Abstract
The aim of the work was to compare the levels of carboxymethylation on cotton with what was obtained previously on viscose fibers under the same treatment conditions. In addition to carboxymethylation levels, their performance was also compared in terms of their propensity for sorption of methylene blue, a cationic dye that is a putative wastewater contaminant. Under comparable conditions of treatment, the degree of carboxymethylation in cotton was 30–90% of that in viscose but the molar ratio of dye sorption to carboxyl groups was 100–230% of that in viscose. In other words, as compared to cotton, viscose fibers showed higher degrees of carboxymethylation but lower accessibility of the carboxyl groups to dye molecules. The divergence between the fiber types is attributed to differences in the spatial distribution of substituted carboxymethyl groups within their structures, as well as the inherently greater swelling propensity of the viscose as compared to cotton. Further work is under way to determine if the order of performance as measured through dye sorption (cotton better than viscose) will also hold true if the performance metric is changed to water and moisture sorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The supplanting of synthetic polymers derived from petroleum-based resources with those derived from biobased, renewable resources is of significant interest. Among biobased polymers, cellulose is a prime candidate to act as substitute for synthetic polymers due to its natural abundance and the fact that it is largely unusable for food or feed. To improve the suitability of cellulose as replacement, it may be necessary to enhance or suppress inherent properties of the polymer. That may be achieved by modifying the chemical structure of the polymer, primarily through reactions at the three hydroxyl groups per D-glucose residue.
The subject of this paper is carboxymethylation of cellulose, which is a Williamson-type etherification of a cellulosic hydroxyl group through nucleophilic substitution with an alkyl halide (monochloroacetic acid) in presence of alkali (Mischnick and Momcilovic 2010). That enhances water/moisture absorption and complexation/binding capabilities and thus the modified polymer (carboxymethyl cellulose, CMC) is of interest as substitute for acrylate-based polymers in water/moisture absorbent products (Miyamoto et al. 2018; Chen et al. 2022) or as contaminant binding agents in wastewater remediation (Wang et al. 2019, 2023).
Different avenues are investigated for the employment of CMC in the above-listed applications, viz. to deposit and fix CMC on a carrier (Ibrahim et al. 2020), to spin fibers from CMC dope and assemble them into fibrous networks (Wang et al. 2023), or first to assemble neat cellulose fibers into networks and then to subject them to chemical modification treatments (Rácz et al. 1995). The latter avenue is of interest for the textiles and nonwovens sectors, as technological capabilities exist for the chemical processing of fibrous networks on a commercial-scale. Here, the treatments are undertaken under heterogeneous conditions, i.e., on solid fibrous substrates in liquid media.
Both native and regenerated cellulosics may be processed in the textiles and nonwovens industries, and although composed of the same polymer, they differ in their supramolecular structure and morphology. The morphology of native cellulosics, e.g., cotton, is characterized by a ‘lamellar’ structure in the fiber bulk, that is comprised of winding layers of cellulose fibrils surrounding a hollow core, termed the lumen (Wakelyn et al. 2007; Hearle 2007). Viscose fibers, which account for about 79% of the annual production of regenerated cellulosic fibers (Textile Exchange 2023), are characterized by a ‘shell/core’ morphology, with a relatively dense shell surrounding a sponge-like core (Abu-Rous et al. 2006). The supramolecular structure of cellulosics is characterized by the presence of pores or voids, and in heterogeneous reactions, the accessibility of reagents to polymer chains is governed by the porosity (i.e., number of pores and their sizes). Typically, regenerated cellulosics exhibit a higher total pore volume as compared to cotton, but the mean pore sizes are approximately in the same range (Bredereck and Hermanutz 2005). There are also differences in the distribution of pores through fibers. In cotton, the inter-lamellar spaces (which are distributed throughout the fiber bulk) and the central lumen constitute the pores. In viscose fibers, a majority of pores are found in the core region whereas the shell region contains only few numbers of small-sized pores. These differences in pore spatial distributions are clearly evident in transmission electron micrographs of fiber cross-sections (Abu-Rous et al. 2006).
The focus of our investigations is to gain understanding of what influence may be exerted by the supramolecular structure and morphological features of cellulosic fibers (e.g., spatial distribution of pores) on the progress of chemical modifications such as carboxymethylation under heterogeneous conditions, and what impact that may have on performance of the resulting product.
The carboxymethylation of viscose fibers with sodium monochloroacetate (MCA) from aqueous alkaline solutions was examined in previous work (Bogner et al. 2024). The highest yields in cellulose carboxymethylation are obtained from alcohol-rich reaction media (Gu et al. 2012; Heinze et al. 2018; Li et al. 2022; Liao et al. 2022; Liu et al. 2023; Thakur et al. 2023). However, processing operations in textiles and nonwovens sectors involve treatments in open-to-air reaction vessels, which do not favor the use of alcohols in large quantities. Thus, the investigations were limited only to aqueous reaction media, where the primary treatment variables were reaction temperature (30 °C, 50 °C) and alkali content (0.5 mol/L and 4.0 mol/L of NaOH). The ‘performance’ of the fibers after carboxymethylation was measured through their propensities for sorption of a cationic dye, methylene blue, which is often used in wastewater remediation studies as a representative contaminant (Rafatullah et al. 2010; Wang et al. 2023). Greater levels of carboxymethylation were observed with 4.0 mol/L NaOH as compared to 0.5 mol/L NaOH, and a higher temperature favored carboxymethylation in reaction media with 0.5 mol/L NaOH but disfavored carboxymethylation in reaction media with 4.0 mol/L NaOH. Despite the higher carboxymethylation, the levels of methylene blue sorption were lower in fibers from reaction media containing 4.0 mol/L NaOH as compared to 0.5 mol/L NaOH. That was attributed to lower accessibility of dye cations to the fiber carboxyl groups, due to differences in the spatial distribution of the carboxymethyl groups. The aim in the present investigation was to repeat the same treatments on cotton fibers and compare the degrees of carboxymethylation and methylene blue dye sorption propensities with what was previously obtained on viscose.
