Abstract
Curcumin, a major bioactive component of turmeric, has diverse therapeutic effects such as anti-inflammatory, antioxidant, anticancer, and antinociceptive activities. The acid-sensing ion channels (ASICs), which can be activated by acute drops in the extracellular pH, play an important role in nociception. However, very little is known about the interaction between ASICs and curcumin in nociception of inflammation. In our study, we investigated whether the antinociceptive effects of curcumin are mediated via ASICs using an orofacial nociceptive model and in vitro western blotting, immunofluorescence, whole-cell patch-clamp recordings in the trigeminal system. Intraperitoneally administered curcumin at a dose of 50 mg/kg can reduce hyperalgesia in both the phases of a formalin-induced orofacial nociceptive model. Curcumin reduced the amplitude of ASICs currents in a dose-dependent manner in trigeminal ganglion (TG) neurons, and curcumin also reduced the protein quantity but did not change the distribution of ASICs in TG. Thus, our results indicate that curcumin can reduce formalin-induced ASICs activation and thus inhibit ASICs-mediated inflammatory pain hypersensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Curcumin (1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a major bioactive component of turmeric, which has been used as a coloring agent in food. It has been used as a herbal medicine for centuries in India (Agrawal and Mishra 2010; Zhou et al. 2013). Recently, curcumin has received a great deal of attention for its anti-inflammatory activity. Research has shown curcumin to be a highly pleiotropic molecule capable of interacting with numerous molecular targets involved in inflammation (Jurenka 2009). Based on cell culture and animal research, clinical trials indicate that curcumin may have potential as a therapeutic agent in diseases such as inflammatory bowel disease, pancreatitis, arthritis, and chronic anterior uveitis, as well as inflammatory response in certain types of cancer (Aggarwal et al. 2007; Chan et al. 1998; Hsu and Cheng 2007). It is reported that curcumin exhibits anti-inflammatory activity by inhibiting a number of different mediators including phospholipase, lipoxygenase, cyclo-oxygenase-2, leukotrienes, thromboxane, prostaglandins, and nitric oxide (Banerjee et al. 2003; Chainani-Wu 2003; Goel et al. 2001; Xu et al. 1997). However, the exact mechanism of action remains controversial.
The acid-sensing ion channels (ASICs) belong to the degenerin/epithelial Na+ channel superfamily (DEG/ENaC), which can be activated by acute drops in the extracellular pH (Krishtal 2003). Six subunits encoded by four genes (ASIC1–4) have been identified: ASIC1a–b, 2a–b, ASIC3, and ASIC4. ASICs are voltage-independent, low-conductance channels that mainly conduct Na+ (Chen et al. 1998). ASICs are widely expressed in peripheral and central nervous system and participate in many important physiological and pathological processes, such as learning and memory, synaptic plasticity, cerebral ischemia, and inflammation (Lingueglia 2007; Mamet et al. 2002).
Multiple studies verified the close relation between inflammation and ASICs. Duan demonstrated that ASIC channels in SDH neurons are essential for inflammation-induced pain hypersensitivity and spinal neuron sensitization (Duan et al. 2007). Our earlier works also indicated that ASICs contribute to the orofacial inflammatory pain and genetic deletion of ASIC1 gene attenuates the inflammatory response of mice (Fu et al. 2016). But whether ASICs also participate in the anti-inflammatory effect of curcumin is unknown.
Our present study aimed to investigate whether the antinociceptive effects of curcumin are mediated via ASICs. Several different experiments were used to investigate the relationship between ASICs and curcumin in nociception of inflammation. The results show that intraperitoneally administered curcumin blocked the nociceptive response in both the phases of a formalin-induced orofacial inflammation model. Curcumin reduced the amplitude of ASICs currents in a dose-dependent manner in trigeminal ganglion (TG) neurons, and curcumin 10 μmol/l also reduced the protein quantity but did not change the distribution of ASICs in TG.
