Abstract
Excessive allostatic load as a consequence of deregulated brain inflammation participates in the development and progression of multiple brain diseases, including but not limited to mood and neurodegenerative disorders. Inhibition of the peripheral and brain Renin–Angiotensin System by systemic administration of Angiotensin II AT1 receptor blockers (ARBs) ameliorates inflammatory stress associated with hypertension, cold-restraint, and bacterial endotoxin administration. The mechanisms involved include: (a) decreased inflammatory factor production in peripheral organs and their release to the circulation; (b) reduced progression of peripherally induced inflammatory cascades in the cerebral vasculature and brain parenchyma; and (c) direct anti-inflammatory effects in cerebrovascular endothelial cells, microglia, and neurons. In addition, ARBs reduce bacterial endotoxin-induced anxiety and depression. Further pre-clinical experiments reveal that ARBs reduce brain inflammation, protect cognition in rodent models of Alzheimer’s disease, and diminish brain inflammation associated with genetic hypertension, ischemia, and stroke. The anti-inflammatory effects of ARBs have also been reported in circulating human monocytes. Clinical studies demonstrate that ARBs improve mood, significantly reduce cognitive decline after stroke, and ameliorate the progression of Alzheimer’s disease. ARBs are well-tolerated and extensively used to treat cardiovascular and metabolic disorders such as hypertension and diabetes, where inflammation is an integral pathogenic mechanism. We propose that including ARBs in a novel integrated approach for the treatment of brain disorders such as depression and Alzheimer’s disease may be of immediate translational relevance.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
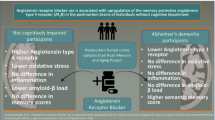
Mood and neurodegenerative disorders are devastating diseases of high prevalence and poorly understood etiology, without adequate treatment. These diseases are the consequence of failure to maintain homeostasis, a condition associated with multiple constellations of factors on a background of genetic vulnerability (Fig. 1).
Brain inflammation participates in the development and progression of brain disorders. Excessive inflammation is one of the multiple factors increasing allostatic load. Superimposed on a background of genetic vulnerability, brain inflammation contributes to loss of homeostasis, and this may lead to neuropsychiatric diseases such as mood and neurodegenerative disorders (modified from Benicky et al. 2011)
A major influence in the development and progression of many brain disorders is the failure of the mechanisms to regulate and control the necessary, adaptive inflammatory responses in the brain (Dantzer et al. 2008; Rivest 2010). When not adequately restricted, brain inflammation may lead to altered behavior and neuronal damage, depression, progressive loss of cognition, and reduced neurological performance (Pascoe et al. 2011; Marchesi 2011; Anisman 2009; Leonard 2007; Tansey and Goldberg 2010). Unfortunately, at present there are no effective and safe treatments to control deregulated inflammatory processes in the brain (Editorial 2007; Nimmo and Vink 2009). For these reasons the search for novel, safe, and effective central anti-inflammatory drugs is of major interest.
The present review summarizes our observations demonstrating that inhibition of the peripheral and brain Renin–Angiotensin System (RAS) by systemic administration of Angiotensin II AT1 receptor blockers (ARBs) ameliorates brain inflammatory stress. ARBs are safe compounds that while commonly used for the treatment of cardiovascular and metabolic disorders where inflammation is a major pathogenic factor (Savoia and Schiffrin 2007; Barra et al. 2009), may have other uses that have not been adequately tested. For example, there is accumulating evidence that ARBs are not only neuroprotective in stroke and diabetes, but also ameliorate age-related cognitive loss, anxiety, and depression (Saavedra et al. 2011). Because of their safety and demonstrated central anti-inflammatory effects, we propose to utilize ARBs as a novel additional component of an integrative treatment of brain disorders such as depression and Alzheimer’s disease, a concept of potential immediate translational value.
What follows is a description of our research findings leading to this proposal, and a brief description of supportive pre-clinical and clinical evidence obtained by other laboratories.
For both our pre-clinical experiments using rodents and for our studies on human circulating monocytes, we used the ARB candesartan at doses and concentrations in the same order of magnitude than those used in clinical settings, supporting the clinical relevance of our findings (Lee et al. 1995; Nishimura et al. 2000a, b; Weinberg et al. 2004; Benicky et al. 2011).
The Brain Angiotensin II System
Angiotensin II was discovered as a circulating pro-hypertensive peptide of renal origin, the active principle of the RAS. Circulating Angiotensin II, through activation of its physiological AT1 receptors, was characterized as a major regulator of vascular tone and fluid metabolism (Skrbic and Igic 2009). Inhibition of peripheral Angiotensin II AT1 receptors may be achieved with the use of ARBs, nonpeptidic, orally active, and well-tolerated compounds (Timmermans et al. 1993). ARBs are currently a mainstream treatment for cardiovascular and metabolic diseases, including essential hypertension and diabetes (McFarlane 2009). The beneficial effects of ARBs are not only limited to reduction of vasoconstriction, but also include a significant decrease in vascular and end-organ inflammation, the result of excessive AT1 receptor activation and a major participant in the pathogenesis of hypertension and diabetes (Savoia and Schiffrin 2007; Marchesi et al. 2008) (Fig. 2).
Angiotensin II and its physiological AT1 receptors. Angiotensin II is the main mediator of the Renin–Angiotensin System, and the activity of this system is determined by the degree of AT1 receptor stimulation. The physiological functions of AT1 receptors include, but are not restricted to, regulation of vascular tone and blood volume, the integrity of the blood brain barrier, cerebrovascular autoregulation, the innate immune response, insulin sensitivity, sensory information, the response to stress and multiple aspects of behavior and cognition. Consequently, AT1 receptor overactivity is involved in hypertension, blood brain barrier breakdown, vulnerability to ischemia as a consequence of loss of cerebrovascular compliance, metabolic alterations including diabetes, neuropathic pain, stress disorders, anxiety, and depression. A result of AT1 receptor overactivity is increased peripheral and brain inflammation. AT1 receptor overactivity may be normalized by ARB administration
Initially, the effects of Angiotensin II in the brain were thought to be restricted to regulation of thirst and sodium appetite (Skrbic and Igic 2009). Later, multiple local RAS systems were described in all tissues studied, including the brain (Bader 2010; Saavedra 1992). Subsequent studies demonstrated that brain Angiotensin II is a pleiotropic neuroregulator, and that brain AT1 receptors are involved in multiple functions including, but not limited to the control of the cerebral circulation, hormonal, and autonomic regulation and in particular the regulation of the response to stress and behavior (Tsutsumi and Saavedra 1991; Saavedra 1992; Saavedra 2005; Saavedra et al. 2011) (Fig. 2).
