Abstract
Aquaporin 4 (AQP4), a water-specific channel protein locating on the astrocyte membrane, has been found to be antagonist, agonist and undergone closely related to epilepsy. Our previous study showed that inhibition of an N-methyl-d-aspartate receptor (NMDAR) subunit NR2A can suppress epileptic seizures, suggesting that AQP4 is potentially involved in NR2A-mediated epilepsy treatment. In this study, we aimed to explore the relevance of AQP4 in NR2A-mediated seizures treatment in pentylenetetrazol (PTZ)-induced rat models. We performed electroencephalogram (EEG) recording and examined AQP4 expression at mRNA and protein levels, and the downstream molecules of AQP4 as well. It showed that AQP4 expression was increased after the induction of seizures. Lateral ventricle pretreatment of NR2A inhibitor could mitigate the PTZ-induced seizures severity and counterbalance the increase of AQP4 expression. In contrast, NR2A activator that resulted in seizures aggravation could further augment the seizure-related elevations of AQP4 expression. Pharmacological inhibition of AQP4 alone could also suppress the PTZ-induced seizure activities, with decreased expressions of NF-κB p65, interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α in the brain. The results indicated that increased expression of AQP4 might be an important mechanism involved in NR2A of NMDAR-mediated treatment for epileptic seizures, enlightening a potentially new target for seizures treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy, a common neurological disorder with an estimated 65 million people affected all over the world, is characterized by repeated spontaneous seizures and abnormal synchronized discharges in neurons (Devinsky et al. 2018). Seizure generation is determined by multiple factors, including N-methyl-d-aspartate receptors (NMDAR), one of the most widely studied receptors of excitatory neurotransmitter glutamate. NMDAR plays a crucial role in the pathogenesis of seizures. The activation of NMDAR mediates neuronal hyper-excitability and excitotoxicity (Lemke et al. 2013). NMDAR non-selective antagonist MK-801 inhibits seizures activities and reduces cell damage caused by status epilepticus (Lemke et al. 2013; Menezes and Da 2017). However, adverse effects of MK-801 made it inappropriate for clinical applications (Xie and Huang 2018; Zhou et al. 2018). More efforts are needed to optimize the NMDAR strategy for epilepsy treatment.
NMDAR is widely distributed throughout the central nervous system and consists of four subunits, NR2A, NR2B, NR2C, and NR2D. The expression pattern of different subunits of NMDA receptors in the brain and their roles in physiology and pathology are different (Kohr 2006; Paoletti and Neyton 2007). Activation of NR2A promoted the expression of brain-derived neurotrophic factor and NR2A antagonist NVP-AAM 077 (PEAQX) prolonged the latent period of seizure onset (Chen et al. 2007). Our previous studies also suggested that PEAQX treatment in rats prolonged the seizure latency and seizure duration (Deng et al. 2014). However, the detailed mechanism underlying this regulation remains obscure. Therefore, this study will further explore how the inhibition of NR2A exerts its anti-seizure effects.
NMDA receptors were activated after traumatic brain injury, triggering massive Ca2+ influx, intracellular calcium overload (Rozov and Burnashev 2016) and excitotoxicity (Mei et al. 2018). Meanwhile, it was found that the expression of aquaporin 4 (AQP4), a water-specific channel protein locating on the astrocyte membrane, was significantly increased after traumatic brain injury. Interestingly, the elevation of AQP4 subsided if pretreated with NMDAR inhibitor MK-801 (Chen et al. 2018). AQP4 is a water-specific channel protein, which is abundantly expressed in the central nervous system. AQP4 plays an important role in maintaining the balance between water and ion metabolism in the brain and is involved in the neural signal transduction, astrocyte migration, and neuroimmunological functions (Medici et al. 2011; Verkman et al. 2017). Changes in AQP4 expression were found in different neurological diseases, such as Parkinson’s disease (Michel et al. 2016), Alzheimer’s disease (Burfeind et al. 2017), brain injury, and particularly epilepsy (Das et al. 2012). In epileptic animal models, the upregulation of AQP4 was found in the pathophysiological process of eclamptic seizure-induced cell death (Han et al. 2018), while pharmacological inhibition of AQP4 relieved seizure-induced brain edema and protected neurons from damage (Hubbard et al. 2017). Our previous studies also indicated that acetazolamide (AZA), an AQP4-specific antagonist had anti-epileptic effects (Chen et al. 2015; Yu et al. 2015, 2016). However, it is not clear whether NR2A signal pathway and seizure activities have potential links with the regulation of AQP4 expression.
Localizing in astrocytes, AQP4 is known to act as a neuroimmunological inducer. It is well accepted that neuroinflammation and seizures may interact as both cause and effect. A considerable amount of literature provided evidence that inflammation contributes to epileptic seizures (Borges et al. 2003; Choi et al. 2009; Mercado-Gomez et al. 2018). Nuclear factor-κB (NF-κB), a central mediator of inflammatory processes, targets several pro-inflammatory genes, including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6) (Ridder and Schwaninger 2009). Several studies reported that the expression levels of NF-κB p65, IL-1β, IL-6, and TNF-α in the brains of patients and rats with seizures were higher than that of the healthy control group (Patterson et al. 2015; Shi et al. 2018). In AQP4 knockout mice, the production of NF-κB p65, IL-1β, IL-6, and TNF-α in the brain was decreased (Dai et al. 2018; Sun et al. 2018). Therefore, we hypothesized that AQP4 might act as a homeostatic regulator in the CNS, involved in NR2A-mediated seizures activities and excitotoxicity-induced neuroinflammation.