Materials
Raw cotton fibers (American Pima, average staple length of 3.4 cm) were kindly donated by Gebr. Otto Baumwollfeinzwirnerei GmbH + Co. KG (Dietenheim, Germany). For scouring and bleaching, technical grade NaOH (50% w/w) was used along with wetting agents and stabilizers from BASF, Germany (Kieralon B, Prestogen PL, Leophen M). The methylene blue was of pharmaceutical grade, and all other reagents were of analytical grade. Ultrapure water (LiChrosolv® grade) purchased from Merck KGaA (Germany) was used in conductometric titration, and unless otherwise mentioned, deionized water of conductivity less than 10 µS/cm was employed in all other experiments.
Methods
Fiber preparation
The raw cotton was scoured, bleached, and demineralized before use in experiments. The scouring and bleaching procedures were based on descriptions in literature (BASF AG 1976). For scouring, the fibers were boiled in a solution of 10 g/L NaOH with 0.5 g/L Kieralon B wetting agent at a material-to-liquor ratio of 1:10 for 4 h followed by a rinse under running hot water at 60–70 °C for 10 min. Then the fibers were drained, immersed in a solution of 0.5% w/w HCl for 15 min to neutralize any residual alkali, rinsed with soft water to wash off residual acidity and allowed to dry in ambient atmosphere.
The scoured fibers were bleached in a solution of 6 mL/L of 35% H2O2, 2 g/L NaOH, 1.5 g/L Prestogen PL as stabilizer and 1 g/L Leophen M as wetting agent with a material-to-liquor ratio of 1:30 at the boil for 3 h. The fibers were then drained, rinsed under running hot water at 60–70 °C for 10 min, followed by three rinses in deionized water, and allowed to dry in ambient atmosphere.
The fibers after bleaching were demineralized by immersion in 0.5% w/w HCl with a material-to-liquor ratio of 1:35 at 40 °C for 1 h. Then the fibers were drained, rinsed twice with deionized water, and immersed in a solution of 1 g/L sodium acetate with material to liquor ratio of 1:35 at 40 °C for 1 h, drained, rinsed thrice in deionized water, and dried in ambient atmosphere.
Carboxymethylation
All carboxymethylation treatments were performed on 2 g fiber in 120 mL reaction media composed of NaOH and sodium chloroacetate (MCA) in deionized water, under constant stirring. At the end of treatment, the fibers were separated from the media, neutralized by immersion for 1 h in 1 mol/L acetic acid, drained, rinsed with deionized water for removal of residual salts, drained of excess water, and dried in ambient atmosphere.
The compositions of treatment solutions, reaction temperatures and durations of treatment are listed in Table 1. The primary variables of interest were the NaOH concentration in reaction media (0.5 mol/L and 4.0 mol/L) and the reaction temperature (30 °C and 50 °C). It was also of interest to obtain a broad range in degrees of carboxymethylation, and that was achieved by varying the MCA content or duration of treatment.
For reactions in 0.5 mol/L NaOH, the MCA content was maintained always at 0.644 mol/L but the treatment durations were of 24 h, 72 h and 168 h. These conditions were derived from results of preliminary experiments described in the following section. For reactions in 4.0 mol/L NaOH, a near total hydrolysis of MCA was observed within 24 h at 30 °C and within 4 h at 50 °C in previous work (Bogner et al. 2024). Hence, in this work, the duration of treatment was set at 24 h and 4 h for all reactions at 30 °C and 50 °C respectively, but the content of MCA in reaction media was varied from 0.081 mol/L to 0.644 mol/L, beyond which the reagent is difficult to dissolve in aqueous alkaline media.
Analyses of reaction media
The reaction media after fiber-treatment were analyzed for residual contents of NaOH with acid–base titration, and high–performance liquid chromatography (HPLC) was employed to assess unreacted MCA, glycolic acid (GA) and diglycolic acid (dGA), as described previously (Bogner et al. 2024). “No-fiber” blank measurements were also performed, i.e., the contents of alkali, MCA, GA and diglycolic acid dGA were measured in reaction media subjected to different times/temperatures, but without any fiber.
Fiber analyses
Detailed descriptions of the procedures for all analyses were reported previously (Bogner et al. 2024), and are only briefly summarized below.
The degree of carboxymethylation on fibers was quantified through determination of the carboxyl group content with the back titration method of TAPPI standard T237 om-08 (TAPPI 2008). To validate these results, the carboxyl content was determined also with conductometric titration on a smaller set of fibers.