In the brain and the periphery, Angiotensin II has been proposed to stimulate, in addition to AT1 receptors, a second receptor type, the AT2 receptor. AT2 receptor stimulation by Angiotensin II has been proposed to play a protective role and to balance AT1 receptor stimulation. However, the emerging consensus is that the AT2 receptor expression in the human brain is limited, the receptor can be activated without Angiotensin II participation, published results are controversial, and there is no clear mechanistic model for its signal transduction (Porrello et al. 2009; De Gasparo and Siragy 1999; Allen et al. 1999; Saavedra 2005; Rompe et al. 2010). For these reasons the proposed role of AT2 receptors in the neuroprotective effects of ARBs will not be discussed here.
The Consequences of Enhanced Brain Angiotensin II AT1 Receptor Activity
Evidence accumulated in many laboratories including ours demonstrated an association of enhanced brain Angiotensin II activity with increased allostatic load leading to vulnerability to stress, anxiety, depression, brain ischemia with associated cerebrovascular remodeling and loss of compliance, and inflammation (Castrén and Saavedra 1988; Xang et al. 1993; Edwards et al. 1999; Nishimura et al. 2000a; Saavedra and Benicky 2007; Zhang et al. 2010; Saavedra et al. 2011) (Fig. 2). Some of the initial information was obtained in a rodent model of essential, genetic hypertension, the Spontaneously Hypertensive Rat (SHR). In this model, enhanced Angiotensin II AT1 receptor expression is indicative of increased activation and it is associated with cerebrovascular stiffness and inflammation (Nishimura et al. 2000a).
The Results of AT1 Receptor Blockade
Since it was demonstrated that ARBs reverse peripheral vascular inflammation and excessive vascular remodeling in hypertension, atherosclerosis, and diabetes (Savoia and Schiffrin 2007), we hypothesized that the beneficial effects of ARBs may extend to the brain circulation in hypertensive animals. To test our hypothesis we asked:
Are ARBs able to reverse the cerebrovascular alterations characteristic of genetic hypertension? First, we demonstrated that systemically administered ARBs crossed the blood brain barrier and blocked AT1 receptors in the brain parenchyma (Nishimura et al. 2000b). We subsequently found that systemic administration of the ARB candesartan protected cerebrovascular flow, reversing cerebrovascular remodeling and inflammation in SHR, effects independent of its blood pressure-lowering effects (Ito et al. 2002; Yamakawa et al. 2003; Ando et al. 2004; Zhou et al. 2005). The end result of ARB therapy was to reduce stroke damage (Fig. 3).
ARBs decrease cerebrovascular remodeling and inflammation in genetic hypertension. Genetic hypertension, such as that developing in SHR, is associated with pathological growth and fibrosis of cerebral arteries (remodeling, right figure) limiting compliance to changes in cerebrovascular flow and increasing vulnerability to brain ischemia and stroke. Cerebrovascular inflammation and macrophage infiltration of the brain parenchyma (center figure) aggravates this condition. Experimental stroke in vulnerable SHR produces major loss of brain tissue (left figure). ARB administration reverses cerebrovascular remodeling improving vascular compliance and protecting blood flow to the brain (right figure), ameliorates macrophage infiltration into the brain parenchyma (center figure), reducing inflammation, and reduces stroke damage (left figure) (modified from Nishimura et al. 2000a; Ando et al. 2004)
Parallel experiments in our laboratory revealed that systemic candesartan administration completely prevented the hormonal and sympathoadrenal response to isolation stress in normotensive rats (Armando et al. 2001, 2007) and the stimulation of central sympathetic activity during cold-restraint stress in SHR (Bregonzio et al. 2008). Although anti-stress properties of ARBs were of a major interest, we recognized that elimination of the stress response to psychogenic stimuli may not necessarily be a beneficial effect. To establish whether or not ARBs manifested anti-stress properties of therapeutic benefit, we asked:
May systemic administration of ARBs prevent a stress-induced disorder? To answer this question we used a rodent model of cold-restraint gastric ulceration. We found that systemic candesartan effectively abolished gastric ulceration in stress-vulnerable SHR (Bregonzio et al. 2003). A mechanistic analysis revealed that in addition to prevention of circulatory vasoconstriction by reduction of the stress-induced sympathoadrenal activation, candesartan significantly prevented the inflammatory response to stress in the gastric mucosa (Bregonzio et al. 2003) (Fig. 4). It was of interest that, while ARBs prevented the HPA axis response to the psychogenic stress of isolation, they did not influence the glucocorticoid production and release during cold-restraint stress (Bregonzio et al. 2003). We interpreted this observation, initially surprising, as further evidence of selective, beneficial anti-stress effects of ARBs, since production and release of glucocorticoids, major anti-inflammatory hormones, is essential for the protection of the gastric mucosa during stress (Bregonzio et al. 2003).
ARBs prevent development of stress-induced gastric ulcers. Cold-restraint stress produces massive acute gastric ulcerations (right figure), the consequence of enhanced sympathoadrenal-mediate local vasoconstriction and inflammation (left figure, representing neutrophil infiltration to the gastric mucosa). ARBs prevent gastric ulcer formation, reducing gastric mucosal vasoconstriction, and ameliorating the local inflammatory response (modified from Bregonzio et al. 2003)
Thus, two independent lines of evidence strongly suggested major beneficial anti-inflammatory effects of ARBs, ameliorating inflammation in the cerebral vasculature during hypertension and in the gastric mucosa during stress. For these reasons we hypothesized that ARBs might possess general anti-inflammatory properties, not only in the periphery but also in the brain, and that amelioration of pathological inflammation might be beyond their direct cardiovascular effects.
AT1 Receptor Blockade in Normotensive Rats
To rule out the participation of blood pressure changes on the anti-inflammatory effects of ARBs, we asked: Does ARB administration ameliorate brain inflammation unrelated to alterations in blood pressure? To test our hypothesis, we selected a well-characterized model of inflammation, the activation of the innate immune response in the periphery and the brain by systemic administration of the bacterial endotoxin lipopolysaccharide (LPS) (Dantzer et al. 1998; Rivest 2010). Brain inflammatory processes are necessary for the maintenance of homeostasis. The brain innate immune response, the initial reaction to inflammatory challenges, is an essential mechanism to restore homeostasis in response to stress, infection, or neuronal injury (Dantzer et al. 1998; Rivest 2010). Exaggerated, disturbed inflammatory responses, however, lead to chronic inflammation and neuronal damage (Dantzer et al. 1998; Rivest 2010). It was therefore of interest to establish whether systemic ARB administration controlled, but not eliminated, the innate immune response to LPS.