Methods and Materials
Animals
Adult healthy Sprague–Dawley (SD) male rats, weighing 280 ± 30 g, were housed in the Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine under standard temperature and humidity control environment with free access to food and water. All animal experiments in this study were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol was reviewed and approved by the Experimental Animal Care and Ethics Committee of Experimental Animal Center of Guangzhou Medical University.
Establishment of PTZ-Induced Acute Seizure Model
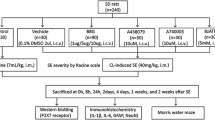
The seizure model was established by i.p. injection of pentylenetetrazol (PTZ) according to a previous study (Kouis et al. 2014). In brief, rats were first injected with 40 mg/kg PTZ, and their behavior was monitored. If rats did not show typical seizure behavior, they were injected with half of the first dose (20 mg/kg) after 10 min and subsequently 10 mg/kg PTZ every 10 min until seizure onset. Rats in the control group were injected with the same amount of physiological saline. According to the Racine scale (Racine 1972), the severity of seizures was divided into the following categories: Grade 0: No seizure; Grade I: rhythmic mouth and facial twitching; Grade II: rhythmic nodding or tail flicking; Grade III: single limb twitch; Grade IV: bilateral anterior limb rigidity or twitching with standing; Grade V: comprehensive tonic–clonic with fall. The successful criterion of seizure model was that animals exhibited grade IV to V seizures at least 3 times within 30 min (The animal grouping and treatments were shown in Table 1).
Lateral Ventricle Injection
Rats were weighed and anesthetized by i.p. injection of 2% pentobarbital sodium (30 mg/kg). The head was fixed on a stereotaxic apparatus; the hair was shaved, and the head skin was cut longitudinally, and the anterior fontanelle was marked. According to the mapping of “The Rat Brain in Stereotaxic Coordinates” by George Paxions et al. (Paxinos and Watson 1996) (0.9 mm post-anterior bregma, 1.6 mm beside the sagittal suture and 4.0 mm below the subdural surface), drugs were injected into the lateral ventricle by a microinjector. The microinjector's needle remained for 10 min and was withdrawn slowly. The skin of the head was sutured and disinfected with vitality iodine.
Electroencephalogram (EEG) Recording
After lateral ventricle injection, the hippocampus (5.6 mm posterior to bregma, 4.5 mm lateral, 2.6 mm ventral to the dura mater) was located. The skull was drilled, and a stainless steel bipolar copper core electrode was inserted into the subdural 3.0 mm. EEG in each rat was recorded by the BL-420E biological function experimental system simultaneously.
Detection of AQP4 by Immunohistochemistry
Brains were dissociated after transcardial perfusion and fixed in 4% paraformaldehyde for 72 h. Frozen tissues were cut into 30-μm-thick sections and soaked in 3% H2O2 (10% methanol: 0.3% H2O2) for 30 min. Sections were incubated in normal goat serum blocking solution for 1 h, and subsequently in rabbit anti-AQP4 primary antibody (1:80, Cell Signaling Technology, USA) for overnight at 4 ℃, and in goat anti-mouse IgG (1:200, BIOSS, Beijing, China) for 1 h at room temperature. Sections were washed thoroughly with PBS. Finally, sections were developed in the DAB color solution for 5–10 min, followed by patching, conventional dehydration, transparent, and sealing. The negative control group was incubated with PBS instead of primary antibody. Under the same light intensity and magnification (× 40), five fields in hippocampus CA3 subregion were randomly selected for each section. Image J software was used for the semi-quantitative analysis of AQP4 expression.
Western Blot Detection
Rat hippocampus was dissected and homogenized in tissue lysis buffer. The homogenates were centrifuged at 12,000 rpm for 15 min, and the supernatant was collected. The BCA method was used to measure protein concentration. Protein samples were loaded at 50 μg/well, subjected to 12% SDS-PAGE vertical electrophoresis, and then electrotransferred to the PVDF membrane. The membranes were incubated at room temperature for 1 h with 5% skim milk, washed by TBST, then incubated with rabbit anti-AQP4 antibody (1:1000, Cell Signaling Technology, USA) and rabbit NF-κB p65 (1:1000, Cell Signaling Technology, USA) overnight at 4 ℃, washed by TBST, incubated with goat anti-rabbit secondary antibodies (1:5000, Bioss, Beijing, China) for 1 h at room temperature, and washed by TBST thoroughly. Proteins were then exposed to ECL (Bioworld, USA) by the imager (Bio-Rad, USA). Band densities were digitally quantified by Image J software, and internal reference was used for normalization; the expression levels of target proteins were shown as relative ratios.