Materials and Methods
Materials
Curcumin and amiloride were purchased from Sigma-Aldrich (MO, USA). Primary antibodies of ASICs (ASIC1, 2a, and 3) were purchased from Alomone Labs (Jerusalem, Israel). β-actin was purchased from Santa Cruz (CA, USA). The goat antirabbit fluorescein isothiocyanate (FITC)-conjugated secondary antibody used in immunofluorescence assay and the secondary antibody used in western blotting were purchased from Santa Cruz (CA, USA), too.
Dulbecco’s modified Eagle’s medium DMEM/F12 was obtained from Gibco Invitrogen Corporation (Carlsbad, CA, USA). Other general agents were purchased from commercial suppliers. All the drugs were prepared as stock solutions. Amiloride was dissolved in distilled water. Curcumin was dissolved in dimethyl sulfoxide (DMSO). All stock solutions were stored at −20 °C. These stock solutions were diluted to the final concentrations with the extracellular solution or saline before application. In electrophysiological experiments, the final concentration of DMSO was <0.05 %.
Animals
The experimental protocols were approved by the Institutional Animal Care and Use Committee of Guangdong Medical College, and were conducted in accordance with the recommendations of the NIH Guide for the Care and the Use of Laboratory Animals. The adult male Sprague–Dawley (SD) rats (250 ± 20 g) were housed in plastic cages with free access to food and water and were maintained in climate-controlled (23 ± 1 °C) and light-controlled (12/12-h dark/light cycle with lights on at 8:00 a.m.) units for at least 10 days before the experiments.
Orofacial Formalin Test
The procedure of orofacial formalin test was the same as described previously (Raboisson and Dallel 2004). The animals were acclimatized to the laboratory environment for at least 1 h before use. Rats received a subcutaneous injection of 50 μl diluted formalin (as formalin only group) or the same volume vehicle (as control group) into the center of the right vibrissa pad. Solutions were prepared from commercially available stock formalin (an aqueous solution of 37 % formaldehyde) and further diluted in isotonic saline to 4 %. Curcumin was intraperitoneally injected 30 min before formalin injection (as formalin + curcumin group), at a dose of 50 mg/kg. Analysis of the behavior was conducted by an investigator who was blinded to the animal’s group assignment. The mice did not have access to food or water during the test. After injection, the animals were immediately placed back in the test box for a 45-min observation period. The recording time was divided into 15 blocks with 3-min intervals, and a nociceptive score was determined for each block by measuring the number of seconds that the animals spent grooming the injected area with the ipsilateral fore- or hindpaw or bilateral flinching of the hind quarters.
Western Blotting
One day after formalin or saline injection, trigeminal ganglia were taken from adult SD rats. The tissue was homogenized in 80 μl of ice-cold lysis buffer (50 mM Tris–HCl (pH 7.4), 1 mM EDTA, 100 mM NaCl, 20 mM NaF, 3 mM Na3VO4, and 1 mM phenylmethanesulfonyl fluoride, with 1 % (v/v) Nonidet P-40). Samples were then centrifuged at 12,000×g for 15 min at 4 °C. Protein concentration was determined using the BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). After denaturation, protein samples (30 μg) were separated by 10 % SDS-PAGE and then transferred to nitrocellulose membranes using a transfer cell system (Bio-Rad, USA). After blocking with 5 % nonfat dried milk in 0.1 %Tris-buffered saline Tween-20 (TBST) for 1 h at room temperature, membranes were probed with the appropriate antibodies against ASICs (ASIC1, 2a, and 3; 1:200 dilution) overnight at 4 °C. Membrane-bound primary antibodies were detected using secondary antibodies conjugated with horseradish peroxidase (1:10,000). Immunoblots were developed on films using the enhanced chemiluminescence technique (ECL; Pierce, Rockford, USA). Normalization of results was ensured by running western blots with β-actin (1:3000 dilutions). All assays were performed at least three times.
