Abstract
The authors investigated the protective effects of a novel astrocyte-modulating agent, arundic acid, in a 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine (MPTP) mouse model of Parkinson’s disease. Male mice received four intraperitoneal (i.p.) injections of MPTP (20 mg/kg) at 2 h intervals. The content of dopamine and its metabolites in the striatum was reduced markedly 7 days after MPTP treatment. The delayed treatment with arundic acid (30 mg/kg, i.p.) administered 3, 4, 5 and 6 days after MPTP treatment did not affect the depletion of dopamine and its metabolites in the striatum. Our immunohistochemical study with anti-tyrosine hydroxylase antibody, anti-neuronal nuclei antibody, anti-glial fibrillary acidic protein antibody, anti-S100β antibody and anti-nestin antibody showed that the delayed treatment with arundic acid had a protective effect against MPTP-induced neuronal damage in the striatum and the substantia nigra of mice. Furthermore, this agent ameliorated the severe reductions in number of isolectin reactive microglia in the striatum and the substantia nigra 7 days after MPTP treatment. These results demonstrate that the inhibition of S100β synthesis in astrocytes may be the major component of the beneficial effect of arundic acid. Thus, our present findings provide that the therapeutic strategies targeted to astrocytic modulation with arundic acid offers a great potential for restoring the functional capacity of the surviving dopaminergic neurons in individuals affected with Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive, age-related, neurodegenerative disease characterized by bradykinesia, resting tremor and gait disturbance. PD is produced by progressive degeneration of dopaminergic neurons in the substantia nigra, thereby depleting a neurotransmitter dopamine in the striatum. It has been proposed that parkinsonian clinical signs appear at the point when dopaminergic neuronal cell loss exceeds a critical threshold: 70–80% of striatal nerve terminals and 50–60% of the substantia nigra par compacta perikaryons (Bernheimer et al. 1973; Agid 1991). Treatment with levodopa, supplying the precursor of dopamine, alleviates major symptom of PD. However, long-term treatment with levodopa is often complicated by the development of adverse effects. There have been additional anti-parkinsonian drugs, such as dopamine agonists, but the available therapies do not protect against dopaminergic neurodegeneration. The patients begin not to respond well to treatment, and start to suffer disabilities that cannot be controlled with existing medical therapies. The prevalence of PD is likely to increase in the coming decades as the number of elderly people will increase. Therefore, it is of utmost importance to develop new drugs that show or halt the rate of progression of PD.
MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is a neurotoxin that produces a parkinsonian syndrome in both humans and experimental animals. Its neurotoxic effects also appear to involve energy depletion and free radical generation. MPTP is converted to its metabolite 1-methyl-4-phenyl-pyridinium ion (MPP+) by monoamine oxidase B. MPP+ is selectively accumulated by high affinity dopamine transporters and taken up into the mitochondria of dopaminergic neurons, where it disrupts oxidative phosphorylation by inhibiting complex I of the mitochondrial electron transport chain (Tipton and Singer 1993). This leads to impairment of ATP production, elevated intracellular calcium levels and free radical generation, thereby exhibiting dopaminergic neurotoxicity (Hasegawa et al. 1990: Sriram et al. 1997).
A recent interesting study has shown that a novel astrocyte-modulating agent, arundic acid ((R)-(−)-2-propylocatanoic acid, ONO-2506), has a potent neuroprotective effect in a rat model of cerebral infarction, providing evidence that astrocytes can be a new target of neuroprotection (Tateishi et al. 2002). We have also demonstrated that arundic acid prevented the depletion of dopamine in the striatum and the neuronal loss of dopaminergic neurons in the substantia nigra in a mouse model of PD caused by the administration of MPTP (Kato et al. 2003). Furthermore, we recently reported that arundic acid can ameliorate neurological deficits caused by MPTP treatment (Kato et al. 2004). However, little is known about the exact effect of post-treatment with arundic acid against MPTP neurotoxicity in mice. The purpose of this study was, therefore, to study exactly the neuroprotective effects of delayed treatment with arundic acid against MPTP neurotoxicity in mice.
