryo rescue on MS medium (Murashige and Skoog 1962). Following the doubling of chromosomes of the wheat–rye hybrids F1 with a solution containing 0.05 % colchicine, 1.5 % dimethylsulfoxide and 5 % MS medium (An et al. 2003), eight amphidiploid plants were obtained and karyotyped by cytological examination. The plants with somatic cell chromosome numbers 2n = 56 were tested for resistance to a mixture of Bgt isolates prevalent in northern China. Five resistant plants were obtained and back-crossed as females with the wheat parent Xiaoyan 6. The BC1F1 offspring were screened for resistance to powdery mildew and self-crossed. The BC1F2 plants were again tested for resistance to powdery mildew and the resistant offspring were continually self-pollinated for an additional six generations. During development, the offspring were selected based on resistance to powdery mildew, wheat-like plant type, and high seed set and then karyotyped by cytological examination. Finally, a fertile self- and genetically stable genotype with chromosome numbers 2n = 42, designated as WR41-1, was selected before molecular cytogenetic characterization. The line exhibited a high level of resistance to powdery mildew and had a desirable fertility.
Evaluation of agronomic performance
The wheat–rye derivative WR41-1 and its parents Xiaoyan 6 and German White were hand planted in early October and harvested in the middle of June next year. Each plot consisted of six 1.5-m-long rows, 30 seeds per row with inter-row spacing of 0.25 m. The plots were arranged in a randomized block design with three replications.
At the physiology maturity stage, 15 whole plants were manually harvested from the center of the three inner rows. Measurement and counting were done on plant height, spike length, spike number per plant, spikelet number per spike, sterile spikelet number per spike, kernel number per spike, thousand-kernel weight (TKW), and grain yield per plant.
Assessment of resistance to powdery mildew
Powdery mildew inoculation experiments and disease assessments at seedling stage were performed (Si et al. 1992). The seedlings of WR41-1 were tested for its reactions to 23 single-pustule-derived powdery mildew virulent isolates by separate artificial inoculation in a temperature-controlled greenhouse at the Institute of Plant Protection, the Chinese Academy of Agricultural Sciences, Beijing, China. Each isolate contains different virulence to resistance genes (Zhou et al. 2002), and their virulence pattern is shown in Table 1. A set of 38 lines carrying known Pm genes or gene combinations (Table 1), Xiaoyan 6, German White, Mingxian 169, and Huixianhong were also included in the tests. At the one-to two-leaf stage, seedlings were inoculated with fresh spores using the dusting method and then transferred to a greenhouse at 18 °C/12 °C (day/night) with a photoperiod of 12–14 h of light per day. Infection types (IT) were scored 14–15 days after inoculation when pustules were fully developed on the susceptible controls, Mingxian 169 and Huixianhong. IT of each plant was recorded based on a 0–4 scale, of which 0 = no visible symptoms and signs; 0; = necrotic flecks without sporulation; 1 = sparse aerial hypha and little sporulation, with diameter of colonies less than 1 mm; 2 = moderate aerial hypha and sporulation, with diameter of colonies less than 1 mm; 3 = thick aerial hypha and abundant sporulation, with diameter of colonies more than 1 mm; and 4 = abundant sporulation with more than 80 % of the leaf area covered with aerial hypha. Plants with an IT score of 0–2 were considered resistant, while those with an IT score of 3–4 were considered as susceptible (Si et al. 1992). The mildew response was scored on new seedlings for each isolate.
Adult plant reactions to powdery mildew were tested on WR41-1 and its parents in field condition using a mixture of Bgt isolates prevalent in northern China. These isolates are virulent to wheat genotypes with powdery mildew resistance genes Pm1, Pm3a, Pm3b, Pm3c, Pm3d, Pm3e, Pm3f, Pm5, Pm6, Pm7, Pm8, Pm17, and/or Pm19, and avirulent to resistance genes Pm2, Pm4a, Pm4b, Pm12, Pm13, Pm16, Pm20, and/or Pm21, as determined by testing them on differential genotypes (Duan et al. 1998). The tests with the mixture of the isolates were conducted using the procedures described by Sheng and Duan (1991) at Luancheng Agro-Ecological Experimental Station, Chinese Academy of Sciences, Shijiazhuang, China. Disease reaction was assessed on a 0–9 scale, where 0–4 was considered resistant and 5–9 susceptible. The adult plant reactions test was repeated in the following year's growing season using the same procedure.
GISH analysis
GISH analysis was conducted to detect rye chromatin in WR41-1. Seeds were germinated on moistened filter paper in petri dishes. Actively growing roots were removed from seedlings and placed in gas treatment for 2 h, fixed in 90 % acetic acid, and stored in 70 % v/v ethanol. Chromosome spread preparation was done as previously described (Han et al. 2006). Genomic DNA of German White rye was isolated (Sharp et al. 1988) and labeled with fluorescein-12-dUTP by nick translation method and used as a probe. Detection and visualization were performed as described (Han et al. 2009). The cells with good hybridization signals were captured by DVC CCD digital camera. The images captured for each color channel were merged using program Image-Pro Plus 4.0.