Fourier Transform Infrared Spectroscopy (FTIR) was used to determine the proportion (or “density”) of carboxyl groups in samples, from the ratio of absorbance intensity in the region of 1600 cm−1 attributed to absorbance of deprotonated carboxyl groups (Mohkami and Talaeipour 2011; Li et al. 2020), to the absorbance intensity in the region of 1156 cm−1 attributed to absorbance of the C–O–C glycosidic linkages due to asymmetric stretching (Marchessault and Liang 1960; Carrillo et al. 2004). These measurements were performed both in Attenuated Total Reflectance (ATR) mode on intact fibers and in transmission mode after milling fibers and pelletizing them with spectroscopic grade KBr. All fibers were subjected to a deprotonation step prior to the measurements.
The levels of methylene blue dye sorption by fibers from solutions buffered to pH 8.5 were determined photometrically. The reflectance spectra of fibers after methylene blue sorption were measured for assessment of absorbance intensities (or color depth) with the Kubelka–Munk function (Eqs. 1 and 2), of shifts in their wavelengths of peak absorbance (Eq. 3), as well as of changes in their color coordinates as per the CIE 1976 system.
where,
- K/Sλ:
-
K/S at wavelength λ.
- Rλ:
-
fractional reflectance at wavelength λ.
- K/Sav:
-
average K/S.
- nλ:
-
number of measurements in the 400–740 nm range (= 35).
λscspectral centroid, measure of shift in absorbance peak (Stone 1967).
Results and discussion
Preliminary experiments
Preliminary experiments were performed to decide on the treatment durations with 0.5 mol/L NaOH. Reaction media were prepared with all components except fibers and maintained at 30 °C and 50 °C for a range of durations, and the residual contents of reactants and reaction products (NaOH, MCA, GA and dGA) were periodically analyzed with HPLC. The results are available in Table S1. At 30 °C, the degree of MCA hydrolysis was about 12.9% after 24 h and 47.0% after 168 h (see Fig. S1). In reactions at 50 °C, the degree of MCA hydrolysis appeared to plateau at about 74.1% after 120 h, which was attributed to insufficient residual NaOH (measured to be 0.009 mol/L). To further increase hydrolysis levels, and thereby to increase levels of fiber carboxymethylation, more alkali was added after 25 h (5 mL from a solution of 9.5 mol/L NaOH). With that, a degree of MCA hydrolysis of about 95.7% was obtained after 72 h.
Analyses of reaction media
The attempt was to obtain a measure of fiber carboxymethylation levels from analyses of residual concentrations of NaOH and unreacted MCA in reaction media, to support the results from analyses of fibers. No significant differences in residual levels of reagents were found between blank experiments (i.e., with no fiber in media) and fiber treatments (see Table S2). It indicates the presence of fiber had no measurable impact on consumption levels of NaOH or MCA, and is attributed to the low degrees of fiber carboxymethylation attained under the reaction conditions employed.
Fiber analyses results
Results of the fiber analyses are shown in Table 2. The carboxyl contents, as measured with back titration, was on average 18.7 mmol/kg in the cleaned fibers, which after treatment with 4.0 mol/L NaOH alone (alkali treated reference), increased by about 2%. In carboxymethylated fibers, the carboxyl contents ranged on average between 54.8–436.8 mmol/kg, which corresponds to average degrees of substitution of 0.009–0.073. The conductometric measurements of carboxyl group content, performed on selected samples to validate the back titration results, yielded very similar values across the range of fiber carboxymethylation levels (see Fig. S2).
In treatments with media containing 0.5 mol/L NaOH, the carboxyl contents increased with longer treatment durations and higher temperatures. The alkali spike in reactions at 50 °C also improved the carboxyl content. In treatments with media containing 4.0 mol/L NaOH, the carboxyl contents changed in proportion to MCA content, but decreased with rise in temperature.
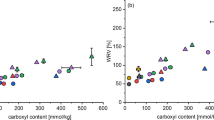
The methylene blue sorption measurements were performed to assess fiber “performance” after carboxymethylation, and the results are shown in Fig. 1. The mechanism of interaction is that of ion-exchange between the dye cation and fiber carboxylate anion at a 1:1 stoichiometric ratio (Stone and Bradley 1967). The observed molar ratios of dye sorbed to fiber carboxyl groups on average was 0.27 for cleaned fibers and fibers treated only in 4 mol/L NaOH, 0.38–0.46 for fibers carboxymethylated in 0.5 mol/L NaOH, and 0.22–0.34 for fibers carboxymethylated in 4 mol/L NaOH. The observed stoichiometries may be regarded as indications of dye cation accessibility to fiber carboxylate anions. Thus, there was greater accessibility of dye cations to carboxylate anions in fibers reacted in 0.5 mol/L NaOH as compared to 4 mol/L NaOH.
Methylene blue dye sorption levels as function of carboxyl content determined by back titration. The diagonal solid line represents a 1:1 ratio between the abscissa and ordinate. The marker colors represent treatment sets: cleaned fibers (
 ), fibers treated only in 4.0 mol/L NaOH (
), fibers treated only in 4.0 mol/L NaOH (
 ), in 0.5 mol/L NaOH at 30°C (
), in 0.5 mol/L NaOH at 30°C (
 ), in 0.5 mol/L NaOH at 50°C (
), in 0.5 mol/L NaOH at 50°C (
 ), in 4 mol/L NaOH at 30°C (
), in 4 mol/L NaOH at 30°C (
 ) and in 4 mol/L NaOH at 50°C (
) and in 4 mol/L NaOH at 50°C (
 ). In some instances, data point sizes exceed those of the error bars
). In some instances, data point sizes exceed those of the error bars
The absorbance intensities related to the deprotonated carboxyl group, normalized with respect to that of the glycosidic linkage, from FTIR spectroscopy in ATR and transmission modes, are shown in Fig. 2. The carboxylate group absorbance peak occurred at 1591 cm−1 in ATR spectra and at 1600 cm−1 in transmission measurements. Small peak shifts between ATR and transmission spectra are attributed to the effects of sample refractive index and its thickness on ATR measurements (Harrick and du Pré 1966; Nunn and Nishikida 2008).