Materials and Methods
Experimental Animals
Male C57BL/6 mice (Nihon SLC Co., Shizuoka, Japan), 8 weeks of age, were used in this study. The animals were housed in a controlled environment (23 ± 1°C, 50 ± 5% humidity) and were allowed food and tap water ad libitum. The room lights were on between 8:00 and 20:00. The mice were injected intraperitoneally (i.p.) four times with MPTP (20 mg/kg) at 2 h intervals, the total dose per mouse being 80 mg/kg, as described previously (Muramatsu et al. 2003; Kurosaki et al. 2004). Control animals received four injections of physiological saline. All experiments were performed in accordance with the Guidelines for Animal Experiments of the Tokushima University School of Medicine.
Treatment with Arundic Acid
Arundic acid was provided by Ono Pharmaceutical, Osaka, Japan. Arundic acid at a dose of 30 mg/kg was administered i.p. 3, 4, 5 and 6 days after the last MPTP injection. This dose (30 mg/kg) was the most effective dose found in our previous study (Kato et al. 2003). Control animals received saline injection in each experiment.
Western Blot Analysis
The mice were killed by cervical dislocation 7 days after MPTP treatment. The striatal tissues were homogenized in HEPES-buffered sucrose (0.32 M sucrose containing 4 μg/ml pepstatin, 5 μg/ml aprotinin, 20 μg/ml trypsin inhibitor, 4 μg/ml leupeptin, 0.2 mM phenylmethanesulfonyl fluroride, 2 mM EDTA, 2 mM EGTA, and 20 mM HEPES, pH 7.2) using a microtube homogenizer. Protein concentrations were determined using a BCA kit (PIERCE, IL, USA). The homogenates were solubilized in Laemmli’s sample buffer. Ten micrograms of protein from each sample were separated on 5–20% SDS-PAGE gel using constant current. Separated proteins were electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (ATTO, Tokyo, Japan) for 1.5 h with semi-dry blotting system. The PVDF membranes were incubated for 1 h at room temperature with Tris-buffered saline containing 0.1% Tween 20 (TBST) and 5% skim milk, followed by overnight incubation at 4°C with desired antibodies. The anti-tyrosine hydroxylase (TH) antibody (1:1,000, Chemicon International, Inc., Temecula, CA, USA) and anti-glial fibrillary acidic protein (GFAP) antibody (1:3,000, Sigma, Saint Louis, MO, USA) were diluted in TBST containing 3% skim milk. Membranes were washed three times for 10 min at room temperature and incubated with horseradish peroxidase-conjugated secondary antibody in TBST containing 3% Skim milk for 1 h. Immunoreactive bands were visualized by enhanced chemiluminescent autoradiography (ECL Kit, Amersham, IL, USA), according to manufacturer’s instructions. α1 Subunit of Na+/K+-ATPase protein (Upstate Biotechnology, NY, USA) was used as a house keeping protein to confirm that equal amounts of protein were loaded in each line (Nakai et al. 2003; Mori et al. 2005). Each group consisted of 2–3 mice. The densities of imunoreactive bands were analyzed an image analysis software (Dolphin-1D) after capturing the digital image with Dolphin-DOC imaging densitometer (KURABO, Osaka, Japan). The protein revels of TH and GFAP per striatal protein were calculated as a ratio with α1 subunit of Na+/K+-ATP.
Measurement of Dopamine, DOPAC and HVA Levels
The mice were killed by cervical dislocation at 7 days after MPTP treatment. After cervical dislocation, the striata were rapidly dissected out and sonicated in ice-cold 0.2 M perchloric acid containing 100 ng/ml isoproterenol as an internal standard. Dopamine, DOPAC and HVA (homovanillic acid) were quantified by high-performance liquid chromatography (HPLC) with an electrochemical detector (ECD) (Eicom, Kyoto, Japan). Concentrations of dopamine and its metabolites are expressed as μg/g tissue weight, as described previously (Araki et al. 2001; Kurosaki et al. 2003). Each group consisted of 5–7 mice.