Sequential multicolor FISH analysis
After rinsing the GISH hybridization probe signals, mc-FISH was performed with two highly repeated DNA sequences, pAs1 (or pHvG38) labeled with digoxigenin-11-dUTP and pSc119.2 labeled with biotin-11-dUTP, respectively (Zheng et al. 2006). Two probes were mixed at the ratio of 1:1 before hybridization. After hybridization, anti-digoxigenin-FITC and avidin rhodamine were used for detection of the two probes simultaneously. The slides were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Detection and visualization were performed as described above. The clone pAs1 contains a 1-kb repetitive DNA sequence from Aegilops tauschii (2n = 2x = 14) (Rayburn and Gill 1986), clone pSc119.2 contains highly repeated sequence from rye (Mcintyre et al. 1990), and clone pHvG38 contains the GAA-satellite sequence from barely (Hordeum vulgare L., 2n = 2x = 14) (Pedersen and Langridge 1997). Using two repeated sequences, pSc119.2 and pAs1, as probes, seven rye chromosomes and 17 of 21 chromosome pairs of hexaploid wheat can be identified (Mukai et al. 1993), whereas, with two probes, pAs1 and pHv38, the entire chromosome complement of hexaploid wheat can be discriminated (Pedersen and Langridge 1997).
PCR analysis
In the present study, PCR amplification was used to detect the alien chromatin in wheat–rye derivatives. Total genomic DNA was isolated from seedling using the phenol/chloroform method (Sharp et al. 1988). Two expressed sequence tag-simple sequence repeat (EST-SSR) markers, KSUM62 (F: 5′-GGAGAGGATAGGCACAGGAC-3′, and R: 5′-GAGAGCAGAGGGAGCTATGG-3′) and MAG1424 (F: 5′-TGAACATCAAGGGGCTGC-3′, and R: 5′-ACGACAGACATAAAGAAGAGCG-3′), respectively, specific for rye chromosome arms of 4RS and 4RL (Xu et al. 2012), were used to detect rye chromosome in line WR41-1.
DNA amplification was conducted in a 10 μl reaction volume containing 1× PCR buffer, 1.0 U of Taq DNA polymerase, 2 pmol of each primer, 2 nmol of each deoxyribonucleotide, 15 nmol of MgCl2, and 30 ng template DNA. The PCR was performed by using a GeneAmp 9700 PCR System (PE Applied Biosystems, PerkinElmer, USA) for 1 cycle at 94 °C for 5 min, 38 cycles at 94 °C for 1 min, 62 °C (KSUM62) or 52 °C (MAG1424) for 1 min (Xu et al. 2012), and 72 °C for 1 min, with a final extension at 72 °C for 10 min. The PCR products were separated in 8 % nondenaturing polyacrylamide gels with a 19:1, 25:1, or 39:1 ratio of acrylamide and bisacrylamide and then silver-stained (Tixier and Sourdille 1997) and photographed.
Results
Agronomic performance of WR41-1
After six consecutive generations of selfing, no segregation was observed in wheat–rye line WR41-1, neither in morphology nor in cytology. The plants of WR41-1 were awned, resembling common wheat (Fig. 3a). It was vigorous and had a compact plant type. WR41-1 seeds were superiorly plump and the average thousand-kernel weight (TKW) was 31.4 g, which was higher than German White but less than Xiaoyan 6 (P < 0.05) (Table 2). WR41-1 was similar to its parent Xiaoyan 6 in plant height, spike number per plant, and grain yield but showed superior performance on spike length and kernel number per spike. The sterile spikelet number per spike of WR41-1 was less than both parents (Table 2). WR41-1, therefore, possessed desirable fertility (Fig. 3b).
Reaction to powdery mildew of WR41-1
For identification with powdery mildew, the seedlings of WR41-1, German White rye, Xiaoyan 6, Mingxian 169, Huixianhong, and 38 wheat genotypes that carry known Pm gene or gene combination were tested for reaction to 23 virulent Bgt isolates. The results of the mildew response from the differential set of wheat genotypes are presented in Table 1. German White showed immunity to all 23 virulent isolates with an IT score of 0. In contrast, the susceptible controls Mingxian 169 and Huixianhong were highly susceptible to these isolates with IT scores of 4. Xiaoyan 6 was susceptible to these isolates, including isolate E09 (Fig. 3c). WR41-1 was resistant to isolates E01, E02, E07, E09, E11, E13, E15, E17, E20, E23-(1), E23-(2), E26, and E30-(1) but susceptible to the remianing 10 isolates. Among 38 wheat genotypes with known Pm gene(s), TAM104/Thatcher with Pm20 derived from rye chromosome 6RL was resistant to 13 mildew isolates and susceptible to the remaining 10 isolates; Kavkaz with Pm8 from rye chromosome 1RS was resistant to isolates E01, E23-(1), E32, E49, and E50, and susceptible to the other 18 isolates; and Amigo with Pm17 from rye chromosome 1RS was resistant only to isolate E06, and susceptible to the other 22 isolates, while CI14189 with Pm7 from rye chromosome 2RL was susceptible to all the 23 isolates. By comparing the resistance reactions of all the wheat genotypes used here, we found that the reaction pattern of WR41-1 was different from those of 38 genotypes with known Pm genes or gene combinations (Table 1). Therefore, the resistance gene carried by the alien chromosome line WR41-1 appeared to be unique.
