Abstract
Aminoalkylalkoxysilane copolymers (co-AAAS) have the ability to simultaneously deacidify and strengthen paper documents. Nevertheless, despite enhancing the tensile strength, they generally fail to improve the folding endurance of the degraded groundwood pulp-rich papers. Focusing on that specific type of papers, several ways to overcome their lack of reinforcement were investigated. Promoting the polymerization of AAAS using catalysts and thermal steps was one of them. A monitoring with FTIR spectroscopy showed that the thermal step was the most efficient in speeding up the hydrolysis and polycondensation of 3-aminopropylmethyldiethoxysilane (AM) monomer in aqueous solutions. The progress of the two-steps reaction in terms of degree of polymerization of AAAS polymers was then studied in-situ after heating the paper, using Cross Polarization—Magic Angle Spinning 29Si solid-state NMR, and was shown to be enhanced as well. The other optimization route explored was to stabilize the paper before treatment. Our previous research had shown that the presence of oxidized groups in paper hampered the strengthening. Sodium borohydride, a known bleaching agent in paper conservation, was used prior to the AAASs treatment to decrease the amount of carbonyls in paper. The reduction and the thermal step were compared in terms of the mechanical properties (folding endurance and tensile strength) upon AAASs treatment of several lignocellulosic papers, including three newsprints dated 1911–1923. The use of sodium borohydride led to a significant improvement of the folding endurance of the samples, an unprecedented result. It enabled at the same time to counteract the yellowing induced by the AAAS and to build a larger alkaline reserve.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In archives and libraries, the preservation of the collections from the 19th to mid-twentieth centuries that contain papers made of unpurified woodpulp is a prime concern because of their poor state of conservation. These papers contain lignin, a biopolymer that produces acids mainly through its oxidation (Dupont et al. 2007), as well as alum-rosin sizing, where the aluminum sulfate salts are a notorious source of acidity (Williams 1981; Banik and Bruckle 2011). Acid-catalyzed hydrolysis and oxidation reactions cause the decrease of the degree of polymerization (DP) of cellulose (Whitmore and Bogaard 1994; Area and Cheradame 2011), the chief biopolymer constituent of paper. As a result, ultimately, the mechanical properties of paper weaken.
To lengthen the lifetime of these papers, treatments based on aminoalkylalkoxysilanes (AAASs) have been developed, with a dual purpose of deacidifying and strengthening in a single step. The treatments were tested on various types of papers using tri-alkoxy functionalized AAAS monomer (3-aminopropyltriethoxysilane (AP)) and di-alkoxy functionalized ones (3-aminopropylmethyldiethoxysilane (AM) or N-(2-aminoethyl)-3-aminopropylmethyldimethoxysilane (DIA)), as well as their mixtures (Piovesan et al. 2014, 2017, 2018b). The deacidification and the deposition of an alkaline reserve are achieved via the amine groups. The in-situ random polycondensation of the tri- and di- functional AAASs in ambient conditions leads to a copolymer network (co-AAAS), which improves both the Folding Endurance (FE) and the Tensile Strength (TS) of paper.
Shortly after the treatment that usually deposits 5–12% (wt/wt) AAAS in the paper, the alkaline reserve is close to 0.40 mol kg−1 (Piovesan et al. 2014, 2017, 2018b), in compliance with the ISO standard 9706, and the tensile strength increases with AP/DIA in 50/50 proportion (wt%) from 13 to 48% (Ferrandin-Schoffel et al. 2021). These improvements are observed in all types of papers, regardless of their constituents and degradation state. In addition, in written historical documents the readability is maintained after treatment, with no feathering or change of color of the inks (Piovesan et al. 2018a). On the other hand, an increase in the folding endurance is observed with certain papers only (Piovesan et al. 2018b; Ferrandin-Schoffel et al. 2021). Folding endurance (FE) is widely used for the evaluation of paper permanence as it is more sensitive to early modifications than tensile strength measurements and better simulates the stresses that paper undergoes when handled, hence the importance of improving it. For instance, independently of their degradation state, FE of lignin-free papers (rag pulp and bleached woodpulp) is in general significantly improved. In contrast, when degraded, i.e., when the number of double folds (N(FE)) falls below 20, lignin-containing papers are more recalcitrant. The presence of lignin was identified as a critical parameter hindering the consolidation (Piovesan et al. 2017, 2018b; Ferrandin-Schoffel et al. 2020, 2021). With the aim to better understand the reasons of this impairment, the chemical reactions that take place between lignin and AAAS were investigated (Ferrandin-Schoffel et al. 2020). Among lignin’s functional groups that can react with the amine function, besides the obvious carboxylic acid functions through acid–base reactions, carbonyls were shown to react rapidly, forming secondary imines, i.e., Schiff bases. In degraded papers, the larger quantity of oxidized functions was held responsible for the lack of fold resistance reinforcement, as well as for some yellowing after treatment. Conversely, the hydrogen bonds forming between the amine groups of AAAS and cellulose have been shown to support the strengthening, so much that hindering their formation would be a possible cause of treatment impairment (Dupont et al. 2010; Souguir et al. 2011). To optimize the treatment, the occurrence of the side reactions between oxidized moieties in paper and AAAS should be avoided as much as possible.
It has also been observed that the mechanical properties of papers treated with AAASs are subject to slow changes over time. For instance, the tensile strength of lignocellulosic papers treated with AP/DIA (50/50) increased over the course of several years, up to + 27% after 7 years of storage in standard conservation conditions (Ferrandin-Schoffel et al. 2021). The in-situ polycondensation was also shown to progress at a slow pace, as evidenced by 29Si NMR. The polymerization kinetics was therefore identified as another key factor affecting the strengthening.