RT-qPCR
The total RNA of hippocampus was extracted with Trizol (Invitrogen, Shanghai, China) and reversely transcribed into cDNA by ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Japan). The oligo-dT primers were synthesized by Invitrogen (Shanghai, China) and the sequences of the primers used in this study are shown in Table 2. Quantification of gene transcripts was performed with the Quantstudio 5 PCR instrument (Life Technologies, USA) using the SYBR Green Real-time PCR Master Mix (TOYOBO, Japan). qPCR reaction conditions were 95 ℃ for 60 s, PCR stage including 95 ℃ for 15 s, 60 ℃ for 15 s, 72 ℃ for 45 s,40 cycles. The PCR products were subjected to melting curve analysis. The expression of the target genes was calculated using the difference between the target gene and β-actin RNA cycle threshold (Ct) (Δ Ct). The fold change between the experimental group and the control group (ΔΔ Ct), the expression change fold was 2−ΔΔCt. The relative expression (relative quantity, RQ) of the control group was 1, and the experimental group was a fold of changes relative to 1.
Detection of AQP4 and NF- κB p65 by Immunofluorescence
Rat brain sections were permeabilized with 1% Triton X-100 in PBS solution for 10 min at room temperature, washed thoroughly with PBS, blocked with 5% bovine serum albumin (BSA) for 30 min, incubated in rabbit anti-AQP4 antibody (1:200, CST, USA) and mouse anti NF-κB p65 antibody (1:100, CST, USA) overnight at 4 ℃, washed thoroughly with PBS, incubated in dark in goat anti-rabbit Secondary Antibody Alexa Fluor® 594 (1:200, Invitrogen, Shanghai, China) and Goat anti-mouse Secondary Antibody Alexa Fluor® 488 (1:200, Invitrogen, Shanghai, China) for 1 h at room temperature, and washed thoroughly with PBS. Finally, the sections were stained with 1 μg/ml DAPI (1:500, Invitrogen, Shanghai, China) in the dark for 5–10 min, washed thoroughly with PBS and mounted. Stained sections were examined and imaged under confocal microscopy (Leica, Germany). Image J software was used for semi-quantitative analysis.
ELISA
The standard working solution and samples were added to the microplate at 100 μl per well, coated with film and incubated at 37 ℃ for 90 min. The solution was then discarded, and the samples were dried without washing. The biotinylated antibody/antigen working solution (100 μl) was added to each well immediately, mixed evenly and incubated at 37 ℃ for 1 h. After washing, the enzyme conjugate working solution was added into the plates (100 μl per well), mixed evenly, coated with film, and incubated at 37 ℃ for 30 min. After washing, each well was added with 90 μl substrate solution (TMB), mixed evenly, coated with film, and incubated at 37 ℃ for about 15 min in the dark. The reaction was terminated by adding 50 μl of termination solution per well. The optical density (OD value) of each well was measured immediately at 450 nm wavelength by enzyme labeling instrument.
Statistical Analysis
Data were analyzed using SPSS 16.0 software. The statistical difference between two groups was assessed by unpaired t test. P<0.05 was considered statistically significant.
Results
AQP4 Expression in Rat Hippocampus was Increased After PTZ-Induced Seizures
To investigate the dynamic changes of AQP4 expression in rat hippocampus after seizures, the expression of AQP4 was assessed by immunohistochemistry, Western blot, and RT-qPCR at 0 h, 12 h, 24 h, 48 h, and 72 h after seizure induction. The AQP4 expression in the hippocampal CA3 region at different time points after PTZ-induced seizure was assessed by immunohistochemistry. We found that AQP4 expression began to increase at 12 h following seizure induction, and reached the highest level at 24 h. AQP expression levels went down at 48 h and 72 h, but still higher than the control (Fig. 1a). The results obtained from Western blot and RT-qPCR showed that the dynamic changes in AQP4 protein and mRNA were consistent with the immunohistochemistry results.
Dynamic changes in AQP4 expression in rat hippocampus after PTZ-induced seizure. a (left) Expression of AQP4 assessed by immunohistochemistry in the hippocampal CA3 region at different time points after PTZ-induced seizure. Scale bar: 100 μm. (right) The morphometric analysis of the mean positive immunoreactivity density of the AQP4 staining. *P < 0.05 compared to Control. Data are represented as Mean ± SD (n = 8 in each group). b Western blot and densitometric analyses of AQP4/β-tubulin in the hippocampus at different time points after PTZ-induced seizure. *P< 0.05 compared to Control. c Relative gene expression of AQP4 in the hippocampus at different time points after PTZ-induced seizure. *P< 0.05 compared to Control. Data are represented as Mean ± SD (n = 8 in each group)
Effects of NR2A Signaling on PTZ-Induced Seizures
To investigate whether NR2A signal strength might be involved in the PTZ-induced seizure, NR2A antagonist NVP-AAM 077 (PEAQX) or agonist rapastinel (GLYX-13) were administered by lateral ventricle injection before seizure induction. Behavioral observations (Fig. 2a) showed that PEAQX pretreatment significantly prolonged seizure latency and shortened seizure duration, while NR2A activation by GLYX-13 led to increases in seizure sensitivity indicated by latency and duration.
Effects of NR2A antagonist /agonist pretreatment in the rat model of PTZ-induced seizure. a The histograms of the latent period and duration of seizure in each group. *P < 0.05 compared with the SE group, #P < 0.05 compared with the GLYX-13 + SE group. Data are represented as Mean ± SD (n = 8 in each group). b Hippocampal EEG recording in each group. c The histogram of the brain wave amplitude in each group. *P < 0.05 compared with the control group, #P < 0.05 compared with the SE group. Data are represented as Mean ± SD (n = 8 in each group)
NR2A Signaling Affected AQP4 Expression
To investigate if dynamic regulation of AQP4 expression was involved in NR2A-mediated alteration in seizure sensitivity, we assessed the expression of AQP4 protein and mRNA in the hippocampus of the rats pretreated with NR2A antagonist and agonist and undergone the PTZ-induced seizures. Western blot and RT-qPCR results (Fig. 3a, b) showed that the expression of AQP4 protein and mRNA decreased after PEAQX pretreatment and increased after GLYX-13 pretreatment, suggesting a tight link between NR2A signaling and AQP4 expression.
