Abstract
The mechanism of sevoflurane preconditioning-induced neuroprotection is poorly understood. This study was aimed at identifying microRNAs (miRNAs) involved in the protective effect of sevoflurane preconditioning against hypoxic injury using the miRCURYTM LNA Array. The screened differentially expressed miRNAs were further validated using qRT-PCR. Finally, after transfection of miRNA (miR-101a or miR-34b) mimics or inhibitor, MTT and flow cytometry assays were used to evaluate cell survival and apoptosis in sevoflurane preconditioning. qRT-PCR confirmed the changes in expression of differentially expressed miRNAs that were screened by the microarray: down-regulation of rno-miR-101a, rno-miR-106b, and rno-miR-294 and up-regulation of rno-miR-883, rno-miR-16, and rno-miR-34b. MiR-101a and miR-34b were the most differentially expressed miRNAs. Sevoflurane preconditioning-inhibited apoptosis and preconditioning-enhanced cell viability of PC12 cells were significantly attenuated by transfection of miR-101a mimetic or miR-34b inhibitors, but were significantly enhanced by transfection of miR-34b mimetic. Therefore, a number of miRNAs, including miR-101a and miR-34b, might play important roles in the neuroprotection induced by sevoflurane preconditioning. Such miRNAs might provide novel targets for preventive and therapeutic strategies against cerebral ischemia–reperfusion injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral ischemia is characterized by obstruction of blood flow to the brain and is the leading cause of death and chronic disability worldwide (Zhong et al. 2014). There are three main pathological types of cerebral ischemia: (1) ischemic stroke, (2) primary intra-cerebral hemorrhage, and (3) subarachnoid hemorrhage (Clarkson 2007). Ischemic stroke is the most common form of cerebral ischemia and accounts for approximately 80 % of all stroke lesions (Wolf 1998). Cerebral ischemia has been considered as an untreatable pathology with no effective drug treatments (Clarkson 2007). Increasing evidence suggests that suffering from a stroke may increase the risk of developing suicidal ideation and dying by suicide (Pompili et al. 2012). Many patients with stroke suffered from depression before dying by suicide suggesting that mood disorder may significantly contribute to increasing the risk of suicide (Pompili et al. 2012). Neuronal death is partly a result of cell damage that results from hypoxic stress. Several studies have focused on the development of new strategies to prevent hypoxic injury and to promote recovery, however, with only limited success as they focused on the acute phase of brain damage and neglected that a significant amount of neuronal tissue damage develops progressively after the initial insult (Lehane et al. 2013). It was suggested that neuroprotective drugs with the potential to reduce cell hypoxic injury should improve clinical outcome (Heiss and Graf 1994).
In recent years, researchers have found that application of volatile anesthetics after brain ischemia provides neuroprotection in adult animals (anesthetic preconditioning) and human subjects (Yellon and Dana 2000; Yang et al. 2011). Volatile anesthetic preconditioning is a promising strategy for neuroprotection. Sevoflurane is a widely used inhalational anesthetic in clinical practice that can induce ischemic tolerance both in vitro and in vivo (Kitano et al. 2007; Yang et al. 2011). The mechanisms underlying sevoflurane preconditioning have been associated with inhibition of apoptosis, attenuation of inflammation, release of reactive oxygen species (ROS), and improving blood–brain-barrier integrity (Codaccioni et al. 2009; Wang et al. 2011). However, the precise mechanism underlying sevoflurane preconditioning is not fully understood.
MicroRNAs (miRNAs), which were discovered in 1993, are a class of small (19–23 nucleotides long) noncoding RNAs with important posttranscriptional regulatory functions. The mature miRNAs recognize complementary sites in the 3′-untranslated regions (UTR) of target genes, resulting in down-regulation of a wide variety of proteins (Shukla et al. 2011; Bartel 2009). Numerous miRNAs have been shown to be involved in neurogenesis and neuronal maturation in the central nervous system during development (Kosik 2006). Recently, miR-34a was shown to have a neuroprotective effect against neuron cell apoptosis (Hu et al. 2012). MiR-101 enhanced apoptosis induced by cisplatin in the HepG2 cell line (Xu et al. 2013), and promoted breast cancer cell apoptosis by targeting Janus kinase 2 (Wang et al. 2014). Given the multifaceted nature of the cellular effects of sevoflurane preconditioning, we hypothesized that miRNAs may be differentially expressed under hypoxic conditions.