Immunohistochemistry
For the immunohistochemical study, the mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) 7 days after MPTP treatment, and the brains were perfusion-fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) following a heparinized saline flush. The brains were removed 1 h after perfusion fixation at 4°C and were immersed in the same fixative until they were embedded in paraffin. Paraffin sections (5 μm) of the striatum and substantia nigra were used for immunohistochemistry. In addition, the mice that received treatment with MPTP and arundic acid (30 mg/kg, i.p.) were also treated in the same way. Each group contained 5–6 animals.
The sections were stained immunohistochemically with anti-tyrosine hydroxylase (TH) antibody (Chemicon International, Inc., Temecula, CA, USA, 1:200), anti-neuronal nuclei (NeuN) antibody (Chemicon International, Inc., Temecula, CA, USA, 1:200), anti-glial fibrillary acidic protein (GFAP) antibody (Chemicon International, Inc., Temecula, CA, USA, 1:200), anti-S100β antibody (Dako, Carpinteria, CA, USA, 1:150) or anti-nestin antibody (Chemicon International, Inc., Temecula, CA, USA, 1:150) using the ABC method (Vectastain elite ABC kit, Vector Laboratories, Burlingame, CA, USA), according to the supplier’s recommendations. In brief, after deparaffinization, the sections were incubated in phosphate buffered saline (PBS) containing 10% methanol and 0.3% H2O2 for 20 min for the blocking of endogenous peroxidase activity. The sections were preincubated with 10% normal serum in PBS for 30 min. They were then incubated with one of the primary antibodies in PBS containing 10% normal serum and 0.3% Triton X-100 overnight at 4°C. The sections were then incubated with biotinylated secondary antibody for 1 h, followed by avidin–biotin–peroxidase complex for 30 min at room temperature. Lastly, the sections were reacted with 0.05% 3,3′-diaminobenzidine and 0.02% H2O2 in Tris–HCl buffer (pH 7.6) for color development. Negative control study was performed using nonimmuned IgG or by omission of the primary antibody, which showed no stable staining. Microglial cells were visualized with peroxidase-labeled isolectin B4 from Griffonia simplicifolia (Sigma), as described previously (Kurosaki et al. 2004). Briefly, the sections were incubated with isolectin (20 μg/ml) in PBS with cations at 4°C overnight. Then, the sections were reacted with 0.05% 3,3′-diaminobenzidine and 0.02% H2O2 in Tris–HCl buffer (pH7.6) for color development. Each group consisted of 5–6 mice.
For immunohistochemical stainings, changes of the densities of these immunostainings or changes in number of immunopositive cells in stained sections were evaluated with a light microscope at a magnification of ×400 without the examiner knowing the experimental protocols, using a computer-associated image analyzer software (WinRoof Version 5, Mitani Corporation, Fukui, Japan), as described recently (Hayakawa et al. 2007). All values were expressed as mean ± SD and statistical significance was evaluated by one-way analysis of variance (ANOVA) followed by Fisher’s PSLD multiple comparison test for parametric analysis or Dunnett’s multiple comparison test for nonparametric analysis.
Results
Striatal TH and GFAP Protein Analysis
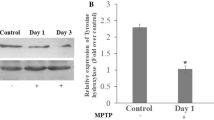
As shown in Fig. 1, four administrations of MPTP at 2 h intervals to mice produced the remarkable loss of TH protein levels 7 days after MPTP treatment. Furthermore, our Western blots with levels of GFAP protein in the striatum showed markedly the increase of GFAP protein levels 7 days after MPTP treatment. In addition, α1 subunit of Na+/K+-ATPase protein was detected as a house keeping protein to confirm that equal amounts of protein were loaded in each line. In the present study, therefore, we elected to use the mice 7 days after MPTP treatment to evaluate the neuroprotective effects of delayed treatment with arundic acid.
(A, B) Western blot analysis using anti-TH antibody and anti-GFAP antibody in the mouse striatum 7 days after MPTP treatment. α1 Subunit of Na+/K+-ATPase protein was detected as a house keeping protein to confirm that equal amounts of protein were loaded in each line. (A) Western bolt, (B) Optical density. n = 2–3
Dopamine and its Metabolites Content Analysis
As shown in Table 1, the striatal dopamine, DOPAC and HVA levels were significantly decreased 7 days after MPTP treatment. Post-treatment of arundic acid did not ameliorate the reductions of dopamine, DOPAC and HVA levels in the striatum 7 days after MPTP treatment. In contrast, arundic acid alone did not affect the striatal dopamine, DOPAC and HVA levels.