For adult plant tests with powdery mildew in the field, the plants of WR41-1, German White, and Xiaoyan 6, along with five controls, including Mingxian 169, Huixianhong, Kavkaz (Pm8), CI14189 (Pm7), and Amigo (Pm17), were inoculated with the mixture of Bgt isolates collected from northern China in two consecutive wheat growing seasons. Xiaoyan 6 and the five controls were covered by powdery mildew spores and all showed high susceptibility with a disease reaction type ranging from 8 to 9 (Sheng and Duan 1991), whereas German White rye developed no symptoms of powdery mildew with a disease reaction score of 0. WR41-1 showed an IT of 1 and, therefore, was regarded as highly resistant.
GISH analysis of WR41-1
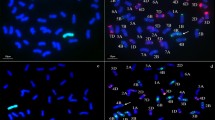
The GISH analysis was done to determine the chromosome constitution of WR41-1 using rye genomic DNA as a probe. WR41-1 was found to have 42 chromosomes, among which two pairs of chromosome arms displayed bright green hybridization signals, showing that the DNA in these chromosome arms was hybridized by the labeled probes from rye. This confirmed that WR41-1 contains chromosomes translocated from German White rye to wheat. The breakpoints of the translocations were clear and appeared to be in the centromeric region of wheat chromosomes forming two wheat–rye centromeric fusion chromosomes (Fig. 1a and c). The GISH analysis also revealed that most chromosomes at the mitotic metaphase showed only the blue signals counterstained with DAPI, indicating that they were not hybridized with the probe and, obviously, these were chromosomes that originated from the wheat parent, Xiaoyan 6, that is, WR41-1 was proved to be a wheat–rye translocation line (Fig. 1a and c). Different progeny plants of WR41-1 were examined by using the GISH analysis and confirmed the results, indicating that WR41-1 was a genetically stable wheat–rye line involving four chromosome translocations.
a and c Genomic in situ hybridization (GISH) analysis of the wheat–rye translocated chromosomes in WR41-1 show bright green hybridization signals evenly distributed on rye chromosome arms, with rye genomic DNA as a probe and Chinese Spring DNA as a blocker. The wheat chromosomes were counterstained with DAPI (blue). b Sequential multicolor fluorescence in situ hybridization (mc-FISH) on the same metaphase after GISH analysis (a) of translocation line WR41-1 by pAs1 (red) and pSc119.2 (green) simultaneously. d mc-FISH on the same metaphase after GISH analysis (c) of translocation line WR41-1 by pAs1 (red) and pHvG38 (green) simultaneously. Long arrows indicate one pair of T4BL·4RL translocated chromosomes and short arrows indicate one pair of T7AS·4RS translocated chromosomes
Mc-FISH analysis of WR41-1 after GISH
Following GISH analysis, mc-FISH with three probes, pSc119.2, and pAs1 (or pHvG38), was used to further determine the chromosome composition of WR41-1. The dispersed patterns were observed on the short and long arms of four translocated chromosomes in WR41-1 when labeled with pSc119.2. There were three specific pSc119.2 hybridization bands on the translocated rye chromosome arms: one strong telomeric band was on the short arm of two translocated rye chromosomes, and one strong intercalary band and one faint intercalary band were on the long arm of other two translocated rye chromosomes. No pAs1 hybridization signal was detected on the four arms of translocated rye chromosomes (Fig. 1b). These results indicated that the short arms of translocated rye chromosomes were 4RS and the long arms were 4RL (Mukai et al. 1993). Three specific pSc119.2 hybridization bands were also observed on the long arms of translocated wheat chromosome in WR41-1, one was a strong telomeric band, one was a strong intercalary band, and the other was a weaker band between them (Fig. 1b), indicating that the long arms of two translocated wheat chromosomes were 4BL (Mukai et al. 1993). No pAs1 hybridization signal was detected on the four arms of translocated wheat chromosomes (Fig. 1b). Based on the FISH patterns, the short wheat chromosome arms in the translocations originated from A-genome. With further use of the pHvG38 probe, we observed one faint GAA band at the terminal position of the translocated wheat chromosomes (Fig. 1d), indicating that the short arms of the translocated wheat chromosomes were 7AS (Pedersen and Langridge 1997). Thus, two pairs of translocation chromosomes were 4BL·4RL and 7AS·4RS, respectively, and WR41-1 was a 4R chromosome translocation based on the pSc119.2, pAs1, and pHvG38 diagnostic banding pattern.