To avoid confounding cardiovascular effects we administered LPS to normotensive rats at a subseptic dose not influencing blood pressure (Sánchez-Lemus et al. 2008). These concentrations are similar to those found in humans affected by metabolic disorders (Schwartz et al. 2010), supporting the clinical relevance of our findings.
Systemic LPS stimulates peripheral target cells, namely vascular endothelial cells and circulating and tissue macrophages located in multiple organs (Guha and Mackman 2001; Quan and Banks 2007; Rivest 2010). In turn, these cells produce and release to the circulation large amounts of pro-inflammatory cytokines directly affecting the brain (Rivest 2010). Circulating pro-inflammatory cytokines and LPS target cerebrovascular endothelial cells, stimulating inflammatory cascades that promote further inflammation and microglia activation in the brain parenchyma (Quan and Banks 2007). If the inflammatory response is not balanced, it leads to neuronal injury (Guha and Mackman 2001; Quan and Banks 2007; Rivest 2010). In addition, systemic LPS strongly activates the HPA axis, a characteristic inflammatory stress with enhanced production and release of pro-inflammatory aldosterone from the adrenal gland (Sánchez-Lemus et al. 2008). The HPA axis reaction to LPS includes enhanced production and release of anti-inflammatory corticosterone, responsible for the feedback control and regulation of the hormonal and systemic inflammatory responses (Sánchez-Lemus et al. 2008). The LPS-induced peripheral and brain inflammation is associated with an initial behavioral reaction or “sickness syndrome” with fever, diminished locomotion, decreased social interactions, and anorexia leading to body weight loss and depression (Dantzer et al. 1998).
Effects of ARBs on the Peripheral and Brain Innate Immune Response
Consequently, we tested the effects of ARB administration on the LPS-induced innate immune response, and we asked the following questions: Does ARB administration influence circulating biomarkers of inflammation? In normotensive rats, a subseptic LPS dose not affecting systemic blood pressure produced a major increase in inflammatory factors in the general circulation (Benicky et al. 2011). The ARB candesartan decreased or eliminated LPS induction of circulating inflammatory factors without affecting LPS induction of anti-inflammatory factors. This significantly decreased the LPS-induced pro-inflammatory profile in the general circulation (Benicky et al. 2011; Sánchez-Lemus et al. 2008, 2009a, b) (Fig. 5).
ARBs decrease bacterial endotoxin production and release of inflammatory factors affecting the brain. Systemic LPS administration markedly induces circulatory biomarkers of inflammation, including but not limited to IL-6, IL-1β, PGE2, and aldosterone. Anti-inflammatory factors (corticosterone, IL-10) are also increased by LPS. Pretreatment with the ARB candesartan significantly decreases pro-inflammatory factors without affecting levels of anti-inflammatory markers. The net result is a significant decrease in the LPS-induced pro-inflammatory profile in the circulation (modified from Benicky et al. 2011; Sánchez-Lemus et al. 2008)
Does ARB administration influence peripheral inflammatory responses in target organs, indirectly affecting the brain? Circulating markers of inflammation have their origin in target cells for LPS, namely endothelial cells in the vasculature, macrophages in peripheral organs and components of the HPA axis. We studied the pituitary and adrenal glands, major components of the HPA axis, and the macrophage-rich spleen, a major target for systemic LPS (Sánchez-Lemus et al. 2008, 2009a, b). LPS-induced profound inflammatory responses in all tissues studied. In the adrenal gland, candesartan prevented LPS-induced upregulation of pro-inflammatory aldosterone production without affecting that of anti-inflammatory glucocorticoids (Sánchez-Lemus et al. 2008). In the adrenal gland, pituitary gland, and spleen, candesartan significantly reduced the LPS-induced upregulation of all inflammatory pathways studied, including cytokine production, COX-2 and iNOS transcription, ROS formation and NF-κB activation (Sánchez-Lemus et al. 2008, 2009a, b) (Fig. 6). This indicates that the anti-inflammatory effect of ARBs was widespread throughout the organism, and amelioration of peripheral inflammation was not the consequence of hemodynamic effects.
ARBs decrease inflammatory stress in LPS target organs. ARBs significantly reduce LPS induction of gene expression of all inflammatory factors studied in adrenal gland, pituitary gland, and spleen, including but not restricted to IL-6 and COX-2, and PGE2 formation in the spleen (modified from Sánchez-Lemus et al. 2008, 2009a, b)
We had demonstrated that systemic ARB administration reduces inflammation in peripheral tissues and diminishes production of circulating inflammatory factors affecting the brain. The reduction in peripheral inflammation may decrease inflammatory stress to the brain (Dantzer et al. 2008; Rivest 2010). To answer this question, we asked:
Does systemic ARB administration influence brain inflammation? LPS increased AT1 receptor mRNA in the PVN, suggesting a participation of AT1 receptor activation in inflammatory stress (Sánchez-Lemus et al. 2009b). Systemic candesartan administration blocking not only peripheral but also brain AT1 receptors significantly decreased LPS-induced upregulation of inflammatory cytokines, their receptors, adhesion molecules, iNOS and COX-2 production, c-fos and NF-κB induction, and microglia activation (Benicky et al. 2009; Benicky et al. 2011) (Figs. 7, 8). The effects of LPS were widespread, and were documented not only in the PVN and SFO, characteristic brain targets for circulating LPS and pro-inflammatory cytokines, but also in the prefrontal cortex, amygdala and hippocampus (Benicky et al. 2011) (Figs. 7, 8). In all cases, candesartan significantly limited LPS inflammatory effects (Benicky et al. 2011). In parallel with the peripheral anti-inflammatory effects and decreased circulating inflammatory factors, systemic administration of candesartan reduced brain inflammation.