Absorbance peak intensities at 1591 cm-1 and 1600 cm-1 from ATR and transmission spectra respectively, normalized with respect to the corresponding intensities at 1156 cm-1. The circles are data from ATR spectra, and the triangles from transmission spectra. The marker colors represent treatment sets: cleaned fibers (
 ,
,
 ), fibers treated only in 4.0 mol/L NaOH (
), fibers treated only in 4.0 mol/L NaOH (
 ,
,
 ), in 0.5 mol/L NaOH at 30°C (
), in 0.5 mol/L NaOH at 30°C (
 ,
,
 ), in 0.5 mol/L NaOH at 50°C (
), in 0.5 mol/L NaOH at 50°C (
 ,
,
 ), in 4.0 mol/L NaOH at 30°C (
), in 4.0 mol/L NaOH at 30°C (
 ,
,
 ) and in 4.0 mol/L NaOH at 50°C (
) and in 4.0 mol/L NaOH at 50°C (
 ,
,
 ). In some instances, data point sizes exceed those of the error bars
). In some instances, data point sizes exceed those of the error bars
In ATR mode, the incident beam penetration depth into samples is limited to between 0.5–5 µm (PIKE Technologies Inc. 2018). The measurements were performed on intact fibers, and thus the normalized intensities are proportional to the proportion of carboxylate groups (or their density) in the vicinity of fiber surfaces. In transmission mode measurements, the specimens from fiber samples were milled before being pelletized with KBr, and thus the normalized intensities are representative of the bulk density of carboxylate groups in samples.
Quasi-linear relationships were obtained between the normalized FTIR absorbance intensities and fiber carboxyl contents (see Fig. 2). Up to about 175 mmol/kg of fiber carboxyl content, generally no significant differences may be observed between the values from ATR and transmission measurements. This region includes all fibers from reactions in 0.5 mol/L NaOH and some from reactions in 4.0 mol/L NaOH. With further rise in carboxyl content, the ratios from ATR measurements are greater than those from transmission measurements. It suggests that in fibers with carboxymethylation levels of up to 175 mmol/kg, the distribution of substituents through fiber structures was more uniform as compared to fibers with higher levels of carboxymethylation, where the surface density of carboxyl contents was greater than the bulk density.
When the levels of methylene blue sorption are plotted against normalized FTIR-ATR absorbance intensities (see Fig. 3), two distinct near-linear relationships may be observed—one corresponding to fibers reacted in 0.5 mol/L NaOH, which has a significantly greater gradient of change as compared to the other, which corresponds to fibers reacted in 4.0 mol/L NaOH. It implies a greater extent of dye interactions per unit surface density of carboxylate anions in fibers from reactions in 0.5 mol/L NaOH as compared to 4.0 mol/L NaOH. This is despite the fact that in Fig. 2, the FTIR-ATR absorbance intensities appear to vary only with the carboxyl content and no influence of the alkali level in treatment is observed.
The levels of methylene blue sorption in fibers plotted vs. their normalized FTIR-ATR intensities. The marker colors represent treatment sets: cleaned fibers (
 ), fibers treated only in 4.0 mol/L NaOH (
), fibers treated only in 4.0 mol/L NaOH (
 ), in 0.5 mol/L NaOH at 30°C (
), in 0.5 mol/L NaOH at 30°C (
 ), in 0.5 mol/L NaOH at 50°C (
), in 0.5 mol/L NaOH at 50°C (
 ), in 4 mol/L NaOH at 30°C (
), in 4 mol/L NaOH at 30°C (
 ) and in 4 mol/L NaOH at 50°C (
) and in 4 mol/L NaOH at 50°C (
 ). In some instances, data point sizes exceed those of the error bars
). In some instances, data point sizes exceed those of the error bars
A probable explanation for the apparent contradiction is that the accessibility to carboxylate anions may not be the same for the dye cation as compared to the incident infrared beam. As discussed above, the depth of incident beam penetration into substrates may extend up to 5 µm, and thus all carboxylate groups in that region will contribute to the absorbance signal in FTIR-ATR spectroscopy. If the accessibility of dye cations is limited to shallower depths, it will be reflected in a lower rate of change in dye sorption per unit change in normalized intensity. It also suggests that the carboxymethylation reaction occurred up to greater depths in treatments with 4.0 mol/L NaOH as compared to 0.5 mol/L NaOH.
The K/S value calculated with Eq. 1 is a measure of the absorbance intensity at each measured wavelength in the range 400–700 nm, and the average calculated with Eq. 2 is a measure of the overall absorbance (or color) intensity. The spectra of K/S values from selected samples after methylene blue sorption are shown in Fig. S3(a). With rise in overall absorbance intensity, a shift in the wavelength of absorbance maxima towards lower wavelengths, i.e., hypsochromic shift, may be observed. That resulted in a change of the color, which is represented on the CIE 1976 chromaticity diagram in Fig. S3(b).