PCR analysis of WR41-1
In this study, the two EST-SSR markers, KSUM62 and MAG1424, specific for 4RS and 4RL of rye chromosome, respectively, were used to identify WR41-1. DNA fragments about 160 and 235 bp were, respectively, amplified from WR41-1 and German White rye (Fig. 2a and b), indicating that WR41-1 contained the DNA region specific for chromosome 4R derived from German White. The corresponding diagnostic fragments were also, respectively, detected in 4RS and 4RL ditelosomic addition lines of ‘CS × Imperial’, both detected in 4R disomic addition line of ‘CS × Imperial’, and the two triticale lines 06CT456 and 06CT461 (AABBRR) but not in the other addition lines of ‘CS × Imperial’, and the other control genotypes, including the three lines of ‘Xiaoyan 6 × German White’ (WR64 and WR81 were T1BL·1RS translocation lines, and WR91 was 2R (2D) substitution line), two T1BL·1RS translocation lines (Lovrin 10 and Lovrin 13) as well as CS and Xiaoyan 6, indicating that they did not contain 4R chromatin of rye (Fig. 2a and b). Therefore, WR41-1 was a T4BL·4RL and 7AS·4RS chromosome translocation line, respectively, supported by analyses of using GISH, mc-FISH, and PCR methods.
PCR amplification of the EST-SSR (expressed sequence tag-simple sequence repeat) markers KSUM62 and MAG1424, respectively, specific for 4RS and 4RL of rye chromosome in wheat–rye lines and controls for detection of 4R in WR41-1. The 160- and 235-bp bands indicate the diagnostic DNA fragments specific for 4RS and 4RL, respectively. Lanes M marker pUC18/MspI, 1 Chinese Spring, 2 Xiaoyan 6, 3 German White, 4 WR41-1, 5 WR64, 6 WR81, 7 WR91 lines derived from ‘Xiaoyan 6 × German White’ without 4R identified by multicolor fluorescence in situ hybridization (mc-FISH). 8 to 14 are 1R–7R addition lines of ‘Chinese Spring × Imperial’, respectively. 15 and 16 are 4RS and 4RL ditelosomic lines of ‘Chinese Spring × Imperial’, respectively. 17 Lovrin 10 and 18 Lovrin 13 are T1BL·1RS chromosome translocation lines. 19 06CT456 and 20 06CT461 are triticale lines (AABBRR)
Discussion
As a cross-pollinated crop, rye contains significant genetic diversity within and between cultivars. In this study, rye cultivar German White showed immunity to all tested 23 virulent Bgt isolates with different virulence patterns at the seedling stage (Table 1) and the mixed Bgt isolates prevalent in northern China at the adult stage. In addition, it has also shown resistances to many other wheat diseases, including stripe rust, leaf rust, and sharp eyespot (Rhizotonia cerealis Van der Hoeven) (data not shown). Therefore, it is a potential source of resistance genes for wheat improvement.
In the present study, a new wheat–rye 4R chromosome translocation line, WR41-1, was produced through wide hybridization and chromosome manipulation between winter wheat cultivar Xiaoyan 6 and winter rye cultivar German White. WR41-1 was highly resistant to the mixture of Bgt isolates prevalent in northern China at the adult growth stage and also highly resistant to 13 of 23 Bgt isolates tested at the seedling stage. Its reaction pattern was different from the known Pm genes, including previously identified Pm7, Pm8, Pm17, and Pm20 derived from rye (Table 1). Therefore, WR41-1 may possess new powdery mildew resistance gene(s) different from the known Pm genes and is an additional source of powdery mildew resistance. Thus, germplasm WR41-1 of 4R chromosome translocation line can be used in the objective of broadening mildew resistance in wheat.