Immunohistochemistry
TH Staining
Representative photographs of TH immunostaining in the striatum and the substantia nigra are shown in Fig. 2A. Dopaminergic neurons with the TH antibody were easily detectable in the substantia nigra of vehicle-treated mice. The bodies or fibers of dopaminergic neurons were intensely stained with evident immunopositive processes in the striatum and the substantia nigra. Seven days after MPTP treatment, TH immunopositive fibers and cell bodies were markedly decreased in the striatum and the substantia nigra, respectively. Post-treatment with arundic acid ameliorated the severe reductions in densities of TH immunoreactivity in the striatum 7 days after MPTP treatment. However, arundic acid did not ameliorate the severe reductions in the number of TH immunopositive neurons 7 days after MPTP treatment (Fig. 2B).
(A, C) Representative microphotographs of TH and NeuN immunostainings in the striatum (a–c) and the substantia nigra (d–f) 7 days after MPTP treatment. (a, d) Control mice; (b, e) 7 days after MPTP treatment; (c, f) 7 days after MPTP and arundic acid treatment. Bar = 1 mm (a–c), bar = 50 μm (d–f). n = 5–6. (B, D) Optical density of TH immunoreactivity in the striatum and changes in the numbers of TH immunopositive cells in the substantia nigra (B) and changes in the numbers of NeuN immunopositive cells in the substantia nigra (C) 7 days after MPTP treatment. All values were given as mean ± SD. ‡ P < 0.05, ‡‡ P < 0.01, compared with MPTP group (Fisher’s PLSD multiple comparison test). n = 5–6
NeuN Staining
Representative photographs of NeuN immunostaining in the substantia nigra are shown in Fig. 2C. Neurons with the NeuN antibody were easily detectable in the substantia nigra of vehicle-treated mice. Seven days after MPTP treatment, NeuN immunopositive cells were significantly decreased in the substantia nigra. Post-treatment with arundic acid ameliorated the severe reductions in the number of NeuN immunopositive neurons in the substantia nigra 7 days after MPTP treatment (Fig. 2D).
GFAP Staining
Representative photographs of GFAP immunostaining in the striatum and the substantia nigra are shown in Fig. 3A. Astrocytes were only slightly immunostained for GFAP in the striatum and the substantia nigra of vehicle-treated mice. GFAP immunoreactivity increased significantly 7 days after MPTP treatment, exhibiting the morphology of reactive astrocytes. Post-treatment with arundic acid ameliorated the severe increases in number of GFAP immunoreactive astrocytes in the striatum and the substantia nigra 7 days after MPTP treatment (Fig. 3B).
(A) Representative microphotographs of GFAP immunostainings in the striatum (a–c) and the substantia nigra (d–f) 7 days after MPTP treatment. (a, d) Control mice; (b, e) 7 days after MPTP treatment; (c, f) 7 days after MPTP and arundic acid treatment. Bar = 100 μm (a–c), bar = 100 μm (d–f). n = 5–6. (B) Changes in the numbers of GFAP immunopositive cells in the striatum and substantia nigra 7 days after MPTP treatment. All values were given as mean ± SD. ‡ P < 0.01, compared with MPTP group (Fisher’s PLSD multiple comparison test). n = 5–6
S100β Staining
Representative photographs of S100β immunostaining in the striatum and the substantia nigra are shown in Fig. 4A. S100β immunoreactivity was recognized moderately in astrocytes in vehicle-treated mouse brains. S100β immunoreactivity was increased significantly in the striatum and the substantia nigra 7 days after MPTP treatment. S100β immunoreactivity was relatively stronger in the substantia nigra than in the striatum after MPTP treatment. Post-treatment with arundic acid ameliorated the increases in number of S100β immunoreactive astrocytes in the striatum and the substantia nigra 7 days after MPTP treatment (Fig. 4B).