A plot of the overall color intensity (K/Sav) against methylene blue dye sorption levels, see Fig. 4a, shows an initial rapid rise in color intensity as dye sorption levels increased from ca. 20 mmol/kg to 40 mmol/kg, followed by a more gradual change as dye sorption levels increased further to ca. 100 mmol/kg. No differences may be discerned between fibers from different treatment conditions in these trends. In opaque solids such as fibers, the color intensity is proportional to colorant amounts at or near their surfaces. The initial, near vertical, rise in color intensity with dye sorption suggests dye accumulation at or near fiber surfaces and the later gradual rise is indicative of dye permeation beyond the surface further into the fiber bulk. The pattern of change in K/Sav appeared not to be influenced by any of the treatment variables during carboxymethylation (alkali level, temperature).
Profiles of (a) color intensity, i.e., K/Sav, and (b) spectral centroid, λsc vs. methylene blue sorption in fibers. The marker colors represent treatment sets: cleaned fibers (
 ), fibers treated only in 4.0 mol/L NaOH (
), fibers treated only in 4.0 mol/L NaOH (
 ), in 0.5 mol/L NaOH at 30°C (
), in 0.5 mol/L NaOH at 30°C (
 ), in 0.5 mol/L NaOH at 50°C (
), in 0.5 mol/L NaOH at 50°C (
 ), in 4 mol/L NaOH at 30°C (
), in 4 mol/L NaOH at 30°C (
 ) and in 4 mol/L NaOH at 50°C (
) and in 4 mol/L NaOH at 50°C (
 ). In some instances, data point sizes exceed those of the error bars
). In some instances, data point sizes exceed those of the error bars
The shifts in wavelength of maximum absorbance may be quantified with the spectral centroid (λsc) as calculated with Eq. 3 (Stone 1967), and that is plotted against dye sorption levels in Fig. 4b. The hypsochromic shift, termed “metachromasy”, is known to occur with methylene blue sorption on cellulose polyanions including carboxymethyl cellulose, and is proportional to the degree of close packing between the sorbed dye molecules (Pal 1958; Bergeron and Singer 1958; Lawton and Phillips 1982). It may be observed in Fig. 4b, that the spectral centroid of the absorbance spectra decreased (i.e., metachromasy levels increased) in a near-linear fashion with rise in levels of dye sorption. Again, no discernible differences may be observed between fibers from different treatment conditions.
Comparisons to results from previous work on carboxymethylation of viscose fibers
In Table 3, comparisons are shown between viscose fibers from previous work and cotton fibers from this work, of the ranges observed in carboxyl contents and in molar ratios of dye sorption to carboxyl groups. Cotton after carboxymethylation exhibited carboxyl contents in the range of ca. 50–440 mmol/kg and viscose in the range of ca. 170–625 mmol/kg. Both fiber types are represented in the carboxyl content range of ca. 170–440 mmol/kg, but values in the range ca. 50–170 mmol/kg were observed only for cotton and those in the range ca. 440–625 mmol/kg only for viscose. Despite limits to the range of carboxyl contents where both fiber types are represented, comparisons between the two yield observations of interest.
The differences in degrees of carboxymethylation between fiber types (cotton and viscose) may be attributed to greater accessibility during reaction. All reactions were performed in alkaline media, where viscose fibers swell to greater levels than cotton (You et al. 2021), which may be attributed to the lower molecular weight (i.e., degree of polymerization) and lower degree of crystallinity typical of viscose in comparison to cotton (Bredereck and Hermanutz 2005). Further, cellulose swelling levels in 4.0 mol/L NaOH are greater than in 0.5 mol/L NaOH (Klemm et al. 1998), to which may be attributed the greater degree of carboxymethylation at the higher levels of alkali content.
However, in comparisons of methylene blue sorption, cotton fibers performed better than the viscose, and fibers treated in 0.5 mol/L NaOH performed better than fibers reacted in 4.0 mol/L NaOH. The comparison between molar ratios of dye sorption to carboxyl content shown in Table 3, are reflected in Fig. S4, where it may be observed that at similar levels of carboxymethylation, the degree of dye sorption increased in the order of fiber/reaction medium: viscose/4.0 mol/L NaOH < viscose/0.5 mol/L NaOH\(\cong\)cotton/4.0 mol/L NaOH < cotton/0.5 mol/L NaOH. As dye sorption stoichiometries may be regarded as a measure of dye cation accessibility to fiber carboxylate anions, it may be accepted that the accessibility changed in the same order. The treatment temperature influenced the degree of carboxymethylation but did not appear to exert influence on their accessibility, and the observed differences appeared to relate primarily to the fiber type and NaOH content in reaction media.
One factor that may limit accessibility of carboxyl groups to dye cations is the depth to which the carboxymethylation occurs within fiber structures. Indications of differences in carboxymethylation depths may be obtained from the normalized FTIR absorbance intensities. The proportionality of normalized FTIR-ATR absorbance intensities to carboxyl content did not differ between cotton and viscose fibers, or between fibers from different NaOH contents in reaction media (Fig. S5). But when the levels of methylene blue sorption are plotted against the normalized FTIR-ATR intensities (Fig. S6), distinctly different proportionalities are evident as function of fiber type and NaOH content in reaction media. That suggests not all of the carboxyl groups detectable with FTIR-ATR spectroscopy are accessible to dye cations, and thus the observed differences in Fig. S6, which mirror the order observed in molar ratios of dye sorption to carboxyl content, result from differences of carboxymethylation depths. It is also reasonable to expect that greater levels of swelling during reaction will allow for greater depths to which reactions can occur within fibers. As stated above, viscose fibers swell to greater levels than cotton in alkali solutions, and cellulose swells to greater extents in 4.0 mol/L NaOH as compared to 0.5 mol/L NaOH (Klemm et al. 1998).