Expressions of AQP4 with NR2A antagonist/agonist pretreatment. a Western blots and densitometric analyses of AQP4/β-tubulin in the hippocampus of rats in each group. *P < 0.05 compared with the Control group, #P < 0.05 compared with the SE group. Data are represented as Mean ± SD (n = 8 in each group). b Relative mRNA expression of AQP4 in the hippocampus of rats in each group. *P < 0.05 compared with the Control group, #P < 0.05 compared with the SE group. Data are represented as Mean ± SD (n = 8 in each group)
Inhibition of AQP4 Signaling Suppressed Seizures
As inhibition of NR2A led to paralleling decreases between AQP4 expression and seizure sensitivity, we next explored the relationship between AQP4 signaling and seizure sensitivity. By analyzing seizure activities and EEG wave amplitude, we found that pro-seizure effects of NR2A antagonist GLYX-13 were counteracted by AQP4 inhibitor AZA, indicated by seizure latency and duration (Fig. 2). The epileptic wave amplitude in EEG recording (Fig. 2b, c) showed similar changes that were consistent with the behavioral observation. Administration of AZA alone could have a significant beneficial effect on seizure sensitivity (Fig. 4). These results suggested that the effects of NR2A signaling on seizures might be achieved by modulating AQP4, and AQP4 signaling strength was associated with seizure sensitivity.
Effects of AQP4 inhibitor AZA in the rat model of the seizure. a Histograms of the latent period and duration of rats in each group. *P < 0.05 compared with the SE group. Data are represented as Mean ± SD (n = 8 in each group). b Hippocampal EEG recording in each group. c The histogram of the brain wave amplitude in each group. *P < 0.05 compared with the Control group; #P < 0.05 compared with the SE group. Data are represented as Mean ± SD (n = 8 in each group)
AQP4 inhibition decreases seizure-induced cytokines
To investigate if AQP4 inhibition could suppress seizure-related neuroinflammation, we assessed the expression of cytokines, including NF-κB p65, IL-1β, IL-6, and TNF-α. After pretreatment of AZA, we firstly examined the expression of AQP4 and NF-κB p65 in the hippocampal CA3 region at 24 h after seizure induction by PTZ using immunofluorescence, Western blot, and RT-qPCR. The expression levels of IL-1β, IL-6, and TNF-α in the hippocampus were assessed by ELISA and RT-qPCR. The immunofluorescence showed that the expression of NF-κB p65, IL-1β, IL-6, and TNF-α was downregulated by AZA pretreatment, indicating that inhibition of AQP4 signaling could suppress seizure-related neuroinflammation (Fig. 5).
Effects of AQP4 inhibition on NF-κB p65, IL-1β, IL-6 and TNF-α levels in rat hippocampus. a Representative immunofluorescent images (left) and morphometric analysis(right) of AQP4 and NF-κB p65 in the hippocampal CA3 region in each group. Scale bar: 100 μm. *P < 0.05 compared with the Control group, #P < 0.05 compared with the SE group. Data are represented as Mean ± SD (n = 8 in each group). b Western blot and densitometric analyses of NF-κB p65/β-tubulin in the hippocampus of each group. *P < 0.05 compared with the Control group, #P < 0.05 compared with the SE group. Data are represented as Mean ± SD (n = 8 in each group). c Relative mRNA expression of NF-κB p65 in the hippocampus of each group. *P < 0.05 compared with the Control group, #P < 0.05 compared with the SE group. Data are represented as Mean ± SD (n = 8 in each group). d Relative levels of IL-1β, IL-6, and TNF-α in the hippocampus of each group. *P < 0.05 compared with the Control group, #P < 0.05 compared with the SE group. Data are represented as Mean ± SD (n = 8 in each group). e Relative mRNA expression of IL-1β, IL-6, and TNF-α in the hippocampus of each group. *P < 0.05 Compared with the Control group, #P < 0.05 compared with the SE group. Data are represented as Mean ± SD (n = 8)
Discussion
In this study, we demonstrated the dynamic changes of AQP4 expression in the hippocampus of rats after seizures and the role of AQP4 in seizure sensitivity and neuroinflammation. The results showed that the expression of AQP4 in the hippocampus of rats increased and the highest expression was observed at 24 h after seizure induction, when neuroinflammation was initiated. NMDAR signaling is correlated to the expression of AQP4 and seizure sensitivity. Inhibition of NR2A led to the concomitant decreases of AQP4 and seizure severity. Additionally, AQP4 inhibitor itself could depress seizures and reduce the production of neuroinflammation cytokines, including NF-κB p65, IL-1β, IL-6, and TNF-α.