Pheochromocytoma-12 (PC12) cells, which adopt a round morphology and proliferate to high density, exhibit a unique sensitivity to change in O2 concentration (Gozal et al. 2005). PC12 cells have been widely used as a model of neuronal differentiation in both neurobiological and neurotoxicological studies to investigate neuronal vulnerability to hypoxia (Gozal et al. 2005; Das et al. 2004). Therefore, we used PC12 cells to describe expression profile of miRNAs following hypoxia and the impact of sevoflurane preconditioning, especially focusing on investigating the effect of miR-101a and miR-34b, which were most changed, on viability and apoptosis of hypoxic cells that were preconditioned with sevoflurane.
Materials and Methods
Cell Culture
PC12 cells (passage number <10) were cultured in high-glucose DMEM (Gibco, USA) containing 5 % FBS and 10 % horse serum; 100 μg/mL of streptomycin and 100 U/mL of penicillin were added. The cells were grown at 37 °C with 5 % CO2 humidified atmosphere and plated at a density 5 × 103 cells/cm2.
Preconditioning with Sevoflurane and Hypoxic Stimulation
PC12 cells were preconditioned with 30 min exposure to 2 % sevoflurane (sevoflurane group), followed by 6 h recovery at 37 °C in an atmosphere of 21 % O2 and 5 % CO2 (normoxia) and subsequent exposure to hypoxia. Hypoxic conditions (1 % O2) were achieved using the Anaeropack for Cell (Mitsubishi Gas Chemical Co., Japan) according to methods previously reported (Kamiya et al. 1998). Anaeropack is a disposable oxygen-absorbing and CO2-generating agent.
For the miRNA microarray, PC12 cells were incubated under hypoxic conditions for 6 h (Beitner-Johnson et al. 2000) and then reoxygenated for 24 h. For the MTT assay, PC12 cells were incubated under hypoxic conditions for four time periods (24, 48, 72, and 96 h). For flow cytometry, PC12 cells were incubated under hypoxic conditions for 24 h. The control incubation was performed under normoxic conditions (humidified 5 % CO2 in air, normoxia group) for the entire experiment.
RNA Extraction
The total RNA of the cells that had exposure to the Anaeropack for 6 h was isolated using TRIzol (Invitrogen, USA) and the miRNeasy mini kit (QIAGEN, Netherlands) according to the manufacturer’s instructions, which efficiently recovered all RNA species, including miRNAs. RNA quality and quantity was measured using a nanodrop spectrophotometer (ND-1000, Nanodrop Technologies, USA).
miRNA Microarray
The 7th generation of the miRCURYTM LNA Array (v.18.0) (Exiqon, Denmark) contains 3100 capture probes, covering all human, mouse, and rat microRNAs annotated in miRBase18.0, as well as all viral microRNAs related to these species. In addition, this array contains capture probes for 25miRPlus™ mouse microRNAs.
After RNA isolation from the samples, the miRCURY™ Hy3™/Hy5™ Power labeling kit (Exiqon, Vedbaek, Denmark) was used according to the manufacturer’s guideline for miRNA labeling. After stopping the labeling procedure, the Hy3TM-labeled samples were hybridized on the miRCURYTM LNA Array (v.18.0) (Exiqon) according to the array manual. Then the slides were scanned using the Axon GenePix 4000B microarray scanner (Axon Instruments, Foster City, CA, USA).
Identification of Differentially Expressed miRNA
Scanned images were then imported into GenePix Pro 6.0 software (Axon, Malta) for grid alignment and data extraction. Replicated miRNAs were averaged and miRNAs with intensities ≥30 in all samples were chosen for calculating the normalization factor. Expressed data were normalized using the median normalization. After normalization, differentially expressed miRNAs were predicted through Fold Change filtering (Fold Change ≥1.5).