Another factor that may contribute to limiting the accessibility of carboxyl groups is resistance to permeation of dye molecules caused by swelling of the carboxymethylated fibers in alkaline dye media (dye solutions were buffered at pH 8.5). Evidence of permeation resistance was observed in the previous work (Bogner et al. 2024), from the results of conductometric titrations on carboxymethylated viscose fibers, and similar differences but to smaller extents were also observed in the conductometric measurements on cotton. Carboxymethylation significantly increases the swelling and gelation propensity of cellulose (Thielking and Schmidt 2006), and the extents vary in direct proportion to the degree of substitution and in inverse proportion to the chain lengths of the cellulose polymer. The uniformity of substitution patterns along the individual polymer chains may also exert an influence (Stigsson et al. 2006). The lower permeation resistance in cotton as compared to viscose, may be attributed to the inherently lower degree of polymerization in viscose as compared to cotton (Bredereck and Hermanutz 2005).
The K/Sav (or color intensity) is proportional to dye contents in the surface regions, and thus if two fibers exhibit similar dye sorption levels but differences of color intensity, it may be inferred there was more surface accumulation of dyes in fibers exhibiting the higher K/Sav. A greater surface accumulation for similar dye sorption levels may be regarded as indication of lower dye permeation. In Fig. S7a, it may be noted that in the region of about 80–100 mmol/kg dye sorption, the color intensities were similar for both cotton and viscose carboxymethylated in 4.0 mol/L NaOH but those for viscose carboxymethylated in 0.5 mol/L NaOH were lower. The metachromasy levels (Fig. S7b) which are related to the degree of dye close-packing, although similar for all carboxymethylated fibers up to about 80 mmol/kg dye sorption, increased in the order of fiber/reaction medium: viscose/0.5 mol/L NaOH < cotton/4.0 mol/L NaOH < viscose/4.0 mol/L NaOH in the region of about 80–100 mmol/kg dye sorption, which suggests dye permeation resistance decreased in the same order. The dye sorption levels of cotton fibers carboxymethylated in 0.5 mol/L NaOH ranged between about 21–76 mmol/kg, and significant differences of metachromasy were not observed in that region between fiber types and NaOH contents in reaction media.
Conclusion
The influence of treatment variables (NaOH content, temperature) on the degree of carboxymethylation in cotton was similar to that observed previously in viscose (Bogner et al. 2024). A rise in NaOH content from 0.5 mol/L to 4.0 mol/L improved carboxymethylation levels, and treatments at 50 °C improved carboxymethylation levels as compared to 30 °C when the NaOH content was lower but the reverse was observed when the NaOH content was higher. These trends are attributed to the effect of treatment variables on the propensity of MCA reaction with cellulose relative to MCA hydrolysis in the reaction medium.
Low levels of carboxymethylation were obtained in the work, and that may be attributed to the choice of treatment conditions. The reaction medium was limited to only aqueous solutions of NaOH without addition of any alcohol, and high liquor ratios (i.e., solution volume per unit fiber mass) were employed. Such conditions favor hydrolysis of the MCA over its reaction with cellulose (Zhang et al. 1993). The restriction on reaction medium composition was because, as explained in the introduction section, the use of alcohols is difficult in large-scale textile treatment operations. A high liquor ratio was employed to ensure thorough and complete wetting of fibers with the treatment solutions. The primary focus of the work was to gain understanding of the influence of cellulose fiber supramolecular structure and morphology, on the progress of carboxymethylation reactions and performance of the carboxymethylated fibers, where the performance metric was sorption levels of a cationic dye, methylene blue. Despite the low levels of carboxymethylation, interesting insights were obtained on comparing the results between treatments on cotton fibers versus viscose fibers.
Not one of the carboxymethylated fibers, either cotton or viscose, exhibited a 1:1 stoichiometry of carboxyl group content to dye sorption level. The highest molar ratio of dye sorption to carboxyl group content was in the region of 0.46, i.e., at best only about half of the carboxyl groups in fibers were available for interaction with dye molecules.
The dye accessibility to fiber carboxyl groups decreased in the following order of fiber/reaction medium: cotton/0.5 mol/L NaOH > cotton/4.0 mol/L NaOH\(\cong\)viscose/0.5 mol/L NaOH > viscose/4.0 mol/L NaOH. The order appears related to the level of fiber swelling in reaction media, as greater swelling levels would allow for greater depths to which carboxymethylation reactions can occur within fiber structures. Another factor contributing to reduced accessibility may be resistance to dye permeation caused by fiber swelling and gelation, and some evidence of that was also observed.