AQP4 is the most abundant aquaporin in the mammalian brain and is mainly present in the astrocyte membrane. A large number of studies have shown that the expression of AQP4 is increased in patients with seizures. For example, Medic et al. determined the expression of AQP4 in the patients with seizures by immunohistochemistry and Western blotting, and the results showed that the expression of AQP4 was increased (Medici et al. 2011). In a mesial temporal seizure rat model, expression of AQP4 in the hippocampus was significantly upregulated (Duan and Di 2017). Here, we found that AQP4 expression increased after an epileptic seizure, and reached a peak at 24 h. Based on this result, we performed the following experiments, including the effects of NR2A antagonist and agonist on AQP4 expression at 24 h after seizure induction. The pathogenesis of epilepsy is complicated. One of the currently recognized essential causes is the imbalance of excitatory amino acids and inhibitory amino acids in the central nervous system (Szepetowski 2017). That is, the excitatory glutamic acid is increased, or the inhibitory γ-aminobutyl acid is reduced. The NMDA receptor is a subtype of the glutamate ion-type receptor, which has many allosteric regulatory sites, and different NMDA receptors correlate with seizure-related excitotoxicity and excitotoxicity-induced neuroinflammation (Luo et al. 2011; Schrattenholz and Soskic 2006). NR2A subunit is the predominant subunit of NMDA receptor in mature neurons (Tovar and Westbrook 1999), while NR2B-containing NMDARs are the predominant subtype at extrasynaptic sites (Liu et al. 2004). Thus, it is not surprising that NR2B inhibition did not result in the expression changes of AQP4 and affected seizure activities. Several studies have shown that downregulating NR2A inhibits epileptic seizures (Wang et al. 2017a, b; Yang et al. 2018), which is consistent with our results. However, there is no research reporting the relationship between NR2A and AQP4. Li X et al. studied the inhibitory effect of magnesium sulfate on eclampsia and found that the pretreatment of magnesium sulfate into the eclampsia-like seizure rat model significantly reduced convulsive seizures and decreased the expression of AQP4 (Li et al. 2017). Verkman’s study found that the threshold of seizures in AQP4 knockout mice was significantly increased, and the incubation period of seizures was prolonged (Verkman et al. 2017). Previous studies showed that inflammatory cytokines upregulate AQP4 expression (Ito et al. 2006; Ohnishi et al. 2014; Wang et al. 2018). In this study, we inhibited the expression of AQP4 with AZA and found that AZA can downregulate the expression of NF-κB p65, reduce the levels of inflammatory factors IL-1β, IL-6, and TNF-α, and depress seizures. Verkman et al. suggested that AQP4 as a potential drug target in neurological disorders (Verkman et al. 2017). Wang suggested that inhibiting the upregulation of AQP4 and inflammatory cytokines can protect neurons from brain ischemia (Wang et al. 2017a, b). Except for decreasing the release of inflammation cytokines, AQP4 as a water channel, plays an important role in the formation of brain edema (van Vliet et al. 2007). Downregulating AQP4 can depress the seizures and relieve the edema after seizures to protect neurons vitality (Shin et al. 2014). Thus, AQP4 could be a better approach to anti-epileptic development. In addition, a recent study showed that the AQP4 inhibitor TGN-020 had attenuated LPS-induced lung injury, reduced pro-inflammatory cytokine release, including IL-1α, IL-1β, IL-6, TNF-α, IL-23, and IL-17A (Guo et al. 2019). Our previous study found that inhibition of AQP4 with AZA inhibited seizures, which is consistent with above mentioned findings (Chen et al. 2015; Yu et al. 2015, 2016). Other studies showed that downregulation of AQP4 decreased the levels of NF-κB p65, IL-1β, IL-6, and TNF-α. Consistent with previous studies (Cai et al. 2016; Sun et al. 2017; Yin et al. 2018), in this study, we found that AQP4 inhibitor AZA reduced the production of NF-κB p65, IL-1β, IL-6, and TNF-α and suppressed epileptic seizures. Our results, together with others, indicate that inhibition of AQP4 may exert anti-epileptic effects by reducing the production of pro-inflammatory cytokines.
In this study, we presented data to highlight a possible signal link between two membrane channels NR2A and AQP4. These data include (1) upregulation of AQP4 after seizures. The time window of the elevations is associated with the seizure-induced neuroinflammation, indicating AQP4 might be involved in the process. (2) NR2A signal modulation led to changes in seizure activities, demonstrating that NR2A signal is highly involved in the PTZ-induced model. (3) NR2A signal modulation could lead to accordant changes in AQP4 expression, suggesting a tight link between NR2A and AQP4 in the seizure model. (4) Functionl modulation of AQP4 did correlate with seizure sensitivity and seizure-induced neuroinflammation.
NMDAR is commonly considered neuron-specific. However, previous studies showed that NMDA receptors are also expressed in glial cells (Skowronska et al. 2019; Wu et al. 2017). Consistently, it is reported that astrocyte could express inducible functional NMDA receptors without the presence of neurons (Zhou et al. 2010). Even NR2A and AQP4 might locate in different types of cells (e.g., neurons and astrocyte), our findings suggested a possible signaling link between NR2A and AQP4. It is well known that glial cells have a direct role in the regulation of synaptic strength and neuronal excitability. Vice versa, inhibition of NR2A signaling might rapidly induce some changes in the membrane of glial cells to become “excitable” glia cells, consequently, lowering seizure threshold and precipitating seizures. In our results, we found a link between NR2A and AQP4 changes involved in seizure activities. Their signaling between NR2A and AQP4 might be either coupled on cell membrane or bridged in a way of glia-neuron communications. It still lacks evidence, so far, to know in which way they might interact. For our hypothesis, the activation of NMDA receptor (NR2A) might lead to the release of ATP (Eyo et al. 2014), which might act on P2Y1 receptor in astrocytes and thus regulate AQP4 expression or function.