Clustering of Normalized Data of miRNA in All Samples
Hierarchical clustering was performed based on normalized data of identified miRNA in all samples using R Script. miRNAs that had Foreground-Background intensities <30 in all samples were filtered.
Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) Analysis
The expression of predicted differentially expressed miRNAs was further validated using qRT-PCR. Samples from three independent experiments were used. Total RNA extracted from hypoxic PC12 cells was transcribed into cDNA and analyzed with an Applied Biosystems 7500 Real-Time PCR system (ABI, USA). The stem-loop primers used for the PCR amplification were synthesized by RiboBio (China). The relative expression levels of miRNAs were normalized against U6 expression level. All primers for miRNAs and U6 are listed in Table 1. Gene expression is shown as relative expression to control. The data shown are representative of three independent experiments.
Transfection of microRNA Mimics
The miR-101a mimics, miR-34b mimics or inhibitor, and miRNA negative control (NC) were purchased from a commercial manufacturer (GenePharm, China). PC12 cells were seeded in a 6-well plate (2 × 105/well) 24 h before transfection and cells were transfected with miRNA mimics (50 nM), inhibitor (50 nM), or miR-NC (50 nM) using FuGENE® HD transfection reagent (Promega, USA) in accordance with the manufacturer’s instruction.
MTT Assay
After transfection with miR-101a mimics, miR-34b mimics/inhibitor, or miRNA NC for 24 h, the effect of preconditioning with sevoflurane on cell survival was detected using the MTT [3-(4,5-dimethyl-thiazol-2-y1) 2,5-diphenyl tetrazolium bromide] (Sigma, USA) colorimetric assay. PC12 cells preconditioned with sevoflurane were seeded in 96-well tissue culture plates at 2 × 104 cells per well and incubated under hypoxic conditions for various periods of time. Cells were first washed with PBS and then incubated in 100 µL of 5 mg/mL MTT solution (Invitrogen) for 3 h. MTT was converted into purple-colored formazan in living cells which were then solubilized with dimethylsulfoxide (DMSO) (Invitrogen) and absorbance of solution was taken at 450 nm using the microplate reader Thermo Plate (Rayto Life and Analytical Science C. Ltd., Germany).
Flow Cytometry
After transfection with miR-101a mimics, miR-34b mimics/inhibitor, or miRNA NC for 24 h, the effect of preconditioning with sevoflurane on cell apoptosis was determined by Annexin V assay. PC12 cells preconditioned with sevoflurane were incubated under hypoxic conditions for 24 h and then collected and stained using the Annexin V-FITC/PI apoptosis detection kit (BestBio, Shanghai, China) following the manufacturer’s instructions. Then, cell suspensions were analyzed by flow cytometry. PI negative and annexin V-FITC positive cells were defined as early apoptotic cells, while late apoptotic cells were defined as PI positive and annexin V-FITC positive. The total apoptotic rate was the sum of the early apoptotic rate and the late apoptotic rate. Each experiment was performed three times, and data were presented as mean ± SD.
Statistical Analysis
For quantitative data, all results are expressed as the mean ± SD unless indicated otherwise from at least three independent experiments. Statistical significance between groups was determined using one-way analysis of variance or an unpaired Student’s t test using SPSS 18.0 (SPSS, USA). A P value of <0.05 was considered statistically significant.
Results
Identification of Differentially Expressed miRNA Using miRNA Microarray
After pre- or non-conditioning with sevoflurane for 30 min, PC12 cells were incubated in the normoxia conditions for 6 h, and subsequently exposed to hypoxic conditions for 6 h. MTT assay revealed that sevoflurane protected cells against hypoxia (Fig. 1a).