ARBs ameliorate inflammatory stress in brain structures controlling the hormonal and behavioral responses to stress. ARBs decrease LPS-induced inflammatory cascades in the brain parenchyma, including but not restricted to amelioration of LPS-induced TNF-α and IL-1β gene expression. These effects are widespread and include the subfornical organ, the hypothalamic paraventricular nucleus, controlling the HPA axis response, and the prefrontal cortex, central nucleus of the amygdala and hippocampus, involved in the behavioral responses to stress (modified from Benicky et al. 2011)
ARBs reduce neuronal and microglia activation in the PVN. Candesartan reduces the number of c-fos positive neurons and the microglia activation in the PVN, as determined by expression of the specific marker OX-42 and microglial morphology (modified from Benicky et al. 2011)
Since systemically administered candesartan blocks both peripheral and brain AT1 receptors (Nishimura et al. 2000b), and all sartans studied, when administered peripherally, are able to cross the blood–brain barrier and enter the brain (Li et al. 1993; Polidori et al. 1998; Wang et al. 2003) the question remained as to the relative role of peripheral and brain AT1 receptor blockade on the anti-inflammatory effects of ARBs. To address this point, we asked:
Is there a central component to the anti-inflammatory effects of systemically administered ARBs in the brain? We believe this question may be answered in the affirmative, since we have earlier demonstrated that brain AT1 receptors were involved in the effects of systemically administered ARBs; orally administered candesartan administration abrogated the drinking response and the increase in blood pressure produced by intracerebral administration of Angiotensin II (Seltzer et al. 2004). In addition, ARBs administered into the cerebral ventricles in preclinical animal studies prevent the effects of peripherally administered Angiotensin II in the brain (Kang et al. 2009). However, since direct infusion of ARBs into the brain is not used in clinical settings, these experiments do not directly clarify whether or not ARBs, as administered in the clinic, have central effects in addition to peripheral actions.
To definitely answer the question of a participation of brain AT1 receptors in the anti-inflammatory effects of systemically administered ARBs, we asked:
Do ARBs reduce inflammation directly in brain cells? We studied the direct effects of candesartan on LPS inflammation in cultured brain cells. We selected rat primary cerebellar granule cells because brain AT1 receptors have a predominant expression in neurons (Tsutsumi and Saavedra 1991), microglia because of their fundamental role in central inflammatory responses (Hanisch 2002), and cerebral microvascular endothelial cells as targets of LPS, circulating pro-inflammatory factors and Angiotensin II (Benicky et al. 2011). Candesartan reduced LPS-induced production of inflammatory cytokines in all cultures, TNF-α release from cerebellar granule cells, and IL-1β release from microglia (Benicky et al. 2011) (Fig. 9). In addition, candesartan prevented NF-κB activation, IL-1β, IL-6, and TNF-α mRNA expression in cerebellar granule cells, iNOS upregulation in cerebral microvascular endothelial cells, and IL-1β mRNA expression in cultured cortical microglia (Benicky et al. 2011). We concluded that ARBs are capable of reducing inflammation directly on cerebrovascular endothelial cells, neurons, and microglia through multiple mechanisms. For this reason, and because orally administered ARBs prevent the effects of centrally administered Angiotensin II (Seltzer et al. 2004), we hypothesize that part of the anti-inflammatory effects of ARBs may be the consequence of direct actions in the brain (Figs. 10, 11).
Direct anti-inflammatory effects of ARBs in brain cells. ARBs reduce inflammatory stress directly on brain targets for circulating inflammatory factors, the cerebrovascular endothelial cells, and in cells within the brain parenchyma, including microglia and neurons. The effects of ARBs include reduction of LPS-induced inflammatory factors, including but not restricted to TNF-α and IL-1β gene expression and release (modified from Benicky et al. 2011)
Decreased LPS-induced inflammatory factors after treatment with ARBs decreases the production of inflammatory cascades in the brain and microglia activation. ARBs decrease circulating inflammatory factors (right figure), and as a consequence there is a decrease in inflammatory cascades in the brain parenchyma (center figure), followed by a decrease in activation of microglia (left figure). Decreased parenchymal inflammation protects neurons from inflammatory injury (modified from Saavedra et al. 2011)
Circulating inflammatory signals stimulate target cells in the brain increasing inflammatory cascades and leading to microglia activation and neuronal damage. Circulating inflammatory factors include Angiotensin II, LPS, PGE2, and pro-inflammatory cytokines activating interacting receptors in the cerebrovascular endothelial cells: AT1, CD14 and TLR4, EP4 and cytokine receptors (1–4). This leads to activation of transcription factors AP-1 and NF-κB (5), with further production of inflammatory cascades within the endothelial cells and additional stimulation of inflammatory factor receptors and adhesion molecules (6–10). Enhanced release of inflammatory factors including but not limited to PGE2, NO, TNF-α, IL-6, and IL-1β into the brain parenchyma leads to activation of microglia (11) further production of inflammatory cascades, and neuronal injury (12). Inflammatory signals generated in brain parenchyma activate microglia in the brain parenchyma and injure neurons in association with or independently of circulating inflammatory factors.
ARB Effects on Behavior
Severe brain inflammation is accompanied by profound mood and behavioral alterations, including enhanced anxiety and sickness behavior, representing depressive symptomatology (Dantzer et al. 1998; Dantzer et al. 2008). We have previously demonstrated that systemically administered ARBs reduced anxiety (Saavedra et al. 2006), an effect associated with a normalization of stress-induced alterations in the cortical corticotrophin-releasing factor 1 receptors, a pro-anxiety regulatory system (Keck and Holsboer 2001; Bravo et al. 2011), and the anti-anxiety benzodiazepine-1 receptors, part of the inhibitory GABAA complex (Domschke and Zwanzger 2008). Again, a separated line of evidence suggested that ARBs may ameliorate anxiety and depression associated with brain inflammation (Saavedra et al. 2006).
To test our hypothesis, we asked:
Does ARB administration influence inflammation-induced sickness behavior and anxiety? We found that candesartan prevented the LPS-induced sickness behavior, the anorexia leading to weight loss, which are core symptoms of depression, and reduced anxiety (Benicky et al. 2011) (Fig. 12). We conclude that ARBs may exert anti-depressant and anti-anxiety properties.
Beneficial behavioral effects of ARBs. ARBs significantly reduce LPS-induced sickness behavior leading to anorexia and weight loss, and decrease anxiety in control and LPS-treated rats, as determined in the elevated plus-maze (modified from Benicky et al. 2011)
Results obtained in pre-clinical experiments are not always substantiated in the clinic. For this reason it was important to consider whether ARBs ameliorated inflammatory stress in normal human cells. To answer this question, we asked:
Do ARBs directly ameliorate inflammation in human cells? We studied human circulating monocytes obtained from healthy volunteers. We used LPS at a concentration substantially below the levels encountered during sepsis (Larrayoz et al. 2009), and similar to the LPS concentrations found in obesity, insulin resistance, diabetes, and those resulting from a high fat and carbohydrate meal (Schwartz et al. 2010). In human monocytes, candesartan significantly reduced LPS-induced inflammation, including a reduction of inflammatory cytokine production and release to the medium, decreased ROS formation and diminishing NF-κB activation, without affecting secretion of the anti-inflammatory cytokine IL-10 (Larrayoz et al. 2009) (Fig. 13). We conclude that ARBs directly ameliorate inflammatory stress in human circulating monocytes.