To date, 61 powdery mildew resistance genes and/or alleles in 46 loci (Pm1–Pm46) (http://wheat.pw.usda.gov/GG2/pubs.shtml; McIntosh et al. 2008; McIntosh et al. 2011; Huang et al. 2012) have been identified and designated in wheat and its wild relatives, including Pm7, Pm8, Pm17, and Pm20 originating from rye that provide isolate-specific resistance to powdery mildew. Of these resistance genes, Pm8 derived from rye cultivar Petkus (Hsam and Zeller 1997) and Pm17 from rye cultivar Insave (Heun et al. 1990), both located on 1RS of rye chromosome, were proved to be allelic genes and widely used in wheat breeding programs as translocations T1BL·1RS and T1AL·1RS, respectively (Rabinovich 1998). In China, there was about 38 % of the wheat cultivars with T1BL·1RS translocation (Zhou et al. 2004). However, the wide and extensive use of isolate-specific resistance genes led to the rapid emergence of new virulent pathogen isolates due to the coevolution of pathogen virulence and host resistance (McDonald and Linde 2002). The cultivars with 1RS translocation successively lost the resistance to powdery mildew (Zhuang and Li 1993; Zhuang 2003). A dominant resistance gene Pm7 derived from 2RL of rye cultivar Rosen was transferred into common wheat as a translocation, T4BS·4BL-2RL (Friebe et al. 1996), but it no longer exhibited resistance to all 23 Bgt isolates tested (Table 1). Another dominant resistance gene, Pm20, derived from rye cultivar Prolific, located on 6RL of rye chromosome, was transferred into common wheat as a translocation, T6BS·6RL (Friebe et al. 1994; Jiang et al. 1994). It was conditioned to be highly resistant against 13 Bgt isolates tested and susceptible to the remaining 10 isolates (Table 1). A new temporarily designated PmCn17, derived from T1BL·1RS translocation of rye Petkus, was proved to be different from Pm8 and Pm17 (Ren et al. 2009). In addition, there were many reports on the identification of new wheat–rye germplasms or powdery mildew resistance genes from rye. These Pm genes (loci) that they possessed were all located on 1R, 2R, and 6R chromosomes of rye, and their reaction patterns were different from the four known Pm genes Pm7, Pm8, Pm17, and Pm20 derived from rye (Li et al. 2004; An et al. 2006; Hysing et al. 2007; Tang et al. 2008; Ren et al. 2009; Wang et al. 2009b; Fu et al. 2010; Wang et al. 2010; Zhuang et al. 2011). The resistance patterns of a set of isogenic wheat–rye addition, substitution, and translocation lines were analyzed in a previous study, and it was found that the rye chromosomes 4R did not condition any powdery mildew resistance against the seven isolates tested (Heun and Friebe 1990), which agreed with another study for chromosomes 4R (Lind 1982). Prior to the present study, no translocation or substitution or addition lines involving rye chromosome 4R that possessed resistance to powdery mildew have been reported.
The chromosome 4R of rye harbored genes for many favorable traits. An independent and dominant locus Alt3 for aluminum tolerance was located on 4RL chromosome of rye (Miftahudin et al. 2002). Genes controlling drought tolerance such as relative water content and general adaptability were detected on chromosomes 4R in rye cultivar Imperial (Koeszegi et al. 1996). Karnal bunt (Tilletia indica Mitra) resistance gene was found on chromosomes 4R of wheat–rye addition lines (Sidhu et al. 2001). A waxy endosperm gene (Wx) was located on a chromosomal segment of 4RL (Korzun et al. 1997). A gene Rfc4 in rye that restored the male fertility of hexaploid wheat with timopheevi cytoplasm was located on 4RL and its genetic mapping showed that Rfc4 was located 16.1 cM from the telomere of 4RL and at least 8.0 cM from the centromere (Curtis and Lukaszewski 1993). The quantitative trait locus (QTL) for the number of rye spikes was also detected on chromosome 4R (Borner et al. 2000).
Nevertheless, few wheat–rye translocation lines involving 4R chromosome have been used in wheat improvement because of agronomic disadvantages of the wheat genetic background, such as CS. The wide usefulness of wheat-alien hybrids depends, to some extent, on their wheat genetic backgrounds. In the present study, we considered not only the transfer of desirable genes of German White rye but also placing them in a valuable background of wheat cultivar Xiaoyan 6. Thus, the 4R chromosome translocation line obtained in this study had a favorable wheat genetic background and possessed superior plump seeds with high percentage of seed set (Fig. 3a and b, Table 2).
a Plants of Xiaoyan 6 and chromosome translocation line WR41-1 (left and right). b Spikes and seeds of German White, Xiaoyan 6, and WR41-1 (left and right). c Powdery mildew reactions of Mingxian 169, Huixianhong, Xiaoyan6, German White, and WR41-1 (from left to right) inoculate with isolate E09 of Blumeria graminis f. sp. tritici (Bgt) at the seedling stage. Reaction to powdery mildew in different genotypes is indicated as R resistant and S susceptible
GISH and mc-FISH techniques showed their advantage in analysis and identification of alien chromosome or chromosome segments introgressed into the wheat genome. Using GISH and mc-FISH, we characterized a new T2BL·1RS wheat–rye translocation line resistant to stripe rust and powdery mildew in a previous study (Wang et al. 2009b). In the present study, combining GISH with mc-FISH using three repeated sequences pSc119.2 and pAs1 (or pHvG38) as probes, WR41-1 was proved to be a 4R translocation line (Fig. 1).