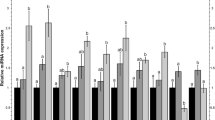
The protection of sevoflurane against hypoxic cell injury and the conformation of screened miRNAs. a MTT assay revealed the protection of sevoflurane against hypoxic cell injury in PC12 cells (n = 3). The validation of predicted differentially expressed miRNAs (n = 3), b down-regulated miRNAs in microarray result; c up-regulated miRNAs in microarray result. The Anaeropack system was used for cell culture to create an hypoxic environment. Control: cells without any volatile anesthetic. The same samples with miRNA array were used for qRT-PCR detection.*P < 0.05 vs. control; and # P < 0.05 vs. hypoxia
To predict the miRNAs involved in the sevoflurane preconditioning against hypoxic injury in PC12 cells, the miRCURYTM LNA Array (v.18.0) was used and the results predicted 14 differentially expressed miRNAs between the sevoflurane group and normoxia group: seven up-regulated miRNAs including rno-miR-300, rno-miR-24, rno-miR-27a, rno-miR-195, rno-miR-16, rno-miR-34b, and rno-miR-883, and seven down-regulated miRNAs including rno-let-7b, rno-miR-207, rno-miR-101a, rno-miR-23a, rno-miR-125a, rno-miR-106b, and rno-miR-294.
Heat Map and Hierarchical Clustering
Hierarchical clustering of expressed miRNAs showed distinguishable miRNA expression profiling between the sevoflurane group and normoxia group (samples pre- or non-conditioned with sevoflurane) (Fig. 2). The results revealed that all the samples preconditioned with sevoflurane and samples non-conditioned with sevoflurane were well separated.
Hierarchical clustering of normalized data of expressed miRNA in PC12 cells pre- or non-conditioned with sevoflurane. The heat map diagram shows the result of the two-way hierarchical clustering of miRNAs and samples (n = 3). The miRNA clustering tree is shown on the left, and the sample clustering tree appears at the top. Cluster analysis revealed that the identified miRNAs were able to clearly distinguish the samples indicating the expression of miRNAs is related to sevoflurane preconditioning. Red indicates high relative expression, and green indicates low relative expression. Sevoflurane preconditioning: sevoflurane group that was preconditioned with sevoflurane (sample1); Controls: normoxia group that was not preconditioned with sevoflurane (sample2) (Color figure online)
Validation of the Expression of Predicted Differentially Expressed miRNA
Using qRT-PCR and samples from three independent experiments, the results revealed some similar changes in expression of miRNAs with the microarray: rno-miR-101a-3p, rno-miR-106b-5p, and rno-miR-294 showed down-regulated expression in the sevoflurane group, and rno-miR-883-5p, rno-miR-16-5p, and rno-miR-34b-3p showed up-regulated expression (Fig. 1b, c). The changes in expression of those miRNAs were confirmed. Because of the great changes in miR-101a and miR-34b expression, we investigated further the effects of miR-101a and miR-34b on sevoflurane preconditioning in hypoxic PC12 cells.
Effect of miR-101a on Sevoflurane Preconditioning in Hypoxic PC12 Cells
After transfection with miR-101a mimetic, cell survival under hypoxic conditions was tested by MTT assay (Fig. 3a). Overexpression of miR-101a significantly decreased the viability of PC12 cells after hypoxic incubation for 48 h compared with NCs in PC12 cells. In addition, the effect of transfection of miR-101a mimetic on cell apoptosis was examined using the Annexin V-FITC/PI assay (Fig. 3b). The results showed that overexpression of miR-101a significantly increased the apoptosis of PC12 cells at 24 h of hypoxic incubation, and enhanced both the levels of early and later apoptosis compared with NCs in PC12 cells (Fig. 3c).