As discussed above, we only provided evidence for the concurrent involvement of NMDAR and AQP4 in seizures. Considering NR2A subunit is the subunit of predominant NMDA receptor in adult rodents, it is reasonable to speculate that NR2B antagonist may not play an effective role in NMDAR-related seizures directly. The interaction between NR2A and AQP4 might be through direct binding, or mediated by other molecules in astrocytes, or most likely mediated by glia-neuron signaling mechanisms. These detailed underlying mechanisms need further investigations. Previous reports have not reported the relationship between NR2A and AQP4, except that Chen et al. suggested that AQP4 expression increased significantly after traumatic brain injury and was downregulated mediates microglia activationted by the NMDAR inhibitor MK-801 (Chen et al. 2018). Our findings might potentially link the two epilepsy-related molecules, NR2A and AQP4. We could not exclude a possibility that the changes in AQP4 might be induced by neuronal activities rather than NR2A signaling directly. In fact, we tend to hypothesize that changes in AQP4 are caused by NR2A-mediated neuronal activities. On the other side, the PTZ-induced seizure activities might trigger uncontrolled, pathological activation of NR2A, leading to excitotoxicity and neuroinflammation via AQP4 pathways. The intermediate signals between NR2A and AQP4 remain largely unknown. Nonetheless, the findings might support the potential function of decreased AQP4 expression is involved in NMDAR activation and seizure activities and can be targeted to reduce seizures.
References
Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R (2003) Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol 182(1):21–34
Burfeind KG, Murchison CF, Westaway SK, Simon MJ, Erten-Lyons D, Kaye JA, Quinn JF, Iliff JJ (2017) The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer's disease. Alzheimers Dement (NY) 3(3):348–359
Cai M, Yu Z, Wang L, Song X, Zhang J, Zhang Z, Zhang W, Li W, Xiang J, Cai D (2016) Tongxinluo reduces brain edema and inhibits post-ischemic inflammation after middle cerebral artery occlusion in rats. J Ethnopharmacol 181:136–145
Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH, Xiong ZQ (2007) Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci 27(3):542–552
Chen L, Yu H, Zhou Y, Ma M, Wang L, Hu J, Lei S, Zhu X (2015) Effect of acetazolamide on the expression of aquaporin-4 in hippocampus of rats with PTZ-induced chronic epilepsy. J Huazhong Univ Sci Technol (Med Sci) 05:510–514
Chen LH, Zhang HT, Xu RX, Li WD, Zhao H, Yang Y, Sun K (2018) Interaction of aquaporin 4 and N-methyl-d-aspartate NMDA receptor 1 in traumatic brain injury of rats. Iranian J Basic Med Sci 21(11):1148–1154
Choi J, Nordli DJ, Alden TD, DiPatri AJ, Laux L, Kelley K, Rosenow J, Schuele SU, Rajaram V, Koh S (2009) Cellular injury and neuroinflammation in children with chronic intractable epilepsy. J Neuroinflamm 6:38
Dai W, Yan J, Chen G, Hu G, Zhou X, Zeng X (2018) AQP4knockout alleviates the lipopolysaccharideinduced inflammatory response in astrocytes via SPHK1/MAPK/AKT signaling. Int J Mol Med 42(3):1716–1722
Das A, Wallace GT, Holmes C, McDowell ML, Smith JA, Marshall JD, Bonilha L, Edwards JC, Glazier SS, Ray SK, Banik NL (2012) Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience 220:237–246
Deng Z, Luo M, Zhao Y, Yu H, Xie L, Chen L, Zhu X, Hu J, Lei S (2014) Effect of GluN2A inhibitor on expression of P-glycoprotein in rats with status. Epilepticus. 2:168–172
Devinsky O, Vezzani A, O'Brien TJ, Jette N, Scheffer IE, de Curtis M, Perucca P (2018) Epilepsy. Nat Rev Dis Primers 4:18024
Duan L, Di Q (2017) Acetazolamide suppresses multi-drug resistance-related protein 1 and P-glycoprotein expression by inhibiting aquaporins expression in a mesial temporal epilepsy rat model. Med Sci Monit 23:5818–5825
Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ (2014) Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J Neurosci 34(32):10528–10540
Guo C, Wu T, Zhu H, Gao L (2019) Aquaporin 4 blockade attenuates acute lung injury through inhibition of Th17 cell proliferation in mice. Inflammation 42(4):1401–1412
Han X, Huang Q, Liu L, Sha X, Hu B, Liu H (2018) Changes in the expression of AQP4 and AQP9 in the hippocampus following eclampsia-like seizure. Int J Mol Sci 19(1):300
Hubbard JA, Szu JI, Binder DK (2017) The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res Bull. 136:118–129