Immunofluorescence Assay
One day after formalin or saline injection, trigeminal ganglia were dissected aseptically from adult SD rats. TG slices (20 μm) that were frozen on dry ice for 24 h were cut with a freezing microtome (CM1900, Leica Microsystems, Wetzlar, Germany). The slices were dried overnight with 30 % sucrose solution. After three rinses with 0.01 M phosphate-buffered saline (PBS, pH 7.4) for 10 min each, they were then fixed in PBS with 0.3 % Triton X-100 for 30 min, followed by 3 %BSA-PBS for 30 min at room temperature. The slices were coincubated with 1:50 anti-ASIC1, 2a, and 3 antibody in PBS/0.3 % Triton X-100/1 %BSA/2 % goat serum overnight at 4 °C. Slices were rinsed in PBS three times for 10 min each, and then coincubated with 1:100 goat antirabbit fluorescein isothiocyanate (FITC)-conjugated secondary antibody in PBS containing 0.3 % TritonX-100, 2 % goat serum, and 1 % BSA for 1 h at room temperature. Afterward, the slices were washed three times in PBS, were mounted on glass slides with 10–15 % glycerin, and imaged using a confocal laser scanning microscope (FV500; Olympus, Tokyo, Japan).
Cell Preparation
TG neurons were cultured from adult SD rats as described previously (Deval et al. 2011). Trigeminal ganglia were dissected aseptically and collected in 0.1 % collagenase (type Xl-S, Sigma, St Louis, USA) and 20 U/ml papain (Roche, Basel, Swiss) in modified Hank’s solution (pH 7.4). After incubating for 20–30 min at 37 °C, individual cells were dissociated by triturating the tissue through a fire-polished glass pipette. After trituration and centrifugation at 118×g for 6 min, these cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 supplemented with 10 % fetal bovine serum (heat-inactivated), and 100 units/ml penicillin–streptomycin. The cells were plated on poly-d-lysine (1 mg/ml)-coated glass coverslips (15 mm diameter) and cultured for 1–3 h at 37 °C in a water-saturated atmosphere with 95 % O2 and 5 % CO2.
Electrophysiological Experiments
The procedure for whole-cell patch-clamp recording of ASICs was consistent with our previous reports (Hu et al. 2010). The whole-cell patch-clamp was performed in a voltage-clamp mode, and membrane currents were recorded with HEKA EPC-10 patch-clamp amplifier (HEKA, Germany) which was connected to a compatible computer via D/A and A/D converter. Pulse+pulsefit software 8.6 (HEKA, Germany) was used to produce protocols, and acquire and process data. The glass pipettes (150F-10; Clark Instruments, Salisbury, UK) were made by a two-stage puller (pp-10, Narishige Instruments, Tokyo, Japan) from star-bore capillary tubes (GG-17) and had resistance of no more than 5 M after the pipette solution was perfused. The series resistance (Rs) was electrically compensated to minimize the capacitive surge on the current recording, and the series resistance was compensated at least 75 % in most experiments. TG neurons were voltage-clamped at −80 mV throughout the experiments. A multi-barrel perfusion system was used to achieve a rapid exchange of extracellular solutions. The sampling rate and filter rate were 10 and 2 kHz, respectively. The amplitude of current was normalized to the maximum evoked currents of ASICs. If the uncompensated series resistance resulted in voltage-clamp errors of >15 M, the results were excluded. All experiments were carried out at room temperature (20–25 °C).
The pipette solution contained the following (in mM): KCl 140, NaCl 10, and MgCl2∙6H2O 1, EGTA 5, MgATP 2, HEPES 10, pH 7.2 with 1 mM Tris-OH. The external solution contained the following (in mM): NaCl 150, KCl 5, MgCl2∙6H2O 1, CaCl2 2, Glucose 10, HEPES 10, pH adjusted to 7.4 with 1 mM Tris-OH. MES was used instead of HEPES to buffer bath solution with pH ranging from 6.0 to 5.0.