Effect of miR-101a mimics on cell viability and apoptosis during hypoxia in PC12 cells preconditioned with sevoflurane. After transfection of miR-101a mimetic or miRNA negative control (NC) for 24 h, cell survival (a) and apoptosis (b, c) of hypoxic PC12 cells preconditioned with sevoflurane were tested by MTT assay and Annexin V-FITC/PI flow cytometry, respectively. Quadrant statistics: viable cells in lower left (LL) and early apoptotic cells in lower right (LR), necrotic cells in upper left (UL), late apoptotic cells in upper right (UR). c Proportion of apoptotic cells was measured. *P < 0.05 miR-101a mimics group vs. NC group
Effect of miR-34b on Sevoflurane Preconditioning in Hypoxic PC12 Cells
Using MTT assay, overexpression of miR-34b significantly enhanced the viability of PC12 cells after hypoxic incubation for 48 h, and miR-34b inhibitor significantly decreased the viability for the same duration compared with NCs in PC12 cells (Fig. 4a). Using Annexin V-FITC/PI flow cytometry (Fig. 4b), the results showed that overexpression of miR-34b significantly decreased the apoptosis of PC12 cells at 24 h of hypoxic incubation at both levels of early and later apoptosis compared with NCs in PC12 cells (Fig. 4c). In addition, the administration of miR-34b inhibitor significantly enhanced the apoptosis of hypoxic PC12 cells at both levels of early and later apoptosis.
Effect of miR-34b mimics on cell viability and apoptosis during hypoxia in PC12 cells preconditioned with sevoflurane. After transfection of miR-34b mimetic or miRNA negative control (NC) for 24 h, cell survival (a) and apoptosis (b, c) of hypoxic PC12 cells preconditioned with sevoflurane were tested by MTT assay and Annexin V-FITC/PI flow cytometry, respectively. Quadrant statistics: viable cells in lower left (LL) and early apoptotic cells in lower right (LR), necrotic cells in upper left (UL), late apoptotic cells in upper right (UR). c Proportion of apoptotic cells was measured. *P < 0.05 vs. NC group
Discussion
The protective effects of anesthetic preconditioning were first reported both in vitro and in vivo models of myocardial ischemia (Cope et al. 1997, Boutros et al. 1997). In recent years, several studies showed that preconditioning by anesthetics exerted neuroprotective effects against cerebral ischemia in vitro and in vivo (Kawaguchi et al. 2004, Kaneko et al. 2005). As one of the important inhalational anesthetics, sevoflurane has been investigated in various models using both immediate and delayed preconditioning paradigms (Li et al. 2014, Yang et al. 2012). Sevoflurane preconditioning shows a neuroprotective effect by reduction in cellular injury and inhibition of neuronal apoptosis (Toner et al. 2001). The main finding of this study was that the down-regulation of miR-101a and up-regulation of miR-34b play important roles in the protective effect of sevoflurane preconditioning against hypoxic injury.
In this study, PC12 cells were exposed to Anaeropack-induced hypoxia (1 % O2). MTT assay revealed that sevoflurane protected against hypoxic cell injury in PC12 cells. Using miRNA microarray, we concluded that changes in miRNA expression in the sevoflurane group were caused only by the influence of sevoflurane anesthesia itself. Hierarchical clustering of expressed miRNA revealed that all the samples preconditioned with sevoflurane and samples non-conditioned with sevoflurane separated, indicating that the expression of miRNAs is related to sevoflurane preconditioning.
We further validated these screened miRNAs using qRT-PCR. The down-regulation of rno-miR-101a-3p, rno-miR-106b-5p, and rno-miR-294 and the up-regulation of rno-miR-883-5p, rno-miR-16-5p, and rno-miR-34b-3p were consistent with the microarray data (Fig. 1). However, the expression of rno-let-7b-5p, rno-miR-207, rno-miR-23a-3p, and rno-miR-125a-5p was significantly up-regulated, which was inconsistent with the microarray data, and that of rno-miR-300-5p and rno-miR-27a-3p were slightly but significantly down-regulated. rno-miR-195-5p expression did not significantly change (Fig. 1). It has been reported that miRNAs played an important role in apoptosis in various cells. miR-101 enhanced apoptosis induced by cisplatin in the HepG2 cell line, and promoted breast cancer cell apoptosis by targeting Janus kinase 2 (Wang et al. 2014). It has also been reported that MiR-106b modulated the apoptosis and angiogenesis in myocardial infraction (Liu et al. 2012). MiR-34a has been shown to have a neuroprotective effect against neuron cell apoptosis (Hu et al. 2012). MiR-34b directly conserved p53 target genes. Ectopic miR-34 expression has been found to induce apoptosis in tumor cells (Hermeking 2010). As the difference in expression of miR-101a and miR-34b was the largest between the sevoflurane group and normoxia group, we investigated further the function of miR-101a and miR-34b in the mechanism of the protective effect of sevoflurane preconditioning against hypoxic injury.