(A, C) Representative microphotographs of S100β immunostaining and isolectin stainings in the striatum (a–c) and the substantia nigra (d–f) 7 days after MPTP treatment. (a, d) Control mice; (b, e) 7 days after MPTP treatment; (c, f) 7 days after MPTP and arundic acid treatment. Bar = 50 μm (a–c), bar = 50 μm (d–f). n = 5–6. (B, D) Changes in the numbers of S100β immunopositive cells and isolectin positive cells in the striatum and the substantia nigra 7 days after MPTP treatment. All values were given as mean ± SD. ‡ P < 0.05, ‡‡ P < 0.01, compared with MPTP group (Fisher’s PLSD multiple comparison test). n = 5–6
Isolectin Staining
Representative photographs of isolectin B4 staining in the striatum and the substantia nigra are shown in Fig. 4C. Resting microglia were only slightly stained with isolectin in vehicle-treated mice. Activation of microglia, with an increase in staining intensity and morphological changes, was observed in both striatum and substantia nigra 7 days after MPTP treatment. Post-treatment with arundic acid ameliorated the increases in number of isolectin reactive microglia in the striatum and the substantia nigra 7 days after MPTP treatment (Fig. 4D).
Nestin Staining
Representative photographs of nestin immunostaining in the striatum are shown in Fig. 5A. Astrocytes were only slightly stained with nestin in the striatum of vehicle-treated mice. Nestin immunoreactivity increased significantly in the striatum 7 days after MPTP treatment, exhibiting the morphology of reactive astrocytes. However, nestin immunoreactivity was not observed in reactive astrocytes of the substantia nigra after MPTP treatment (data not shown). Post-treatment with arundic acid ameliorated the severe increases in number of nestin immunoreactive astrocytes in the striatum 7 days after MPTP treatment (Fig. 5B).
(A, C) Representative microphotographs of nestin immunostaining and double-labeled immunostainings with TH and NeuN in the striatum (a–c) and the substantia nigra (d–f) 7 days after MPTP treatment. (a, d) Control mice; (b, e) 7 days after MPTP treatment; (c, f) 7 days after MPTP and arundic acid treatment. Bar = 50 μm (a–c), bar = 50 μm (d–f). n = 5–6. (B, D) Changes in the numbers of nestin immunopositive cells in the striatum (B) and TH and NeuN double-labeled immunopositive cells in the substantia nigra (D) 7 days after MPTP treatment. All values were given as mean ± SD. ‡ P < 0.05, compared with MPTP group (Fisher’s PLSD multiple comparison test). n = 5–6
TH and NeuN Double-labeled Immunostainings
Representative photographs of TH and NeuN double-labeled immunostaining in the substantia nigra are shown in Fig. 5C. In vehicle-treated animals, both TH and NeuN double-labeled immunoreactivity was observed markedly in the dopaminergic neurons of the substantia nigra. Seven days after MPTP treatment, the TH and NeuN double-labeled immunoreactivity was decreased significantly in the dopaminergic neurons of the substantia nigra. Post-treatment with arundic acid showed a tendency to ameliorate the severe decreases in number of the TH and NeuN double-labeled immunoreactive cells in the substantia nigra 7 days after MPTP treatment (Fig. 5D).
Discussion
Several studies have demonstrated the pharmacological effects of arundic acid on astrocytes (Tateishi et al. 2002). This compound acts selectively on astrocytes and modulates their activation or prevents to much activation that may be harmful to neighboring neurons. Arundic acid does not act on neuronal death in cultures, but also suppresses the changes induced in cultured astrocytes, such as an increase in S100β contents, a secretion of nerve growth factor, a reduction of glutamate transporter (GLT-1 and GLAST) expression and a disappearance of γ-aminobutyric acidA (GABAA) receptors, in a dose-dependent manner without GFAP expression (Katsumata et al. 1999; Shinagawa et al. 1998, 1999). Furthermore, a recent interesting study has demonstrated that arundic acid is neuroprotective in a rat model of focal cerebral ischemia and reduces the volume of cerebral infarction (Tateishi et al. 2002). Moreover, several studies have also reported that enhanced synthesis of astrocytic protein S100β participates in delayed infarct expansion following focal cerebral ischemia in rats (Matsui et al. 2002) and in the dopaminergic neuronal damage of mice after MPTP treatment (Muramatsu et al. 2003). From these observations, it is suggested that the neuroprotective effect of arundic acid in cerebral ischemia is thought to be associated with the inhibition of astrocytic S100β synthesis. However, little is known about the neuroprotective effects of post-treatment with arundic acid against MPTP neurotoxicity in mice.