All evidence in this work point to differences in depth to which the carboxymethylation reactions occurred within fiber structures i.e., from the surface towards the fiber core. The difference in normalized intensities between ATR and transmission modes of FTIR spectroscopy were very similar in both fibers, which suggests similar gradients in distribution of the substituted carboxymethyl groups from the surface to the core. However, there is yet no clarity on how the carboxymethyl groups are distributed through the fiber bulk. It is of interest to know, for example, what is the level of carboxymethylation at the core regions in both fibers and how the distribution of pores may have influenced distribution of the carboxymethyl groups. Investigations of this aspect are ongoing with spatial mapping of the carboxyl groups through fiber cross-sections with atomic force microscopy combined with FTIR spectroscopy. Another area of investigation is to examine if the observed differences in dye sorption to carboxyl content are also reflected in another metric of performance, that of water and moisture sorption. These investigations are also underway.
Further questions of interest worthy of investigation include if similar results will be obtained with other alkali types (e.g., KOH instead of NaOH), and what effects will be observed with other modification reactions (e.g., acetylation instead of carboxymethylation).
Data availability
All data that support the findings of the study are available from the corresponding author, APM, upon reasonable request.
References
Abu-Rous M, Ingolic E, Schuster KC (2006) Visualisation of the fibrillar and pore morphology of cellulosic fibres applying transmission electron microscopy. Cellulose 13:411–419. https://doi.org/10.1007/s10570-006-9052-5
BASF AG (1976) BASF Ratgeber für Cellulosefasern. Schlichten, Vorbehandeln, Färben. BASF Aktiengesellschaft, Ludwigshafen, Germany
Bergeron JA, Singer M (1958) Metachromasy: an experimental and theoretical reevaluation. J Cell Biol 4:433–457. https://doi.org/10.1083/jcb.4.4.433
Bogner P, Bechtold T, Pham T, Manian AP (2024) Alkali induced changes in spatial distribution of functional groups in carboxymethylated cellulose. Cellulose Press. https://doi.org/10.1007/s10570-024-05798-9
Bredereck K, Hermanutz F (2005) Man–made cellulosics. Rev Prog Color Relat Top 35:59–75. https://doi.org/10.1111/j.1478-4408.2005.tb00160.x
Carrillo F, Colom X, Suñol JJ, Saurina J (2004) Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur Polym J 40:2229–2234. https://doi.org/10.1016/j.eurpolymj.2004.05.003
Chen J, Wu J, Raffa P et al (2022) Superabsorbent Polymers: From long-established, microplastics generating systems, to sustainable, biodegradable and future proof alternatives. Prog Polym Sci 125:101475. https://doi.org/10.1016/J.PROGPOLYMSCI.2021.101475
Gu H, He J, Huang Y, Guo Z (2012) Water soluble carboxymethylcellulose fibers derived from alkalization-etherification of viscose fibers. Fibers Polym 13:748–753. https://doi.org/10.1007/S12221-012-0748-X/METRICS
Harrick NJ, du Pré FK (1966) Effective thickness of bulk materials and of thin films for internal reflection spectroscopy. Appl Opt 5:1739–1743. https://doi.org/10.1364/AO.5.001739
Hearle JWS (2007) Physical structure and properties of cotton. In: Gordon S, Hsieh Y-L (eds) Cotton: science and technology. Woodhead Publishing Limited, Cambridge, England, pp 35–67
Heinze T, El Seoud OA, Koschella A (2018) Etherification of Cellulose. In: Heinze T, El Seoud OA, Koschella A (eds) Cellulose derivatives: synthesis, structure, and properties. Springer International Publishing, Cham, pp 429–477
Ibrahim NA, Eid BM, Abd El-Ghany NA, Mabrouk EM (2020) Polyfunctional cotton cellulose fabric using proper biopolymers and active ingredients. J Text Inst 111:381–393. https://doi.org/10.1080/00405000.2019.1634459
Klemm D, Philipp B, Heinze T et al (1998) General considerations on structure and reactivity of cellulose: section 2.2–2.2.3. In: Comprehensive Cellulose Chemistry. Wiley, Ltd, Weinheim, Germany, pp 43–82. https://doi.org/10.1002/3527601929.ch2c
Lawton JB, Phillips GO (1982) The rôle of water in the metachromatic reaction. Die Makromol Chem 183:1497–1509. https://doi.org/10.1002/macp.1982.021830614
Li C, Ma H, Venkateswaran S, Hsiao BS (2020) Sustainable carboxylated cellulose filters for efficient removal and recovery of lanthanum. Environ Res 188:109685. https://doi.org/10.1016/j.envres.2020.109685
Li Z-W, Du Z-M, Wang Y-W et al (2022) Chemical modification, characterization, and activity changes of land plant polysaccharides: a review. Polymers (Basel) 14:4161. https://doi.org/10.3390/polym14194161
Liao Y, Alam N, Fatehi P (2022) Semitransparent films from low-substituted carboxymethylated cellulose fibers. J Mater Sci 57:10407–10424. https://doi.org/10.1007/s10853-022-07262-0
Liu Y, Wei Y, He Y et al (2023) Large-scale preparation of carboxylated cellulose nanocrystals and their application for stabilizing pickering emulsions. ACS Omega 8:15114–15123. https://doi.org/10.1021/acsomega.2c08239
Marchessault RH, Liang CY (1960) Infrared spectra of crystalline polysaccharides III. Mercerized cellulose. J Polym Sci 43:71–84. https://doi.org/10.1002/pol.1960.1204314107