After transfection of miR-101a mimetic, sevoflurane preconditioning inhibition of apoptosis of PC12 cells was significantly attenuated suggesting an important role of miR-101a in the protective effect of sevoflurane preconditioning against hypoxic injury. After transfection of miR-34b mimetic, sevoflurane preconditioning inhibition of apoptosis of PC12 cells was significantly enhanced. We also used an inhibitor against the role of miR-34b which resulted in significantly increased apoptosis. Taken together, overexpression of miR-101a and inhibition of miR-34b expression promotes the protective effect of sevoflurane preconditioning against hypoxic injury.
It is worth noting that in the present study we focused on PC12 cells, which have been widely used in both neurobiological and neurotoxicological studies to investigate neuronal vulnerability to hypoxia. The miRNA targets in combination with the regulatory network with other signal transduction networks that may contribute with the miRNAs against cerebral ischemia–reperfusion injury are still poorly understood (Serafini et al. 2012). Importantly, expression patterns of miRNAs differ in the various brain regions and may be cell-type specific, and should be studied well. Also, further study should be carried out on the specific miRNA sequence polymorphisms.
Conclusion
The current study predicted a number of miRNAs that regulate the protective effect of sevoflurane preconditioning against hypoxic injury, and demonstrated the important roles of miR-101a and miR-34b in the neuroprotection induced by sevoflurane preconditioning. Further elucidation of the relationship between miRNAs and sevoflurane preconditioning might provide a novel target for preventive and therapeutic strategies against cerebral ischemia–reperfusion injury.
References
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Beitner-Johnson D, Rust RT, Hsieh T, Millhorn DE (2000) Regulation of CREB by moderate hypoxia in PC12 cells. Adv Exp Med Biol 475:143–152
Boutros A, Wang J, Capuano C (1997) Isoflurane and halothane increase adenosine triphosphate preservation, but do not provide additive recovery of function after ischemia, in preconditioned rat hearts. Anesthesiology 86:109–117
Clarkson AN (2007) Anesthetic-mediated protection/preconditioning during cerebral ischemia. Life Sci 80:1157–1175
Codaccioni JL, Velly LJ, Moubarik C, Bruder NJ, Pisano PS, Guillet BA (2009) Sevoflurane preconditioning against focal cerebral ischemia: inhibition of apoptosis in the face of transient improvement of neurological outcome. Anesthesiology 110:1271–1278
Cope DK, Impastato WK, Cohen MV, Downey JM (1997) Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology 86:699–709
Das KP, Freudenrich TM, Mundy WR (2004) Assessment of PC12 cell differentiation and neurite growth: a comparison of morphological and neurochemical measures. Neurotoxicol Teratol 26:397–406
Gozal E, Sachleben LR Jr, Rane MJ, Vega C, Gozal D (2005) Mild sustained and intermittent hypoxia induce apoptosis in PC-12 cells via different mechanisms. Am J Physiol 288:C535–542
Heiss WD, Graf R (1994) The ischemic penumbra. Curr Opin Neurol 7:11–19
Hermeking H (2010) The miR-34 family in cancer and apoptosis. Cell Death Differ 17:193–199
Hu K, Xie YY, Zhang C, Ouyang DS, Long HY, Sun DN et al (2012) MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. BMC Neurosci 13:115
Kamiya T, Kwon AH, Kanemaki T, Matsui Y, Uetsuji S, Okumura T, Kamiyama Y (1998) A simplified model of hypoxic injury in primary cultured rat hepatocytes. In Vitro Cell Dev Biol Anim 34:131–137
Kaneko T, Yokoyama K, Makita K (2005) Late preconditioning with isoflurane in cultured rat cortical neurones. Br J Anaesth 95:662–668