Data Analysis
Data are expressed as mean ± SEM or mean ± SD. Student’s t test and ANOVA followed by Student–Newman–Keuls test were used for statistical analysis where appropriate. The SPSS 11.0 software and Sigma Plot 8.0 software were used. Differences were considered to be statistically significant when p < 0.05.
Results
Curcumin Attenuated the Nociceptive Response in a Formalin-Induced Nociception
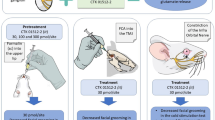
The nociceptive score, which evaluated the nociceptive response after administration of different solutions, is presented in Fig. 1. The time course of orofacial formalin test was biphasic: Phase I was recorded in the first 12 min after formalin injection; Phase II appeared 12–45 min after the formalin injection. At first, ten rats received a 50-μl subcutaneous injection of saline into the center of the right vibrissa pad, as control. As shown in Fig. 1, the nociceptive score had no obvious change after injection of saline, indicating that the injection itself had no effect on the behavior of mice. Then, ten other rats received a 50-μl subcutaneous injection of 4 % formalin into the same site, as formalin model group. The nociceptive score increased obviously in both the phases. But in curcumin + formalin group (intraperitoneal administration of 50 mg/kg curcumin 30 min before formalin), we found that the nociceptive score of Phases I and II significantly reduced compared to formalin-only group.
Curcumin and amiloride attenuated the nociceptive response in rats with formalin-induced orofacial inflammation. a Typical experiment procedure of rats. b The nociceptive response was reinforced after subcutaneous injection of formalin compared to the control group. Injection of curcumin or amiloride before formalin attenuated the formalin-induced nociceptive response in both the phases of formalin test, respectively. Data expressed as mean ± SEM. n = 10; *p < 0.05 versus formalin group; **p < 0.01 versus formalin group
Then we replaced curcumin by amiloride, a nonselective inhibitor of ASICs (Ugawa et al. 2002), in other ten rats; the intraperitoneal administration of amiloride (0.2 %) also reduced the nociceptive score of Phases I and II compared to the formalin group. These data suggest that both curcumin and amiloride have antinociceptive effects in orofacial formalin model and ASICs may contribute to the nociceptive response in orofacial inflammation, but whether ASICs take part in the antinociceptive effect of curcumin cannot be confirmed in this behavior test.
Curcumin Inhibited the Amplitude of ASIC Currents in TG Neurons
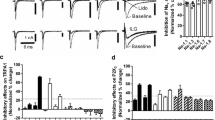
In our previous study, we confirmed the presence of ASICs in TG neurons innervating orofacial region (Fu et al. 2016). So we first tested the function of ASICs under orofacial inflammatory condition in this study. Some kinds of inward currents are activated by rapid lowering of pH from 7.4 to 6.0 and recorded at a holding potential of −80 mV in TG neurons. The ASIC1-like current is activated and inactivated rapidly (Fig. 2a upper), whereas the ASIC3-like current rapidly activated but inactivated slowly (Fig. 2a middle). We found that the amplitude of both ASIC1-like and ASIC3-like current is obviously higher in the formalin group than in the control group (635.36 ± 197.45 vs. 278.67 ± 89.52 pA for ASIC1-like current, 1694.16 ± 486.64 vs. 1047.48 ± 275.53 pA for ASIC3-like current, respectively). However, if we preincubated the neurons with curcumin 10 μmol/l for 5 min in formalin model, this increase can be partially abolished (from 635.36 ± 197.45 to 346.34 ± 122.35 pA for ASIC1-like current, and from 1694.16 ± 486.64 to 1158.88 ± 233.15 pA for ASIC3-like current, respectively). Compared to the formalin, 10 μmol/l curcumin inhibited ASIC1-like current about 45.15 ± 17.54 % (n = 11, p < 0.05) and inhibited ASIC3-like current about 31.67 ± 11.84 % (n = 8, p < 0.05, Fig. 2a). The IC50 are 13.2 ± 0.61 μmol/l for ASIC1-like current and 17.73 ± 1.12 μmol/l for ASIC3-like current (Fig. 2b).