In the present study, four administrations of MPTP to mice caused a severe reduction in the dopamine, DOPAC and HVA content of striatum after 7 days. In contrast, post-treatment with arundic acid did not affect the reductions of dopamine, DOPAC and HVA levels in the striatum 7 days after MPTP treatment. The present results demonstrate that delayed treatment with arundic acid could not affect a significant reduction of dopamine, DOPAC and HVA levels in the striatum 7 days after MPTP treatment.
Our immunohistochemical study showed that delayed treatment with arundic acid can ameliorate the severe reductions in densities of TH immunoreactivity in the striatum 7 days after MPTP treatment. In contrast, this compound did not ameliorate the severe reductions in the number of TH immunopositive neurons of the substantia nigra 7 days after MPTP treatment. Furthermore, the present study indicated that delayed treatment with arundic acid can ameliorate partly the severe reductions in the number of NeuN immunoreactive neurons of the substantia nigra 7 days after MPTP treatment. Moreover, our study with TH and NeuN double-labeled immunostainings demonstrated that post-treatment with arundic acid showed a tendency to protect the severe decreases in the number of TH and NeuN double-labeled immunopositive neurons of the substantia nigra 7 days after MPTP treatment. A recent interesting study demonstrated that nigrostriatal dopaminergic neurons that survive the MPTP lesioning increase TH protein and may repopulate the striatum with axonal growth and branching, indicating neuroplasticity (Jakowec et al. 2004). Thus, our findings suggest that therapeutic strategies including drug therapy may be targeted toward restoring the functional capacity of the surviving dopaminergic neurons in individuals affected with PD. In the present study, however, we did not study exactly the effect of arundic acid on locomotion defect in MPTP-treated mice. Therefore, further studies should be performed to investigate the precise mechanisms responsible for our present findings.
For the changes of astrocytes, the present study showed that the delayed treatment with arundic acid can ameliorate the increases in number of GFAP immunoreactive astrocytes in the striatum and the substantia nigra 7 days after MPTP treatment. The present study also showed that the delayed treatment with arundic acid can ameliorate the increases in the number of S100β immunoreactive astrocytes in the striatum and the substantia nigra 7 days after MPTP treatment. Previous studies have suggested that enhanced synthesis of astrocytic protein S100β participates in delayed infarct expansion following focal cerebral ischemia in rats (Matsui et al. 2002) and in dopaminergic cell damage in MPTP-treated mice (Muramatsu et al. 2003). The neuroprotective action of arundic acid in cerebral ischemia was associated with the inhibition of astrocytic S100β synthesis (Tateishi et al. 2002). The present study demonstrated that neuroprotection of a progressive damage of dopaminergic neurons after MPTP treatment was accompanied by a decline of S100β immunoreactivity in astrocytes. Therefore, we suggest that the overexpression of S100β synthesis in astrocytes may be the major component of the neuroprotective actions of arundic acid.
For the changes of microglia, the present study showed that the delayed treatment with arundic acid can ameliorate the increases in number of isolectin immunoreactive microglia in the striatum and the substantia nigra 7 days after MPTP treatment. A recent study has shown that blockade of microglial activation with minocycline protects against MPTP neurotoxicity in mice (Wu et al. 2002). It is known that arundic acid also inhibits the expression of cyclooxygenese-2 (Cox-2) or iNOS mRNA induced by lipopolysaccharide in cultured glial cells (Shimoda et al. 1998; Shinagawa et al. 1999). Therefore, we speculate the possibility that arundic acid acts on microglia and its protective effects may be partly mediated through the prevention of microglial activation. However, details of mechanisms of action of arundic acid against microglial activation need to elucidated.