Direct anti-inflammatory effects of ARBs on human circulating monocytes. ARBs ameliorate LPS-induced inflammatory responses in human circulating monocytes, including but not restricted to decreased IL-6 gene expression and reduced reactive oxygen species (ROS) formation (modified from Larrayoz et al. 2009)
Additional Supportive Pre-clinical Evidence
Findings from a single laboratory may not be sufficient to consider further investment on potential clinical applications. A consideration of the literature revealed substantial and highly relevant contributions from other laboratories. Early developmental stress produces life-long increases in basal HPA axis and RAS activity (Edwards et al. 1999). Conversely, anti-anxiety effects of ARBs, of potency similar to that of benzodiazepines, and antidepressant effects in mice and rats have been reported by other groups (Kaiser et al. 1992; Gard et al. 2001; Shekhar et al. 2006). Other pre-clinical studies on stroke models confirmed our initial observations of the neuroprotective, anti-inflammatory effects of ARBs (Ozacmak et al. 2007; Hallevi et al. 2007; Jung et al. 2007). These studies also substantiated our hypothesis of beneficial effects of ARBs unrelated to hypertension (Sironi et al. 2004), because they demonstrated that ARBs protect from experimental stroke in normotensive animals (Lou et al. 2004). Other studies revealed that ARBs protect from neuronal injury during retinal inflammation, whole brain irradiation, hypoxia and experimental injury of dopamine neurons (Robbins et al. 2009; Kurihara et al. 2006; Mertens et al. 2010; Grammatopoulos et al. 2007; Nagai et al. 2007; Conner et al. 2010; Saavedra et al. 2011). ARB administration was reported to be neuroprotective in experimental models of autoimmune diseases, such as experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis, where ARBs decrease macrophage infiltration and reduce paralysis (Platten et al. 2009; Lanz et al. 2010). Other research revealed that ARBs decreased the cognitive decline produced by central administration of amyloid-beta (Tsukuda et al. 2009).
Supportive Evidence from Clinical Studies
Clinical reports have been for the most part focused on the role of ARBs on the prevention and treatment of stroke. Multicenter studies demonstrated that ARBs are of superior benefit when compared to other anti-hypertensive therapies for the treatment of stroke and the preservation of cognition (Papademetriou et al. 2004; Zanchetti and Elmfeldt 2006; Julius et al. 2006; Chrysant 2006; Devereux and Dahlöf 2007; Saxby et al. 2008; Lu et al. 2009). Although normalization of blood pressure is the cornerstone for the treatment and prevention of cardiovascular disease, these findings strongly suggest that factors in addition to simple blood pressure normalization account for the superior beneficial effects of ARBs.
Previous uncontrolled studies suggesting that decreased activation of the brain RAS improved the quality of life and exerted beneficial effects on mood (Braszko et al. 2003; Weber 2005) are increasingly confirmed by further clinical observations (Fogari and Zoppi 2004). ARBs have been reported to improve mood and the regulation of the HPA axis in diabetic patients (Pavlatou et al. 2008). Depressed patients medicated with ARBs to treat co-morbid hypertension improve their response to and require lower doses of antidepressants to achieve therapeutic efficacy (Nasr et al. 2011), and a similar finding was earlier reported in patients treated with inhibitors of Angiotensin II formation the Angiotensin Converting Enzyme inhibitors (ACEI) (Braszko et al. 2003; Hertzman et al. 2005).
A recent cohort prospective analysis revealed that ARB administration protects cognition and significantly decreases the progression of Alzheimer’s disease (Li et al. 2010). This indicated that ARBs have not only potent anti-inflammatory but also neuroprotective properties, in agreement with the multicenter clinical studies on stroke (Papademetriou et al. 2004; Zanchetti and Elmfeldt 2006; Julius et al. 2006; Saxby et al. 2008; Lu et al. 2009; Matsumoto et al. 2010). The neuroprotective effects of ARBs are increasingly recognized in the cardiovascular field (Anderson 2010). If the initial data analysis is confirmed by well-controlled studies, the beneficial effects of ARBs on the long-term protection of cognitive function will represent a major innovative finding of immediate translational value.
Cardiovascular, metabolic, neurodegenerative, and mood disorders are substantially influenced by age. This is not surprising because allostatic load is strongly dependent on time. In pre-clinical studies, it is well-recognized that long-term ARB or ACEI administration prolongs life (Linz et al. 2000; Baiardi et al. 2004), a result commonly considered as the consequence of decreased allostatic load to the cardiovascular system. These findings have been recently confirmed by the observation that life-long depletion (Benigni et al. 2009) of AT1 receptors also significantly increases the life span.
We are left with the intriguing question of whether or not long-term ARB administration will extend the life span and improve the quality of life in human populations.
Conclusions
-
1.
Systemic ARB administration ameliorates inflammatory stress in the brain.
-
2.
These effects can be demonstrated in genetically hypertensive rats by the reversal of cerebrovascular inflammation, after stroke, and following systemic administration of LPS to normotensive animals.
-
3.
The anti-inflammatory effects of ARBs can be demonstrated in peripheral organs, the circulation and the brain, and are independent of their hemodynamic effects.
-
4.
In the brain, anti-inflammatory effects of ARBs are widespread, occurring not only in the hypothalamic centers regulating the HPA axis response to stress, but also in regions modulating the behavioral responses to stress, such as the prefrontal cortex and the hippocampus.
-
5.
ARBs reduce the unwanted behavioral consequences of brain inflammation, protecting from anxiety and sickness behavior, core symptoms associated with depression.
-
6.
ARBs reduce stress-induced allostatic load. While these compounds prevent the HPA axis and sympathetic over activation characteristic of emotional stress, they protect from stress-induced disorders such as gastric ulcerations while conserving beneficial aspects of the HPA axis response (secretion of anti-inflammatory glucocorticoids) and decreasing its negative consequences (secretion of pro-inflammatory aldosterone).
-
7.
Associated mechanisms are responsible for the anti-inflammatory effects of ARBs. They include reduction of pro-inflammatory factors in the circulation leading to the production of inflammatory cascades in the brain parenchyma. They also exert direct anti-inflammatory effects on brain cells target for circulating inflammatory factors, such as the cerebrovascular endothelial cells, and in cells located within the brain parenchyma, including microglia and neurons.
-
8.
Amelioration of inflammatory stress in the brain is a factor involved in the neuroprotective effects of ARBs in a number of conditions (stroke, irradiation, hypoxia) leading to neuronal injury.
-
9.
The neuroprotective effects of ARB explain the increasing evidence linking the use of these compounds with the protection of cognition in stroke and Alzheimer’s disease.
-
10.