Mischnick P, Momcilovic D (2010) Chemical structure analysis of starch and cellulose derivatives. Adv Carbohydr Chem Biochem 64:117–210. https://doi.org/10.1016/S0065-2318(10)64004-8
Miyamoto H, Sakakibara K, Wataoka I et al (2018) Interaction of water molecules with carboxyalkyl cellulose. In: Rosenau T, Potthast A, Hell J (eds) Cellulose science and technology. Wiley Ltd, Hoboken, NJ, USA, pp 127–141. https://doi.org/10.1002/9781119217619.ch6
Mohkami M, Talaeipour M (2011) Investigation of the chemical structure of carboxylated and carboxymethylated fibers from waste paper via XRD and FTIR analysis. BioResources 6:1988–2003
Nunn S, Nishikida K (2008) Advanced ATR correction algorithm. ThermoFisher application note AN50581_E 05/08M. Thermo electron scientific instruments LLC, Madison, WI (USA)
Pal MK (1958) Polyelectrolyte chromotropes in metachromacy: Part II. Die Makromol Chemie 28:91–95. https://doi.org/10.1002/macp.1958.020280109
PIKE Technologies Inc (2018) ATR – Theory and applications. https://www.piketech.com/files/pdfs/PIKE_ATR_Theory-Applications.pdf. Accessed 10 Mar 2024
Rácz I, Deák A, Borsa J (1995) Fibrous carboxymethylcellulose by pad roll technology. Text Res J 65:348–354. https://doi.org/10.1177/004051759506500607
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80. https://doi.org/10.1016/J.JHAZMAT.2009.12.047
Stigsson V, Kloow G, Germgård U (2006) The influence of the solvent system used during manufacturing of CMC. Cellulose 13:705–712. https://doi.org/10.1007/S10570-006-9083-Y/METRICS
Stone AL (1967) Aggregation of cationic dyes on acid polysaccharides II. Quantitative parameters of metachromasy. Biochim Biophys Acta - Gen Subj 148:193–206. https://doi.org/10.1016/0304-4165(67)90294-2
Stone AL, Bradley DF (1967) Aggregation of cationic dyes on acid polysaccharides I. Spectrophotometric titration with acridine orange and other metachromatic dyes. Biochim Biophys Acta - Gen Subj 148:172–192. https://doi.org/10.1016/0304-4165(67)90293-0
TAPPI (2008) Test method T 237 cm-08: Carboxyl content of pulp. Technical Association of the Pulp and Paper Industry, Atlanta, Georgia (USA)
Textile Exchange (2023) Materials market report. https://textileexchange.org/app/uploads/2023/11/Materials-Market-Report-2023.pdf. Accessed 24 Jan 2024
Thakur D, Seo S, Hyun J (2023) Three-dimensional triboelectric nanogenerator with carboxymethylated cellulose nanofiber and perfluoroalkoxy films. J Ind Eng Chem 123:220–229. https://doi.org/10.1016/j.jiec.2023.03.037
Thielking H, Schmidt M (2006) Cellulose ethers. In: Ullmann’s encyclopedia of industrial chemistry. Wiley Inc. https://doi.org/10.1002/14356007.a05_461.pub2
Wakelyn PJ, Bertoniere NR, French AD et al (2007) Cotton fiber chemistry and technology. CRC Press, Boca Raton, FL, USA
Wang J, Liu M, Duan C et al (2019) Preparation and characterization of cellulose-based adsorbent and its application in heavy metal ions removal. Carbohydr Polym 206:837–843. https://doi.org/10.1016/j.carbpol.2018.11.059
Wang Z, Jiang Y, Mao L et al (2023) Pilot-scale preparation of cellulose/carboxymethylcellulose composite fiber for methylene blue adsorption. Cellulose 30:3611–3623. https://doi.org/10.1007/s10570-023-05109-8
You X, Chen F, Ma Y et al (2021) Single fiber swelling behavior for natural and man-made cellulose fibers under alkaline treatment. Cellulose 28:11287–11298. https://doi.org/10.1007/S10570-021-04280-0/FIGURES/8
Zhang J, Li D, Zhang X, Shi Y (1993) Solvent effect on carboxymethylation of cellulose. J Appl Polym Sci 49:741–746. https://doi.org/10.1002/app.1993.070490420
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. The research leading to these results was performed in the frame of the project “Textile Competence Center Vorarlberg 2 (Project No. 882502)” funded under the COMET program – Competence Centers for Excellent Technologies – by the Federal Ministry for Climate Protection, Environment, Energy, Mobility, Innovation and Technology (BMK) and the Federal Ministry for Digitization and Business Location (BMDW), with co-financing from the federal province of Vorarlberg. The COMET program is administered by the Austrian Research Promotion Agency (FFG).
Author information
Authors and Affiliations
Contributions
Paul Bogner: Investigation, Methodology, Visualization, Writing—Original Draft; Amalid Mahmud-Ali: Investigation; Thomas Bechtold: Conceptualization, Supervision, Writing—Review & Editing; Tung Pham: Conceptualization, Supervision, Writing—Review & Editing, Funding acquisition; Avinash P. Manian: Conceptualization, Supervision, Writing—Review & Editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable, because this article does not contain any studies with human or animal subjects.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bogner, P., Mahmud-Ali, A., Bechtold, T. et al. Alkali induced changes in spatial distribution of functional groups in carboxymethylated cellulose – comparison of cotton and viscose fibers. Cellulose 31, 7313–7324 (2024). https://doi.org/10.1007/s10570-024-06036-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-024-06036-y