PCR analysis using chromosome-specific primers proved to be a powerful tool for the detection of alien chromatin that had been introgressed into common wheat (Lee et al. 2009; Wang et al. 2009a). EST databases for wheat and rye are valuable for the development of EST-based markers. We designed and developed 31 chromosome-specific EST markers for 1RS-7RL chromosome arms of rye, except for 6RS, using wheat and rye EST sequences (Xu et al. 2012). In the present study, two EST markers of KSUM62 and MAG1424 were used and PCR analysis further indicated that WR41-1 possessed chromosome 4RS and 4RL of rye (Fig. 2a and b).
Therefore, both the cytogenetic identification and molecular analyses supported that WR41-1 line was a chromosome translocation line of wheat (‘Xiaoyan 6’)–rye (‘German White’), and its chromosome composition was 2n = 42 = 38W + DT (4BL · 4RL) + DT(7AS · 4RS) = 19″W + 1″T4BL · 4RL + 1″T7AS · 4RS.
Wheat–rye addition, substitutions as well as translocations have been successfully used in wheat breeding. Translocations are preferred and used directly by wheat breeders because of the smaller amount of alien genetic material, less linkage drag, and regular meiotic behavior (Falke et al. 2009). For example, the transfers of Hessian fly (Mayetiola destructor) resistance from rye to wheat via X irradiation-induced chromosomal translocations (Friebe et al. 1991), gametocidal chromosome-induced alien chromosomal translocations between wheat and rye (Friebe et al. 2000; Masoudi-Nejad et al. 2002), and identification and physical mapping of induced translocation breakpoints involving chromosome 1R in rye (Catarino et al. 2006). To further use the powdery mildew resistance of the 4R translocation line, W41-1, in wheat improvement, we are developing small segmental translocation and introgression lines of chromosome 4R by the 60Coγ radiation-induced approach, the ph1b-induced homoeologous recombination, and the gametocidal chromosome originating from Aegilops (Endo 2007). The molecular markers placed on 4R chromosome, together with GISH and mc-FISH, will be used in screening potential translocation derivatives from progenies by these induced translocation methods. Furthermore, W41-1 will be crossed with susceptible control cultivar Huixianhong and major cultivars to generate the F2 and its family lines for locating the resistance gene and identifying associated molecular markers. Finally, the results will greatly facilitate the transfer of the resistance gene(s) from new 4R translocation line into wheat.
Abbreviations
- Bgt:
-
Blumeria graminis f. sp. tritici
- CS:
-
Chinese Spring wheat
- DAPI:
-
4,6-Diamidino-2-phenylindole
- EST-SSR:
-
Expressed sequence tag-simple sequence repeat
- FISH:
-
Fluorescence in situ hybridization
- FITC:
-
Fluorescence isothiocyanate
- GISH:
-
Genomic in situ hybridization
- IT:
-
Infection type
- mc-FISH:
-
Multicolor FISH
- PCR:
-
Polymerase chain reaction
- TKW:
-
Thousand-kernel weight
References
An DG, Zhong GC, Li JM, Wang ZG, Wang YM, Ji J (2003) Chromosome doubling methods of immature embryo plants of wheat distant hybridization. Acta Agro Sin 6:955–957
An DG, Li LH, Li JM, Li HJ, Zhu YG (2006) Introgression of resistance to powdery mildew conferred by chromosome 2R by crossing wheat nullisomic 2D with rye. J Integr Plant Biol 48:838–847
Borner A, Korzun V, Voylokov AV, Worland AJ, Weber WE (2000) Genetic mapping of quantitative trait loci in rye (Secale cereale L.). Euphytica 116:203–209
Catarino S, Alvarez E, Campa A, Vieira R, Roca A, Giraldez R (2006) Identification and physical mapping of induced translocation breakpoints involving chromosome 1R in rye. Chromosome Res 14:755–765
Curtis CA, Lukaszewski AJ (1993) Localization of genes in rye that restore male fertility to hexaploid wheat with timopheevi cytoplasm. Plant Breeding 111:106–112
Duan XY, Sheng BQ, Zhou YL, Xiang QJ (1998) Monitoring of the virulence population of Erysiphe graminis DC. f. sp. tritici E. Marchal. Acta Phytopathol Sin 25:31–36
Endo TR (2007) The gametocidal chromosome as a tool for chromosome manipulation in wheat. Chromosome Res 15:67–75