Inhibition of ASIC currents in TG neurons by curcumin. The inward currents in TG neurons were activated by rapid lowering of pH from 7.4 to 6.0. a The amplitudes of both ASIC1-like and 3-like currents are increased after formalin subcutaneous injection. But after preincubation with 10 µM curcumin for 5 min, two kinds of ASICs current were partially inhibited(n = 11 for ASIC1-like current and n = 8 for ASIC3-like current; *p < 0.05 vs. control, #p < 0.05 vs. formalin model. Data were shown as mean ± SD.). b The dose–effect curves showed that the inhibitive effects of curcumin on ASIC currents are concentration dependent. c TRPV1 currents can also be inhibited by curcumin in TG neurons (n = 6; *p < 0.05 vs. control, #p < 0.05 vs. formalin model, Data were shown as Mean ± SD.)
However, in some neurons (6 of 45 neurons), we found that acid-induced current is not only ASICs; the TRPV1 receptor is sensitive to pH 6.0 solution as well. Under formalin inflammation condition, the TRPV1 current also increased (from 167.36 ± 57.68 to 318.37 ± 134.87 pA). Similar to ASICs, this augmentation can be partially inhibited by curcurmin (from 318.37 ± 134.87 to 253.93 ± 64.55 pA, see the representative currents and histogram in Fig. 2c). This is in accord with some previous reports. For example, Yeon et al. (2010) found that curcumin produces an antihyperalgesic effect via antagonism of TRPV1.
Curcumin Suppressed the Protein Expression but Did Not Change the Distribution of ASICs
The electrophysiological results revealed that curcumin inhibited the function of ASICs in orofacial inflammation. In the following study, we performed western blotting and immunofluorescence experiments to study whether curcumin changes the protein expression and distribution of ASICs in the same condition.
As shown in Fig. 3, western blot results indicated that the injection of formalin significantly increased the protein expression of ASICs in ipsilateral TG neurons.Pretreatment with curcumin 10 μmol/l partially abolished the formalin-induced enhancement of ASICs protein. Similar to the western blot data, immunofluorescence shows that ASIC1 and ASIC3 fluorescence intensity also increased obviously in TG of the formalin model rats. Pretreatment with curcumin 10 μmol/l partially abolished the increased fluorescence intensity of ASIC1 and ASIC3. However, the distribution of all ASIC subunits did not change in both the formalin group and curcumin + formalin group.
Curcumin decreased the expression of ASICs. a Western blotting results indicated that the injection of formalin significantly increased the protein expression of ASICs in ipsilateral TG neurons, and curcumin 10 μmol/l in the inflammatory model group (as formalin model + curcumin group) partially abolished this formalin-induced enhancement. b The histogram shows the quantification of ASIC1a, ASIC2, and ASIC3 protein density in the control, formalin model, and formalin model + curcumin group. Data are expressed as mean ± SD (*p < 0.05 vs. control group; # p < 0.05 vs. formalin group, n = 5). c ASICs (green) immunoactivity in TG neurons. Scale bars 100 μm. After injection of formalin, the immunoactivity of ASICs became stronger, and curcumin 10 μmol/l partially abolished this formalin-induced enhancement of ASICs. d The histogram shows the quantification of ASIC1a, ASIC2, and ASIC3 in the control, formalin model, and formalin + curcumin group accumulated fluorescence density (*p < 0.05 vs. control group; # p < 0.05 vs. formalin group; **p < 0.01 vs. formalin group; ## p < 0.01 vs. formalin group; NS, no significance; data are expressed as mean ± SD, n = 3)
Discussion
In the present study, we investigated the analgesic effects of curcumin on formalin-induced orofacial pain and testified that ASICs participate in curcumin’s analgesic effect.