ARB-induced neuroprotection may be the combination not only of their anti-inflammatory effects but of a number of additional mechanisms, including protection of the cerebrovascular flow, regulation of the HPA axis response to stress and direct neuroprotective effects in the brain parenchyma.
Relevance
Excessive, poorly controlled inflammation is recognized as a major stress factor increasing allostatic load in peripheral organs, contributing to the loss of homeostasis and cardiovascular and metabolic disease. Regulatory mechanisms and disease factors share multiple points of contact between the periphery and the brain, and allostatic load in the periphery and the brain influence each other. This explains the significant co-morbidity between mood and degenerative diseases of the brain, cardiovascular, and metabolic disorders (Johnson and Grippo 2006; Szczepanska-Sadowska et al. 2010). Because of their pleiotropic anti-inflammatory and beneficial metabolic effects and their excellent safety record, ARBs are increasingly utilized as first line of treatment in hypertension, metabolic syndrome, and diabetes. The willingness to consider alternative and additional research avenues has lead to the utilization of novel therapies for the treatment of hypertension and diabetes, resulting in incremental and sustained improvements in the treatment of these conditions. In contrast, there have been no major advances in the therapy of mood and neurodegenerative disorders during the last decades, and several major pharmaceutical companies have recently made the decision to substantially reduce or eliminate their CNS drug discovery operations (Miller 2010). The treatment of major brain disorders remains incomplete and unsatisfactory.
Several conclusions may be drawn from the above. Research in the mechanisms and treatment of brain disorders should be open to the consideration of novel alternatives. Increasing consideration should be made not only of the fundamental differences between brain and peripheral disorders, but also on their points of contact leading to their recognized co-morbidity (Szczepanska-Sadowska et al. 2010). Considering the above, the findings demonstrating that ARBs ameliorate inflammatory stress in the brain, reduce allostatic load and are neuroprotective remain, while novel, not entirely surprising. However, the use of ARBs in mood and neurodegenerative disorders has not been adequately tested. We propose to consider the ARBs as part of an integrative novel approach for the treatment of brain disorders.
References
Allen AM, MacGregor DP, McKinley MJ, Mendelsohn FA (1999) Angiotensin II receptors in the human brain. Regul Pept 79:1–7
Anderson C (2010) More indirect evidence of potential neuroprotective benefits of Angiotensin receptor blockers. J Hypertens 28:429
Ando H, Zhou J, Macova M, Imboden H, Saavedra JM (2004) Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke 35:1726–1731
Anisman H (2009) Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci 34:4–20
Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terrón JA, Falcón-Neri A, Ito T, Juorio AV, Saavedra JM (2001) Peripheral administration of an angiotensin II AT(1) receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation stress. Endocrinology 142:3880–3889
Armando I, Volpi S, Aguilera G, Saavedra JM (2007) Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res 1142:92–99
Bader M (2010) Tissue renin-angiotensin-aldosterone systems: targets for pharmacological therapy. Ann Rev Pharm Tox 50:439–465
Baiardi G, Bregonzio C, Jezova M, Armando I, Saavedra JM (2004) Angiotensin II AT1 receptor blockade prolongs the lifespan of spontaneously hypertensive rats and reduces stress-induced release of catecholamines, glucocorticoids, and vasopressin. Ann N Y Acad Sci 1018:131–136
Barra S, Vitagliano A, Cuomo V, Vitagliano G, Gaeta G (2009) Vascular and metabolic effects of angiotensin II receptor blockers. Exp Opin Pharmacother 10:173–189
Benicky J, Sánchez-Lemus E, Pavel J, Saavedra JM (2009) Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol Neurobiol 29:781–792
Benicky J, Sánchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM (2011) Angiotensin II AT(1) receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 36:857–870
Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G (2009) Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest 119:524–530
Braszko JJ, Karwowska-Polecka W, Halicka D, Gard PR (2003) Captopril and enalapril improve cognition and depressed mood in hypertensive patients. J Basic Clin Physiol Pharmacol 14:323–343
Bravo JA, Dinan TG, Cryan JF (2011) Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int J Neuropsychopharmacol 14:666–683
Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM (2003) Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am J Physiol Gastrointest Liver Physiol 285:G414–G423
Bregonzio C, Seltzer A, Armando I, Pavel J, Saavedra JM (2008) Angiotensin II AT(1) receptor blockade selectively enhances brain AT(2) receptor expression, and abolishes the cold-restraint stress-induced increase in tyrosine hydroxylase mRNA in the locus coeruleus of spontaneously hypertensive rats. Stress 11:457–466 (erratum in Stress 12:95, 2009)
Castrén E, Saavedra JM (1988) Repeated stress increases the density of Angiotensin II binding sites in rat paraventricular nucleus and subfornical organ. Endocrinology 122:370–372
Chrysant SG (2006) Clinical experience with the use of Angiotensin receptor blockers in patients with cardiovascular, cerebrovascular and renal diseases. Curr Clin Pharmacol 1:139–146
Conner KR, Payne VS, Forbes ME, Robbins ME, Riddle DR (2010) Effects of the AT1 receptor antagonist L-158, 809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat Res 173:49–61
Dantzer R, Bluthé RM, Gheusi G, Cremona S, Layé S, Parnet P, Kelley KW (1998) Molecular basis of sickness behavior. Ann N Y Acad Sci 856:132–138
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56
de Gasparo M, Siragy HM (1999) The AT2 receptor: fact, fancy and fantasy. Regul Pept 81:11–24
Devereux RB, Dahlöf B (2007) Potential mechanisms of stroke benefit favoring losartan in the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study. Curr Med Res Opin 23:443–457
Domschke K, Zwanzger P (2008) GABAergic and endocannabinoid dysfunction in anxiety—future therapeutic targets? Curr Pharm Des 14:3508–3517
Editorial (2007) Ensuring drug safety: lessons from the thiazolidinediones. Lancet 370:1101
Edwards E, King JA, Fray JC (1999) Increased basal activity of the HPA axis and renin-angiotensin system in congenital learned helpless rats exposed to stress early in development. Int J Dev Neurosci 17:805–812