Falke KC, Susic Z, Wilde P et al (2009) Testcross performance of rye introgression lines developed by marker-assisted backcrossing using an Iranian accession as donor. Theor Appl Genet 118:1225–1238
Friebe B, Hatchett JH, Gill BS, Sebesta EE (1991) Transfer of hessian fly resistance from rye to wheat via radiation-induced terminal and intercalary chromosomal translocations. Theor Appl Genet 83:33–40
Friebe B, Heun M, Tuleen N, Zeller FJ, Gill BS (1994) Cytogenetically monitored transfer of powdery mildew resistance from rye into wheat. Crop Sci 34:621–625
Friebe B, Jiang J, Raupp W, McIntosh R, Gill B (1996) Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91:59–87
Friebe B, Kynast RG, Gill BS (2000) Gametocidal factor-induced structural rearrangements in rye chromosomes added to common wheat. Chromosome Res 8:501–511
Fu SL, Tang ZX, Ren ZL, Zhang HQ (2010) Transfer to wheat (Triticum aestivum) of small chromosome segments from rye (Secale cereale) carrying disease resistance genes. J Applied Genetics 51:115–121
Han FP, Lamb JC, Birchler JA (2006) High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. P Natl Acad Sci USA 103:3238–3243
Han FP, Gao Z, Birchler JA (2009) Centromere inactivation and reactivation reveals both epigenetic and genetic components for centromere specification. Plant Cell 21:1929–1939
He ZH, Rajaram S, Xin ZY, Huang GZ (2001) A history of wheat breeding in China. CIMMYT, Mexico
He ZH, Lan CX, Chen XM, Zou YC, Zhuang QS, Xia XC (2011) Progress and perspective in research of adult-plant resistance to stripe rust and powdery mildew in wheat. Sci Agric Sin 44:2193–2215
Heun M, Friebe B (1990) Introgression of powdery mildew resistance from rye into wheat. Phytopathology 80:242–245
Heun M, Friebe B, Bushuk W (1990) Chromosomal location of the powdery mildew resistance gene of Amigo wheat. Phytopathology 80:1129–1133
Hsam SLK, Zeller FJ (1997) Evidence of allelism between genes Pm8 and Pm17 and chromosomal location of powdery mildew and leaf rust resistance genes in the common wheat cultivar ‛Amigo’. Plant Breeding 116:119–122
Huang J, Zhao ZH, Song FJ et al (2012) Molecular detection of a gene effective against powdery mildew in the wheat cultivar Liangxing 66. Mol Breeding 30:1737–1745
Hysing SC, Hsam SLK, Singh RP et al (2007) Agronomic performance and multiple disease resistance in T2BS·2RL wheat–rye translocation lines. Crop Sci 47:254–260
Jiang JM, Friebe B, Gill BS (1994) Recent advances in alien gene transfer in wheat. Euphytica 73:199–212
Koeszegi B, Farshadfar E, Vagujfalvi A, Sutka J (1996) Drought tolerance studies on wheat/rye disomic chromosome addition lines. Acta Agron Hun 44:121–126
Korzun V, Malyshev S, Voylokov A, Borner A (1997) RFLP-based mapping of three mutant loci in rye (Secale cereale L.) and their relation to homoeologous loci within the Gramineae. Theor Appl Genet 95:468–473
Lee TG, Hong MJ, Johnson JW, Bland DE, Kim DY, Seo YW (2009) Development and functional assessment of EST-derived 2RL-specific markers for 2BS·2RL translocations. Theor Appl Genet 119:663–673
Li HJ, Conner RL, McCallum BD et al (2004) Resistance of Tangmai 4 wheat to powdery mildew, stem rust, leaf rust, and stripe rust and its chromosome composition. Can J Plant Sci 84:1015–1023
Li ZS, Li B, Tong YP (2008) The contribution of distant hybridization with decaploid Agropyron elongatum to wheat improvement in China. J Genet Genomics 35:451–456
Lind V (1982) Analysis of the resistance of wheat–rye addition lines to powdery mildew of wheat (Erysiphe graminis f. sp. tritici). Tagungsber Akad Landwirtsch Wiss DDR 198:509–520
Masoudi-Nejad A, Nasuda S, McIntosh RA, Endo TR (2002) Transfer of rye chromosome segments to wheat by a gametocidal system. Chromosome Res 10:349–357
McDonald BA, Linde C (2002) The population genetics of plant pathogens and breeding strategies for durable resistance. Euphytica 124:163–180
McIntosh RA, Yamazaki Y, Dubcovsky J et al. A catalogue of gene symbols for wheat. In: Proceedings of the 11th international wheat genetics symposium, Brisbane, Qld Australia, August 24–29 2008.