Ito H, Yamamoto N, Arima H, Hirate H, Morishima T, Umenishi F, Tada T, Asai K, Katsuya H, Sobue K (2006) Interleukin-1beta induces the expression of aquaporin-4 through a nuclear factor-kappaB pathway in rat astrocytes. J Neurochem 99(1):107–118
Kohr G (2006) NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res 326(2):439–446
Kouis P, Mikroulis A, Psarropoulou C (2014) A single episode of juvenile status epilepticus reduces the threshold to adult seizures in a stimulus-specific way. Epilepsy Res 108(9):1564–1571
Lemke JR, Lal D, Reinthaler EM, Steiner I, Nothnagel M, Alber M, Geider K, Laube B, Schwake M, Finsterwalder K, Franke A, Schilhabel M, Jahn JA, Muhle H, Boor R, Van Paesschen W, Caraballo R, Fejerman N, Weckhuysen S, De Jonghe P, Larsen J, Moller RS, Hjalgrim H, Addis L, Tang S, Hughes E, Pal DK, Veri K, Vaher U, Talvik T, Dimova P, Guerrero LR, Serratosa JM, Linnankivi T, Lehesjoki AE, Ruf S, Wolff M, Buerki S, Wohlrab G, Kroell J, Datta AN, Fiedler B, Kurlemann G, Kluger G, Hahn A, Haberlandt DE, Kutzer C, Sperner J, Becker F, Weber YG, Feucht M, Steinbock H, Neophythou B, Ronen GM, Gruber-Sedlmayr U, Geldner J, Harvey RJ, Hoffmann P, Herms S, Altmuller J, Toliat MR, Thiele H, Nurnberg P, Wilhelm C, Stephani U, Helbig I, Lerche H, Zimprich F, Neubauer BA, Biskup S, von Spiczak S (2013) Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat Genet 45(9):1067–1072
Li X, Liu H, Yang Y (2017) Magnesium Sulfate attenuates brain edema by lowering AQP4 expression and inhibits glia-mediated neuroinflammation in a rodent model of eclampsia. Behavioural Brain Res. 364:403–412
Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT (2004) Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304(5673):1021–1024
Luo T, Wu WH, Chen BS (2011) NMDA receptor signaling: death or survival? Front Biol (Beijing) 6(6):468–476
Medici V, Frassoni C, Tassi L, Spreafico R, Garbelli R (2011) Aquaporin 4 expression in control and epileptic human cerebral cortex. Brain Res 1367:330–339
Mei Z, Qiu J, Alcon S, Hashim J, Rotenberg A, Sun Y, Meehan WR, Mannix R (2018) Memantine improves outcomes after repetitive traumatic brain injury. Behav Brain Res 340:195–204
Menezes FP, Da SR (2017) The influence of temperature on adult zebrafish sensitivity to pentylenetetrazole. Epilepsy Res 135:14–18
Mercado-Gomez OF, Cordova-Davalos L, Garcia-Betanzo D, Rocha L, Alonso-Vanegas MA, Cienfuegos J, Guevara-Guzman R (2018) Overexpression of inflammatory-related and nitric oxide synthase genes in olfactory bulbs from frontal lobe epilepsy patients. Epilepsy Res 148:37–43
Michel PP, Hirsch EC, Hunot S (2016) Understanding dopaminergic cell death pathways in parkinson disease. Neuron 90(4):675–691
Ohnishi M, Monda A, Takemoto R, Fujimoto Y, Sugitani M, Iwamura T, Hiroyasu T, Inoue A (2014) High-mobility group box 1 up-regulates aquaporin 4 expression via microglia-astrocyte interaction. Neurochem Int 75:32–38
Paoletti P, Neyton J (2007) NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 7(1):39–47
Patterson KP, Brennan GP, Curran M, Kinney-Lang E, Dube C, Rashid F, Ly C, Obenaus A, Baram TZ (2015) Rapid, coordinate inflammatory responses after experimental febrile status epilepticus: implications for epileptogenesis. Eneuro. https://doi.org/10.1523/ENEURO.0034-15.2015
Paxinos G, Watson C (1996) The rat brain in stereotaxic coordinates, 3rd edn. Academic Press, New York
Racine RJ (1972) Modification of seizure activity by electrical stimulation II motor seizure. Electroencephalogr Clin Neurophysiol 32(3):281–294
Ridder DA, Schwaninger M (2009) NF-kappaB signaling in cerebral ischemia. Neuroscience 158(3):995–1006
Rozov A, Burnashev N (2016) Fast interaction between AMPA and NMDA receptors by intracellular calcium. Cell Calcium 60(6):407–414
Schrattenholz A, Soskic V (2006) NMDA receptors are not alone: dynamic regulation of NMDA receptor structure and function by neuregulins and transient cholesterol-rich membrane domains leads to disease-specific nuances of glutamate-signalling. Curr Top Med Chem 6(7):663–686
Shi Y, Zhang L, Teng J, Miao W (2018) HMGB1 mediates microglia activation via the TLR4/NF-kappaB pathway in coriaria lactone induced epilepsy. Mol Med Rep 17(4):5125–5131
Shin HJ, Kim H, Heo RW, Kim HJ, Choi WS, Kwon HM, Roh GS (2014) Tonicity-responsive enhancer binding protein haplodeficiency attenuates seizure severity and NF-kappaB-mediated neuroinflammation in kainic acid-induced seizures. Cell Death Differ 21(7):1095–1106