Fogari R, Zoppi A (2004) Effect of antihypertensive agents on quality of life in the elderly. Drugs Aging 21:377–393
Gard PR, Haigh SJ, Cambursano PT, Warrington CA (2001) Strain differences in the anxiolytic effects of losartan in the mouse. Pharmacol Biochem Behav 69:35–40
Grammatopoulos TN, Jones SM, Ahmadi FA, Hoover BR, Snell LD, Skoch J, Jhaveri VV, Poczobutt AM, Weyhenmeyer JA, Zawada WM (2007) Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra. Mol Neurodegener 2:1
Guha M, Mackman N (2001) LPS induction of gene expression in human monocytes. Cell Signal 13:85–94
Hallevi H, Hazan-Halevy I, Paran E (2007) Modification of neutrophils adhesion to human endothelial cell line in acute ischemic stroke by dipyridamole and candesartan. Eur J Neurol 14:1002–1007
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40:140–155
Hertzman M, Adler LW, Arling B, Kern M (2005) Lisinopril may augment antidepressant response. J Clin Psychopharmacol 25:618–620
Ito T, Yamakawa H, Bregonzio C, Terrón JA, Falcón-Neri A, Saavedra JM (2002) Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke 33:2297–2303
Johnson AK, Grippo AJ (2006) Sadness and broken hearts: neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol 57(Suppl 11):5–29
Julius S, Nesbitt SD, Egan BM, Weber M, Michelson EL, Kaciroti N, Black HR, Grimm RH, Messerli FH, Oparil S, Schork A, Trial of Preventing Hypertension (TROPHY) Study Investigators (2006) Feasibility of treating prehypertension with an Angiotensin-receptor blocker. N Engl J Med 354:1–13
Jung KH, Chu K, Lee ST, Kim SJ, Song EC, Kim EH, Park DK, Sinn DI, Kim JM, Kim M, Roh JK (2007) Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J Pharmacol Exp Ther 322:1051–1058
Kaiser FC, Palmer GC, Wallace AV, Carr RD, Fraser-Rae L, Hallam C (1992) Antianxiety properties of the angiotensin II antagonist, DUP 753, in the rat using the elevated plus-maze. Neuroreport 3:922–924
Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J (2009) Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res 82:503–512
Keck M, Holsboer F (2001) Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides 22:835–844
Kurihara T, Ozawa Y, Shinoda K, Nagai N, Inoue M, Oike Y, Tsubota K, Ishida S, Okano H (2006) Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Invest Ophthalmol Vis Sci 47:5545–5552
Lanz T, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L (2010) Angiotensin II sustains brain inflammation in mice via TGFb. J Clin Invest 120:2782–2794
Larrayoz IM, Pang T, Benicky J, Pavel J, Sánchez-Lemus E, Saavedra JM (2009) Candesartan reduces the innate immune response to lipopolysaccharide in human monocytes. J Hypertens 27:2365–2376
Lee JW, Naidong W, Johnson T, Dzerk A, Miyabayashi T, Motohashi M (1995) Development and validation of column-switching high-performance liquid chromatographic methods for the determination of a potent AII receptor antagonist, TCV-116, and its metabolites in human serum and urine. J Chromatogr B Biomed Appl 670:287–298
Leonard BE (2007) Inflammation, depression and dementia: are they connected? Neurochem Res 32:1749–1756
Li Z, Bains JS, Ferguson AV (1993) Functional evidence that the angiotensin antagonist losartan crosses the blood-brain barrier in the rat. Brain Res Bull 30:33–39
Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B (2010) Use of Angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ 340:b5465
Linz W, Heitsch H, Schölkens BA, Wiemer G (2000) Long-term angiotensin II type 1 receptor blockade with fonsartan doubles lifespan of hypertensive rats. Hypertension 35:908–913
Lou M, Blume A, Zhao Y, Gohlke P, Deuschl G, Herdegen T, Culman J (2004) Sustained blockade of brain AT1 receptors before and after focal cerebral ischemia alleviates neurologic deficits and reduces neuronal injury, apoptosis, and inflammatory responses in the rat. J Cereb Blood Flow Metab 24:536–547
Lu GC, Cheng JW, Zhu KM, Ma XJ, Shen FM, Su DF (2009) A systematic review of Angiotensin receptor blockers in preventing stroke. Stroke 40:3876–3878
Marchesi VT (2011) Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J 25:5–13
Marchesi C, Paradis P, Schiffrin EL (2008) Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 29:367–374
Matsumoto S, Shimodozono M, Miyata R, Kawahira K (2010) The Angiotensin II type 1 receptor antagonist olmesartan preserves cerebral blood flow and cerebrovascular reserve capacity, and accelerates rehabilitative outcomes in hypertensive patients with a history of stroke. Int J Neurosci 120:372–380
McFarlane SI (2009) Role of angiotensin receptor blockers in diabetes: implications of recent clinical trials. Expert Rev CardiovascTher 7:1363–1371
Mertens B, Vanderheyden P, Michotte Y, Sarre S (2010) The role of the central renin-angiotensin system in Parkinson’s disease. J Renin Angiotensin Aldosterone Syst 11:49–56
Miller G (2010) Is pharma running out of brainy ideas? Science 329:502–504
Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa Y, Yamashiro K, Inoue M, Tsubota K, Umezawa K, Ishida S (2007) Suppression of diabetes-induced retinal inflammation by blocking the Angiotensin II type 1 receptor or its downstream nuclear factor-kB pathway. Invest Ophthalmol Vis Sci 48:4342–4350
Nasr SJ, Crayton JW, Agarwal B, Wendt B, Kora R (2011) Lower frequency of antidepressant use in patients on renin-angiotensin-aldosterone system modifying medications. Cell Mol Neurobiol 31:615–618
Nimmo AJ, Vink R (2009) Recent patents in CNS drug discovery: the management of inflammation in the central nervous system. Recent Pat CNS Drug Discov 4:86–95
Nishimura Y, Ito T, Saavedra JM (2000a) Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke 31:2478–2486
Nishimura Y, Ito T, Hoe K, Saavedra JM (2000b) Chronic peripheral administration of the angiotensin II AT(1) receptor antagonist candesartan blocks brain AT(1) receptors. Brain Res 871:29–38
Ozacmak VH, Sayan H, Cetin A, Akyildiz-Igdem A (2007) AT1 receptor blocker candesartan-induced attenuation of brain injury of rats subjected to chronic cerebral hypoperfusion. Neurochem Res 32:1314–1321
Papademetriou V, Farsang C, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Skoog I, Trenkwalder P, Zanchetti A (2004) Study on Cognition and Prognosis in the Elderly study group. Stroke prevention with the angiotensin II type 1-receptor blocker candesartan in elderly patients with isolated systolic hypertension: the Study on Cognition and Prognosis in the Elderly (SCOPE). J Am Coll Cardiol 44:1175–1180