Firstly, we took a formalin-induced orofacial nociceptive model (Luccarini et al. 2006). The injection of formalin destroys nerve endings, as well as causes the release of inflammatory mediators. It is a valid and reliable model for evaluation of orofacial inflammatory and neuropathic pain. The locally injured tissue due to formalin subcutaneous injection can produce two disparate reactions: initial acute pain and subsequently persistent noxious stimulation. Acute pain (Phase I) results from direct thermal, mechanical, or chemical activation of particular subsets of primary afferent neurons. However, the persistent component of the pain (Phase II) is associated with the central sensitization and release of multiple inflammatory factors, including neurotransmitters and protons (Julius and Basbaum 2001; Luccarini et al. 2006).
The behavior test presented here clearly demonstrates that curcumin reduced the nociceptive response in both the phases of formalin test. Compared to the model group, the formalin-induced face-rubbing episodes (nociceptive response) decreased significantly in both the phases in curcumin-pretreated rats. Curcumin can not only attenuate the direct irritant effect mediated by peripheral nociception, but also display more tolerance to the combination of sensory input and central sensitization through central nociception mechanism. The behavior test results indicate that curcumin has potential to modulate periphery and central pain processes in inflammation.
However, we found amiloride, a nonselective inhibitor of ASICs, has a similar effect in the formalin test. This suggests that ASICs also play an important role in the inflammatory response of formalin model, but whether curcumin exerts an antinociceptive effect through inhibition of ASICs cannot be verified in the behavior test.
Therefore, we then examined whether curcumin modulates ASICs in the same orofacial inflammation model using a series of in vitro experiments. The electrophysiological results indicate that curcumin partially reduced the amplitude of ASICs currents under the inflammation condition. Accordingly, western blotting and immunofluorescence results suggest that curcumin partially abolished the up-regulation of ASICs protein levels of orofacial inflammatory rats, but it does not change the distribution of ASICs in TG. These results demonstrate that in formalin-induced inflammation, curcumin can inhibit the protein expression and function of ASICs, but it does not change their distribution in TG neurons.
Tissue acidosis is a dominant factor in inflammation and plays an important role in pain and hyperalgesia (Steen and Reeh 1993). Under inflammation conditions, the produced H+ can activate peripheral nociceptors via the activation of some inward cation-selective channels such as ASICs and TRPV1. Previous studies have demonstrated that curcumin exerts its antinociceptive activity by suppressing the capsaicin-induced TRPV1 activity (Yeon et al. 2010). In the present study, the suppression of inflammation-induced TRPV1 current by curcumin was also verified. And interestingly, the whole-cell patch-clamp results firstly show that curcumin also decreased the amplitude of ASIC currents, which are up-regulated by formalin. Subsequently, using western blotting and immunofluorescence techniques, we found that curcumin decreased the protein expression of ASIC1, 2a, and 3, which are up-regulated by formalin, without changing their distribution.
Among all ASIC subunits, ASIC1 is the only channel that conducts both Na+ and Ca2+ (Voilley et al. 2001). By facilitating Na+ inflow, it promotes the depolarization of neurons and generates action potentials (AP). By facilitating Ca2+ inflow, it promotes the release of neurotransmitters. Meanwhile, the depolarization can also open the voltage-dependent calcium channels, and then promotes the release of neurotransmitters and the generation of LTP in dorsal horn and central hyperalgia. The ASIC3 isoform also takes part in the generation of AP, its sustained current contributes to the persistence of AP and the sustain pain of inflammation (Deval et al. 2011). By inhibiting ASIC1 and ASIC3, curcumin can decrease the excitability of sensory neurons (such as TG neurons) and reduce the release of neurotransmitters, such as prostaglandins and interleukins, and thus exert the anti-inflammatory effect.
In conclusion, results from the behavioral testing, electrophysiological, and western blotting show that curcumin down-regulates the function and expression of ASICs, and then leads to decrease the excitability of sensory neurons and reduction of the inflammatory mediator release. This may be a new view about the exact mechanism through which curcumin exerts an obvious antinociceptive effect in inflammation.