Skowronska K, Obara-Michlewska M, Czarnecka A, Dabrowska K, Zielinska M, Albrecht J (2019) Persistent overexposure to N-Methyl-d-Aspartate (NMDA) calcium-dependently downregulates glutamine synthetase, Aquaporin 4, and Kir4.1 channel in mouse cortical astrocytes. Neurotoxicity Res. 35(1):271–280
Sun L, Li M, Ma X, Feng H, Song J, Lv C, He Y (2017) Inhibition of HMGB1 reduces rat spinal cord astrocytic swelling and AQP4 expression after oxygen-glucose deprivation and reoxygenation via TLR4 and NF-kappaB signaling in an IL-6-dependent manner. J Neuroinflamm 14(1):231
Sun L, Li M, Ma X, Zhang L, Song J, Lv C, He Y (2018) Inhibiting high mobility group box-1 reduces early spinal cord edema and attenuates astrocyte activation and Aquaporin-4 expression after spinal cord injury in rats. J Neurotrauma. 36(3):421–435
Szepetowski P (2017) Genetics of human epilepsies: continuing progress. Presse Med. 47(3):218–226
Tovar KR, Westbrook GL (1999) The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci 19(10):4180–4188
van Vliet EA, Da CAS, Redeker S, van Schaik R, Aronica E, Gorter JA (2007) Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain 130(Pt 2):521–534
Verkman AS, Smith AJ, Phuan PW, Tradtrantip L, Anderson MO (2017) The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin Ther Targets 21(12):1161–1170
Wang XX, Li YH, Gong HQ, Liang PJ, Zhang PM, Lu QC (2017a) The subiculum: a potential site of ictogenesis in a neonatal seizure model. Front Neurol 8:147
Wang X, An F, Wang S, An Z, Wang S (2017b) Orientin attenuates cerebral ischemia/reperfusion injury in rat model through the AQP-4 and TLR4/NF-kappaB/TNF-alpha Signaling Pathway. J Stroke Cerebrovasc Dis 26(10):2199–2214
Wang C, Yan M, Jiang H, Wang Q, He S, Chen J, Wang C (2018) Mechanism of aquaporin 4 (AQP 4) up-regulation in rat cerebral edema under hypobaric hypoxia and the preventative effect of puerarin. Life Sci 193:270–281
Wu X, Zhang JT, Li D, Zhou J, Yang J, Zheng HL, Chen JG, Wang F (2017) Aquaporin-4 deficiency facilitates fear memory extinction in the hippocampus through excessive activation of extrasynaptic GluN2B-containing NMDA receptors. Neuropharmacology 112(Pt A):124–134
Xie Y, Huang XF (2018) Commentary: GLYX-13 ameliorates schizophrenia-like phenotype induced by MK-801 in mice: role of hippocampal NR2B and DISC1. Front Mol Neurosci 11:315
Yang Y, Tian X, Xu D, Zheng F, Lu X, Zhang Y, Ma Y, Li Y, Xu X, Zhu B, Wang X (2018) GPR40 modulates epileptic seizure and NMDA receptor function. Sci Adv 4(10):u2357
Yin J, Zhang H, Chen H, Lv Q, Jin X (2018) Hypertonic saline alleviates brain edema after traumatic brain injury via downregulation of aquaporin 4 in rats. Med Sci Monit 24:1863–1870
Yu H, Chen L, Ma M (2015) Effect on the expression of NF- κB p65、IL-1β、IL-6 and TNF-α in the hippocampus of chronic epilepsy rats by acetazolamide. J Apoplexy Nerv Dis 08:730–733
Yu H, Qi GL, Wang J, Chen L, Deng Z, Zhao YS, Lei SS, Zhu XQ (2016) Aquaporin 4 inhibition decreased synthesis of cytokines by acetazolamide in the hippocampus of rats with pentrazol-induced chronic epilepsy. Genet Mol Res 15(3):15039012
Zhou Y, Li HL, Zhao R, Yang LT, Dong Y, Yue X, Ma YY, Wang Z, Chen J, Cui CL, Yu AC (2010) Astrocytes express N-methyl-D-aspartate receptor subunits in development, ischemia and post-ischemia. Neurochem Res 35(12):2124–2134
Zhou D, Lv D, Wang Z, Zhang Y, Chen Z, Wang C (2018) GLYX-13 ameliorates schizophrenia-like phenotype induced by MK-801 in mice: ROLE of hippocampal NR2B and DISC1. Front Mol Neurosci 11:121
Acknowledgements
This work was supported by research grants from the National Natural Science Foundation of China (81771401, 81870992, 81870856, 81603681, U1603281), Natural Science Foundation of Guangdong Province of China (2020A1515010986), Science and Technology Planning Project of Guangdong Province of China (2017ZC0234), a technology project of Guangzhou (2018-1202-SF-0019).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. SL, XZ, and PX designed the study. YH, ZZ, ZL, YL, YH, SD, and XC performed the research. XC analyzed the data. YH wrote the manuscript. XZ and PX revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
All procedures performed in studies involving animals were following the ethical standards of the Experimental Animal Care and Ethics Committee of Experimental Animal Center of Guangzhou Medical University (GY2017-038).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, S., He, Y., Zhu, Z. et al. Inhibition of NMDA Receptors Downregulates Astrocytic AQP4 to Suppress Seizures. Cell Mol Neurobiol 40, 1283–1295 (2020). https://doi.org/10.1007/s10571-020-00813-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00813-6