Pascoe MC, Crewther SG, Carey LM, Crewther DP (2011) Inflammation and depression: why poststroke depression may be the norm and not the exception. Int J Stroke 6:128–135
Pavlatou MG, Mastorakos G, Lekakis I, Liatis S, Vamvakou G, Zoumakis E, Papassotiriou I, Rabavilas AD, Katsilambros N, Chrousos GP (2008) Chronic administration of an angiotensin II receptor antagonist resets the hypothalamic-pituitary-adrenal (HPA) axis and improves the affect of patients with diabetes mellitus type 2: preliminary results. Stress 11:62–72
Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, Phillips LK, Goldstein MJ, Bhat R, Raine CS, Sobel RA, Steinman L (2009) Blocking Angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1-andTH17-mediated autoimmunity. Proc Natl Acad Sci USA 106:14948–14953
Polidori C, Ciccocioppo R, Nisato D, Cazaubon C, Massi M (1998) Evaluation of the ability of irbesartan to cross the blood-brain barrier following acute intragastric treatment. Eur J Pharmacol 352:15–21
Porrello ER, Delbridge LM, Thomas WG (2009) The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front Biosci 14:958–972
Quan N, Banks WA (2007) Brain-immune communication pathways. Brain Behav Immun 21:727–735
Rivest S (2010) Interactions between the immune and neuroendocrine systems. Prog Brain Res 181:43–53
Robbins ME, Payne V, Tommasi E, Diz DI, Hsu FC, Brown WR, Wheeler KT, Olson J, Zhao W (2009) The AT1 receptor antagonist, L-158, 809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int. J Radiat Oncol Biol Phys 73:499–505
Rompe F, Unger T, Steckelings UM (2010) The angiotensin AT2 receptor in inflammation. Drug News Perspect 23:104–111
Saavedra JM (1992) Brain and pituitary Angiotensin. Endocrin Rev 13:329–380
Saavedra JM (2005) Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol 25:485–512
Saavedra JM, Benicky J (2007) Brain and peripheral angiotensin II play a major role in stress. Stress 10:185–193
Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, Sanchez-Lemus E (2006) A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology. 31:1123–1134
Saavedra JM, Sánchez-Lemus E, Benicky J (2011) Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology 36:1–18
Sanchez-Lemus E, Murakami Y, Larrayoz-Roldan IM, Moughamian AJ, Pavel J, Nishioku T, Saavedra JM (2008) Angiotensin II AT1 receptor blockade decreases lipopolysaccharide-induced inflammation in the rat adrenal gland. Endocrinology 149:5177–5188
Sánchez-Lemus E, Benicky J, Pavel J, Larrayoz IM, Zhou J, Baliova M, Nishioku T, Saavedra JM (2009a) Angiotensin II AT1 blockade reduces the lipopolysaccharide-induced innate immune response in rat spleen. Am J Physiol Regul Integr Comp Physiol 296:R1376–R1384
Sánchez-Lemus E, Benicky J, Pavel J, Saavedra JM (2009b) In vivo Angiotensin II AT1 receptor blockade selectively inhibits LPS-induced innate immune response and ACTH release in rat pituitary gland. Brain Behav Immun 23:945–957
Savoia C, Schiffrin EL (2007) Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 112:375–384
Saxby BK, Harrington F, Wesnes KA, McKeith IG, Ford GA (2008) Candesartan and cognitive decline in older patients with hypertension: a substudy of the SCOPE trial. Neurology 70:1858–1866
Schwartz EA, Zhang WY, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD (2010) Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol 30:802–808
Seltzer A, Bregonzio C, Armando I, Baiardi G, Saavedra JM (2004) Oral administration of an AT1 receptor antagonist prevents the central effects of angiotensin II in spontaneously hypertensive rats. Brain Res 1028:9–18
Shekhar A, Johnson PL, Sajdyk TJ, Fitz SD, Keim SR, Kelley PE, Gehlert DR, DiMicco JA (2006) Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J Neurosci 26:9205–9215
Sironi L, Gelosa P, Guerrini U, Banfi C, Crippa V, Brioschi M, Gianazza E, Nobili E, Gianella A, de Gasparo M, Tremoli E (2004) Anti-inflammatory effects of AT1 receptor blockade provide end-organ protection in stroke-prone rats independently from blood pressure fall. J Pharmacol Exp Ther 311:989–995
Skrbic R, Igic R (2009) Seven decades of angiotensin (1939–2009). Peptides 30:1945–1950
Szczepanska-Sadowska E, Cudnoch-Jedrzejewska A, Ufnal M, Zera T (2010) Brain and cardiovascular diseases: common neurogenic background of cardiovascular, metabolic and inflammatory diseases. J Physiol Pharmacol 61:509–521
Tansey MG, Goldberg MS (2010) Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 37:510–518
Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD (1993) Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 45:205–251
Tsukuda K, Mogi M, Iwanami J, Min LJ, Sakata A, Jing F, Iwai M, Horiuchi M (2009) Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension 54:782–787
Tsutsumi K, Saavedra JM (1991) Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol 261:R209–R216
Wang JM, Tan J, Leenen FH (2003) Central nervous system blockade by peripheral administration of AT1 receptor blockers. J Cardiovasc Pharmacol 41:593–599
Weber MA (2005) Angiotensin-II receptor blockers for hypertension and heart failure: quality of life and outcomes. Manag Care Interface 18:47–54
Weinberg AJ, Zappe DH, Ashton M, Weinberg MS (2004) Safety and tolerability of high-dose angiotensin receptor blocker therapy in patients with chronic kidney disease: a pilot study. Am J Nephrol 24:340–345
Xang G, Xi ZX, Wan Y, Wang H, Bi G (1993) Changes in circulating and tissue Angiotensin II during acute and chronic stress. Biol Signals 2:166–172
Yamakawa H, Jezova M, Ando H, Saavedra JM (2003) Normalization of endothelial and inducible nitric oxide synthase expression in brain microvessels of spontaneously hypertensive rats by angiotensin II AT1 receptor inhibition. J Cereb Blood Flow Metab 23:371–380
Zanchetti A, Elmfeldt D (2006) Findings and implications of the Study on Cognition and Prognosis in the Elderly (SCOPE)—a review. Blood Press 15:71–79
Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T (2010) Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience 171:852–858
Zhou J, Ando H, Macova M, Dou J, Saavedra JM (2005) Angiotensin II AT1 receptor blockade abolishes brain microvascular inflammation and heat shock protein responses in hypertensive rats. J Cereb Blood Flow Metab 25:878–886
Acknowledgments
This study was supported by the Division of Intramural Research Programs, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saavedra, J.M. Angiotensin II AT1 Receptor Blockers Ameliorate Inflammatory Stress: A Beneficial Effect for the Treatment of Brain Disorders. Cell Mol Neurobiol 32, 667–681 (2012). https://doi.org/10.1007/s10571-011-9754-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9754-6