References
Aggarwal BB, Sundaram C, Malani N, Ichikawa H (2007) Curcumin: the Indian solid gold. Adv Exp Med Biol 595:1–75. doi:10.1007/978-0-387-46401-5_1
Agrawal DK, Mishra PK (2010) Curcumin and its analogues: potential anticancer agents. Med Res Rev 30:818–860. doi:10.1002/med.20188
Banerjee M, Tripathi LM, Srivastava VM, Puri A, Shukla R (2003) Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacol Immunotoxicol 25:213–224
Chainani-Wu N (2003) Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med 9(1):161–168
Chan MM-Y, Huang H-I, Fenton MR, Fong D (1998) In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol 55:1955–1962. doi:10.1016/S0006-2952(98)00114-2
Chen CC, England S, Akopian AN, Wood JN (1998) A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA 95:10240–10245
Deval E et al (2011) Acid-sensing ion channels in postoperative pain. J Neurosci 31:6059–6066
Duan B et al (2007) Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci 27:11139–11148. doi:10.1523/jneurosci.3364-07.2007
Fu H et al (2016) Acid-sensing ion channels in trigeminal ganglion neurons innervating the orofacial region contribute to orofacial inflammatory pain. Clin Exp Pharmacol Physiol 43:193–202. doi:10.1111/1440-1681.12510
Goel A, Boland CR, Chauhan DP (2001) Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett 172:111–118. doi:10.1016/S0304-3835(01)00655-3
Hsu CH, Cheng AL (2007) Clinical studies with curcumin. Adv Exp Med Biol 595:471–480. doi:10.1007/978-0-387-46401-5_21
Hu Z-L et al (2010) Disruption of PICK1 attenuates the function of ASICs and PKC regulation of ASICs. Am J Physiol 299:C1355–C1362
Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413:203–210
Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14:141–153
Krishtal O (2003) The ASICs: signaling molecules? Modulators? Trends Neurosci 26:477–483. doi:10.1016/s0166-2236(03)00210-8
Lingueglia E (2007) Acid-sensing ion channels in sensory perception. J Biol Chem 282:17325–17329. doi:10.1074/jbc.R700011200
Luccarini P, Childeric A, Gaydier A-M, Voisin D, Dallel R (2006) The orofacial formalin test in the mouse: a behavioral model for studying physiology and modulation of trigeminal nociception. J Pain 7:908–914
Mamet J, Baron A, Lazdunski M, Voilley N (2002) Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22:10662–10670
Raboisson P, Dallel R (2004) The orofacial formalin test. Neurosci Biobehav Rev 28:219–226. doi:10.1016/j.neubiorev.2003.12.003
Steen K, Reeh P (1993) Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett 154:113–116
Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S (2002) Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Investig 110:1185–1190. doi:10.1172/jci15709
Voilley N, de Weille J, Mamet J, Lazdunski M (2001) Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci 21:8026–8033
Xu YX, Pindolia KR, Janakiraman N, Chapman RA, Gautam SC (1997) Curcumin inhibits IL1 alpha and TNF-alpha induction of AP-1 and NF-kB DNA-binding activity in bone marrow stromal cells. Hematop Mol Hematol 11:49–62
Yeon K et al (2010) Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J Dent Res 89:170–174
Zhou T, Chen D, Li Q, Sun X, Song Y, Wang C (2013) Curcumin inhibits inflammatory response and bone loss during experimental periodontitis in rats. Acta Odontol Scand 71:349–356. doi:10.3109/00016357.2012.682092
Acknowledgments
This work was supported by the Medical Scientific Research Foundation of GuangDong (No. A2015356) and the Construct TCM Research Program of GuangDong (No. 20141151) to Dr. H. Fu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, Y., Qin, D., Yang, H. et al. Evidence for the Participation of Acid-Sensing Ion Channels (ASICs) in the Antinociceptive Effect of Curcumin in a Formalin-Induced Orofacial Inflammatory Model. Cell Mol Neurobiol 37, 635–642 (2017). https://doi.org/10.1007/s10571-016-0399-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-016-0399-3