Kawaguchi M, Drummond JC, Cole DJ, Kelly PJ, Spurlock MP, Patel PM (2004) Effect of isoflurane on neuronal apoptosis in rats subjected to focal cerebral ischemia. Anesth Analg 98:798–805
Kitano H, Kirsch JR, Hurn PD, Murphy SJ (2007) Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab 27:1108–1128
Kosik KS (2006) The neuronal microRNA system. Nat Rev 7:911–920
Lehane C, Guelzow T, Zenker S, Erxleben A, Schwer CI, Heimrich B et al (2013) Carbimazole is an inhibitor of protein synthesis and protects from neuronal hypoxic damage in vitro. J Pharmacol Exp Ther 347:781–793
Li X, Luo P, Wang F, Yang Q, Li Y, Zhao M et al (2014) Inhibition of N-myc downstream-regulated gene-2 is involved in an astrocyte-specific neuroprotection induced by sevoflurane preconditioning. Anesthesiology 121:549–562
Liu Z, Yang D, Xie P, Ren G, Sun G, Zeng X, Sun X (2012) MiR-106b and MiR-15b modulate apoptosis and angiogenesis in myocardial infarction. Cell Physiol Biochem 29:851–862
Pompili M, Venturini P, Campi S, Seretti ME, Montebovi F, Lamis DA et al (2012) Do stroke patients have an increased risk of developing suicidal ideation or dying by suicide? An overview of the current literature. CNS Neurosci Ther 18:711–721
Serafini G, Pompili M, Innamorati M, Giordano G, Montebovi F, Sher L et al (2012) The role of microRNAs in synaptic plasticity, major affective disorders and suicidal behavior. Neurosci Res 73:179–190
Shukla GC, Singh J, Barik S (2011) MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol 3:83–92
Toner CC, Connelly K, Whelpton R, Bains S, Michael-Titus AT, McLaughlin DP, Stamford JA (2001) Effects of sevoflurane on dopamine, glutamate and aspartate release in an in vitro model of cerebral ischaemia. Br J Anaesth 86:550–554
Wang H, Lu S, Yu Q, Liang W, Gao H, Li P et al (2011) Sevoflurane preconditioning confers neuroprotection via anti-inflammatory effects. Front Biosci 3:604–615
Wang L, Li L, Guo R, Li X, Lu Y, Guan X et al (2014) miR-101 promotes breast cancer cell apoptosis by targeting Janus kinase 2. Cell Physiol Biochem 34:413–422
Wolf PA (1998) Prevention of stroke. Lancet 352(Suppl 3):SIII15–18
Xu Y, An Y, Wang Y, Zhang C, Zhang H, Huang C et al (2013) miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol Rep 29:2019–2024
Yang Q, Dong H, Deng J, Wang Q, Ye R, Li X et al (2011) Sevoflurane preconditioning induces neuroprotection through reactive oxygen species-mediated up-regulation of antioxidant enzymes in rats. Anesth Analg 112:931–937
Yang Q, Yan W, Li X, Hou L, Dong H, Wang Q et al (2012) Activation of canonical notch signaling pathway is involved in the ischemic tolerance induced by sevoflurane preconditioning in mice. Anesthesiology 117:996–1005
Yellon DM, Dana A (2000) The preconditioning phenomenon: a tool for the scientist or a clinical reality? Circ Res 87:543–550
Zhong X, Lin R, Li Z, Mao J, Chen L (2014) Effects of Salidroside on cobalt chloride-induced hypoxia damage and mTOR signaling repression in PC12 cells. Biol Pharm Bull 37:1199–1206
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript.
Ethical standards
No Human Participants and/or Animals were involved in this research, and we have read and have abided by the statement of ethical standards for manuscript submitted to Cellular and Molecular Neurobiology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Y., Li, Y., Liu, L. et al. Identification of miRNAs Involved in the Protective Effect of Sevoflurane Preconditioning Against Hypoxic Injury in PC12 Cells. Cell Mol Neurobiol 35, 1117–1125 (2015). https://doi.org/10.1007/s10571-015-0205-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0205-7