In the present study, interestingly, nestin immunoreactivity increased significantly in the striatum 7 days after MPTP treatment. Post-treatment with arundic acid ameliorated the severe increases in number of nestin immunoreactive astrocytes in the striatum 7 days after MPTP treatment. A recent study reported that nestin, an embryonic intermediate filament, was up-regulated in reactive astrocytes of the striatum in MPTP-treated mice (Chen et al. 2004, 2006). Expression of nestin reflects the differentiating state of neural progenitor cells in the developing central nervous system (Dahlstrand et al. 1995). Nestin and GFAP expression among astrocytes signifies activated and proliferative cells with the population. These reactive astrocytes in the striatum of the MPTP-treated mice model of PD may exert protective roles on dopamine neurons in the striatum through neurotrophic functions (Ridet et al. 1997; Kernie et al. 2001). However, the molecular mechanisms of nestin up-regulation in the striatum after MPTP treatment is not fully understood. In the present study, GFAP immunoreactivity increased significantly 7 days after MPTP treatment, exhibiting the morphology of reactive astrocytes. The delayed treatment with arundic acid ameliorated the increases in number of GFAP immunoreactive astrocytes in the striatum and the substantia nigra 7 days after MPTP treatment. Based on these observations, we speculate that the action of arundic acid is the suppression of astrocytic activation, resulting in the inhibition of expression of nestin in the striatum after MPTP treatment.
In conclusion, we demonstrate that the delayed treatment with arundic acid provides acceleration in the neuroplasticity of survival nigrostriatal neurons in mice after MPTP treatment. Furthermore, we suggest that the inhibition of S100β synthesis in astrocytes may be the major component of the beneficial effect of arundic acid. These results demonstrate that the therapeutic strategies targeted to astrocytic modulation with arundic acid offers a great potential for restoring the functional capacity of the surviving dopaminergic neurons in individuals affected with PD.
References
Agid Y (1991) Parkinson’s disease: pathophysiology. Lancet 337:1321–1324
Araki T, Mikami T, Tanji H, Matsubara M, Imai Y, Mizugaki M, Itoyama Y (2001) Biochemical and immunohistological changes in the brain of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mouse. Eur J Pharm Sci 12:231–238
Bernheimer H, Birkmayer W, Hornykeiwicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Chen LW, Hu HJ, Liu HJ, Yung KKL, Chan YS (2004) Identification of BDNF in nestin-expressing astroglial cells in the neostriatum of MPTP-treated mice. Neuroscience 126:941–953
Chen LW, Zhang JP, Shum DKY, Chan YS (2006) Localization of nerve growth factor, neurotropin-3, and glial cell line-derived neurotrophic factor in nestin-expressing reactive astrocytes in the caudate-putamen of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated C57/Bl mice. J Comp Neurol 497:898–909
Dahlstrand J, Lardelli M, Lendahl U (1995) Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res 84:109–129
Hasegawa E, Takeshige K, Oishi T, Murai Y, Minakami S (1990) 1-Methylphenylpyridinium (MPP+) induces NADH-dependent superoxide formation and enhances NADH-dependent lipid peroxidation in bovine heart submitochondrial particles. Biochem Biophys Res Commun 170:1049–1055
Hayakawa N, Kato H, Araki T (2007) Age-related of astrocytes, oligodendrocytes and microglia in the mouse hippocampal CA1 sector. Mech Ageing Dev 128:673–677
Jakowec MW, Nixon K, Hogg E, McNeill T, Petzinger GM (2004) Tyrosine hydroxylase and dopamine transporter expression following 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of the mouse nigrostriatal pathway. J Neurosci Res 76:539–550
Kato H, Araki T, Imai Y, Takahashi Y, Itoyama Y (2003) Protection of dopaminergic neurons with a novel astrocyte modulating agent (R)-(-)-2-propyloctanoic acid (ONO-2506) in an MPTP-mouse model of Parkinson’s disease. J Neurol Sci 208:9–15