McIntosh RA, Zhang P, Cowger C, Parks R, Lagudah ES, Hoxha S (2011) Rye-derived powdery mildew resistance gene Pm8 in wheat is suppressed by the Pm3 locus. Theor Appl Genet 123:359–367
Mcintyre CL, Pereira S, Moran LB, Appels R (1990) New Secale cereale (rye) DNA derivatives for the detection of rye chromosome segments in wheat. Genome 33:635–640
Miftahudin, Scoles GJ, Gustafson JP (2002) AFLP markers tightly linked to the aluminum-tolerance gene Alt3 in rye (Secale cereale L.). Theor Appl Genet 104:626–631
Mukai Y, Nakahara Y, Yamamoto M (1993) Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 36:489–494
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Pedersen C, Langridge P (1997) Identification of the entire chromosome complement of bread wheat by two-colour FISH. Genome 40:589–593
Rabinovich SV (1998) Importance of wheat–rye translocations for breeding modern cultivars of Triticum aestivum L. Euphytica 100:323–340
Rayburn AL, Gill BS (1986) Isolation of a D-genome specific repeated DNA sequence from Aegilops squarrosa. Plant Mol Biol Rep 4:102–109
Ren TH, Yang ZJ, Yan BJ, Zhang HQ, Fu SL, Ren ZL (2009) Development and characterization of a new 1BL·1RS translocation line with resistance to stripe rust and powdery mildew of wheat. Euphytica 169:207–213
Sharp PJ, Kreis M, Shewry PR, Gale MD (1988) Location of β-amylase sequences in wheat and its relatives. Theor Appl Genet 75:286–290
Sheng BQ, Duan XY (1991) Improvement of scale 0–9 method for scoring adult plant resistance to powdery mildew of wheat. Beijing Agricultural Sciences 1:38–39
Si QM, Zhang XX, Duan XY, Sheng BQ, Zhou YL (1992) On gene analysis and classification of powdery mildew (Erysiphe graminis f. sp. tritici) resistant wheat varieties. Acta Phytopathol Sin 22:349–355
Sidhu MC, Satija CK, Sharma I (2001) Screening of wheat–rye addition lines for karnal bunt resistance. Crop Improv 28:214–217
Tang ZX, Fu SL, Ren ZL et al (2008) Production of a new wheat cultivar with a different 1B·1R translocation with resistance to powdery mildew and stripe rust. Cereal Res Commun 36:451–460
Tixier MH, Sourdille PMS (1997) Detection of wheat microsallite using no-radioactive silver nitrate staining method. Genet Breed 51:175–177
Wang CM, Li LH, Zhang XT, Gao Q, Wang RF, An DG (2009a) Development and application of EST-STS markers specific to chromosome 1RS of Secale cereale. Cereal Res Commun 37:13–21
Wang CM, Zheng Q, Li LH et al (2009b) Molecular cytogenetic characterization of a new T2BL·1RS wheat–rye chromosome tanslocation line resistant to stripe rust and powdery mildew. Plant Dis 93:124–129
Wang D, Zhuang LF, Sun L, Feng YG, Pei ZY, Qi ZJ (2010) Allocation of a powdery mildew resistance locus to the chromosome arm 6RL of Secale cereale L. cv. 'Jingzhouheimai'. Euphytica 176:157–166
Xu HX, Yin DD, Li LH et al (2012) Development and application of EST-based markers specific for chromosome arms of rye (Secale cereale L.). Cytogenet Genome Res 136:220–228
Zheng Q, Li B, Zhang XY, Mu SM, Zhou HP, Li ZS (2006) Molecular cytogenetic characterization of wheat–Thinopyrum ponticum translocations bearing blue-grained gene(s) induced by r-ray. Euphytica 152:51–60
Zhou YL, Duan XY, Chen G, Sheng BQ, Zhang Y (2002) Analyses of resistance genes of 40 wheat cultivars or lines to wheat powdery mildew. Acta Phytopathol Sin 32:301–305
Zhou Y, He ZH, Zhang GS et al (2004) Utilization of 1BL·1RS translocation in wheat breeding in China. Acta Agron Sin 30:531–535
Zhuang Q (2003) Chinese wheat improvement and pedigree analysis. Chinese Agriculture Press, Beijing
Zhuang Q, Li Z (1993) Present status of wheat breeding and related genetic study in China. Wheat Information Service:1–15
Zhuang LF, Sun L, Li AX, Chen TT, Qi ZJ (2011) Identification and development of diagnostic markers for a powdery mildew resistance gene on chromosome 2R of Chinese rye cultivar Jingzhouheimai. Mol Breeding 27:455–465
Acknowledgments
This research was supported by the National High-Tech Research and Development Program of China No. 2011AA1001, the National Natural Science Foundation of China No. 31171550, and the National Scientific and Technological Supporting Program of China No. 2013BAD01B02.
Conflict of interest
The authors (Diaoguo An, Qi Zheng, Yilin Zhou, Pengtao Ma, Zhenling Lv, Lihui Li, Bin Li, Qiaoling Luo, Hongxing Xu and Yunfeng Xu) declare that our experiments comply with the current laws of China and we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jiming Jiang
Rights and permissions
About this article
Cite this article
An, D., Zheng, Q., Zhou, Y. et al. Molecular cytogenetic characterization of a new wheat–rye 4R chromosome translocation line resistant to powdery mildew. Chromosome Res 21, 419–432 (2013). https://doi.org/10.1007/s10577-013-9366-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-013-9366-8