Kato H, Kurosaki R, Oki C, Araki T (2004) Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice. Brain Res 1030:66–73
Katsumata S, Tateishi N, Kagamiishi Y, Shintaku K, Hayakawa T, Shimoda T, Shinagawa R, Akiyama T, Katsube N (1999) Inhibitory effect of ONO-2506 on GABAA receptor disappearance in cultured astrocytes and ischemic brain. Abstr Soc Neurosci 25:2108
Kernie SG, Erwin TM, Parada LF (2001) Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res 66:317–326
Kurosaki R, Akasak M, Michimata M, Matsubara M, Imai Y, Araki T (2003) Effects of Ca2+ antagonists on motor activity and the dopaminergic system in aged mice. Neurobiol Aging 24:315–319
Kurosaki R, Muramatsu Y, Kato H, Araki T (2004) Biochemical, behavioral and immunohistochemical alterations in MPTP-treated mouse model of Parkinson’s disease. Pharmacol Biochem Behav 78:143–153
Matsui T, Mori T, Tateishi N, Kagamiishi Y, Satoh S, Katsube N, Morikawa E, Morimoto T, Ikuta F, Asano T (2002) Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part I: Enhanced astrocytic synthesis of S-100β in the periinfarct area precedes delayed infarct expansion. J Cereb Blood Flow Metab 22:711–722
Mori A, Ohashi S, Nakai M, Moriizumi T, Mitsumoto Y (2005) Neuronal mechanisms underlying motor dysfunction as detected by the tail suspension test in MPTP-treated C57BL/6 mice. Neurosci Res 51:267–274
Muramatsu Y, Kurosaki R, Watanabe H, Michimata M, Matsubara M, Imai Y, Araki T (2003) The expression of S100 protein is related to neuronal damage in MPTP-treated mice. Glia 42:307–313
Nakai M, Mori A, Watanabe A, Mitsumoto Y (2003) 1-Methyl-4-phenylpyridinium (MPP+) decreases mitochondrial oxidation-reduction (REDOX) activity and membrane potential (Deltapsi(m)) in rat striatum. Exp Neurol 179:103–110
Ridet JL, Malhotra SK, Privat A, Gage FH (1997) Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 20:507–577
Shimoda T, Tateishi N, Shintaku K, Yada N, Katagi J, Akiyama T, Maekawa H, Shinagawa R, Kondo K (1998) ONO-2506, a novel astrocyte modulating agent, suppresses the increase of COX-2 and iNOS mRNA expression in cultured astrocytes and ischemic brain. Abstr Soc Neurosci 24:984
Shinagawa R, Tateishi N, Shimoda T, Maekawa H, Yada N, Akiyama T, Matsuda S, Katsube N (1999) Modulating effects of ONO-2506 on astrocytic activation in cultured astrocytes from rat cerebrum. Abstr Soc Neurosci 25:2108
Shinagawa R, Tateishi N, Shimoda T, Shintaku K, Yada N, Honjyo K, Kagamiishi Y, Kondo K (1998) ONO-2506 ameliorates neurodegenation through inhibition of reduction of GLT-1 expression. Abstr Soc Neurosci 24:984
Sriram K, Pai KS, Boyd MR, Ravindranath V (1997) Evidence for generation of oxidative stress in brain by MPTP: in vitro and in vivo studies in mice. Brain Res 749:44–52
Tateishi N, Mori Y, Kagamiishi Y, Satoh S, Katsube N, Morikawa E, Morimoto T, Matsui T, Asano T (2002) Astrocytic activation and delayed infarct expansion following permanent focal ischemia in rats. Part II. Suppression of astrocytic activation by a novel agent (R)-(-)-2-propyloctanoic acid (ONO-2506) leads to mitigation of delayed infarct expansion and early improvement of neuronal deficits. J Cereb Blood Flow Metab 22:723–734
Tipton KF, Singer TP (1993) Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem 61:1191–1206
Wu DC, Jakson-Lewis M, Vila M, Tieu K, Teismann C, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S (2002) Blocakade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. J Neurosci 22:1763–1771
Acknowledgements
This study was supported in part by the Grant-in-Aid for Scientific Research (12877163, 13671095 and 13670627) from the Ministry of Science and Education in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oki, C., Watanabe, Y., Yokoyama, H. et al. Delayed Treatment with Arundic Acid Reduces the MPTP-induced Neurotoxicity in Mice. Cell Mol Neurobiol 28, 417–430 (2008). https://doi.org/10.1007/s10571-007-9241-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9241-2