In this research, the two above-mentioned obstacles, i.e., the oxidative degradation state of the paper and the slow polymerization of AAAS, were investigated in an attempt to optimize the treatments efficacy. The kinetics of the polymerization was modified with the aim to speed up the formation of longer polysiloxane chains. This was achieved in two ways, by increasing the reaction temperature (Colby et al. 1988; Brochier Salon et al. 2008) and/or using catalysts. The catalysis of the hydrolysis and subsequent polycondensation of alkoxysilanes can be achieved in acidic and in alkaline conditions (Chojnowski and Cypryk 2000), whereas both reactions are very slow at neutral pH (Zhang and Sakka 1999; Bennevault-Celton et al. 2010). Adding acids is out of the scope in paper conservation, and at any rate, corollary to the treatment is the fact that AAASs are alkaline. The conditions to be used are thus those of alkaline catalysis. In addition, two chemical catalysts previously used with various alkoxysilanes, namely diphenyliodonium chloride (Ph2ICl) at a concentration of 1 wt% (Fox et al. 1978), and aluminum acetylacetonate (Al(acac)3) at 0.5 wt% (Zhang et al. 1995; Zhang 1997; Zhang and Sakka 1999; Torry et al. 2006), were tested.
The second obstacle mentioned above, i.e., avoiding side reactions between AAAS and oxidized groups, was achieved by reducing the paper using sodium borohydride (NaBH4) before the application of the AAAS monomers. This reduction agent is commonly used for the stabilization and bleaching of graphic documents (Hey 1977; Burgess 1988; Lienardy and Van Damme 1988; Ďurovič and Zelinger 1993), primarily because it reverts carbonyl groups on cellulose to their native hydroxylated form, thereby also conveniently decreasing the occurrence of yellowing. All carbonylated structures including chromophores in cellulose, hemicelluloses and lignin, namely aldehyde, ketone and quinone bearing-compounds, undergo reduction with NaBH4 (Ďurovič and Zelinger 1993), yielding alcohol functions (Brown et al. 1960; Walker 1976). Our previous research showed that secondary alcohols are unreactive, and primary alcohols have a low-reactivity with AM (Ferrandin-Schoffel et al. 2020). Thus, a reduction step before treatment would circumvent some of the side reactions between AAAS and the oxidized moieties, and would therefore possibly enable a better strengthening of paper. This was investigated in the second part of this work.
Experimental
Chemicals
3-aminopropyltriethoxysilane (AP) (> 98%), 3-aminopropylmethyldiethoxysilane (AM) (97%) and N-(2-aminoethyl)-3-aminopropylmethyldimethoxysilane (DIA) (97%) were purchased from ABCR (Gelest, France) and used as received. Ph2ICl (98%) and Al(acac)3 (97%) were also purchased from ABCR, NaOH from Fluka, NaBH4 from Sigma Aldrich, and absolute ethanol from Fisher. A sample of rosin from the Centre de Recherche sur la Conservation’s resins bank was used.
Kinetics of the polymerization of AM in solution
Hydrolysis and polycondensation of AM were followed with AM/H2O 9/1 wt% solution (Piovesan 2016) in a glass vial. The solution was stirred in ambient conditions. NaOH (0.5%), Ph2ICl (1.0 wt%) or Al(acac)3 (0.5%) were added in some of the vials. Some of the solutions were heated in an oven at a constant temperature (40, 50, 60 or 70 °C). The kinetics were monitored using FTIR spectroscopy, each solution being regularly sampled during one week after its preparation (time (t0)). The spectra were normalized to the Si-CH3 absorption band at 1 255 cm−1 characteristic of Si-CH3 stretching of AM (Socrates 2004), which is not modified by the reactions.
Paper samples
Three newsprint papers deaccessioned from the Bibliothèque Nationale de France, called J1, J2 and J3, dated 1911, 1920 and 1923, respectively, were used. Each one is a different issue of the “Journal des Fabricants de Sucre”, a newsletter published through the late 19th to the early twentieth centuries. The sheets were cut in a similar format (about 12 cm × 25 cm). Their composition (fibers nature, pulp, sizing and fillers) had been previously characterized qualitatively (Ferrandin-Schoffel et al. 2020). Two machine-made laboratory papers containing a mix of bleached sulfite pulp and groundwood pulp, called C3a and C4, were also used in the same format. All the papers contain kaolin fillers, and all are sized with alum-rosin except C4 (unsized). Table 1 gathers several physico-chemical characteristics of the papers.
Reduction step
The papers C3a, J1, J2 and J3 reduced with NaBH4 were called C3a-r, J1-r, J2-r and J3-r, respectively. Four sheets of each (interleaved with a non-woven fabric and stacked) were immersed overnight in a 0.23 wt% NaBH4 absolute ethanol solution (approximately 2 L) under gentle magnetic stirring according to Burgess’ protocol (Burgess 1988). To limit solvent evaporation, the solution was covered with Parafilm®M in which a few holes were punctured to allow the passage of the hydrogen gas produced. The papers were then washed several times in deionized water (approximately 2 L) for 30 min until the pH of the wash bath was neutral. Finally, the papers were dried at room temperature for at least 3 h and gently heated at 40 °C in an oven for 1 h before and placed in the environmental room to equilibrate at 23 °C and 50% relative humidity (RH).
AAAS treatment and post-treatment thermal step
Bicomponent mixtures AP/AM and AP/DIA, 50/50 or 5/95 (wt%), were sprayed undiluted on both sides of the samples using an airbrush (model LN-119, WilTec) with a 0.8 mm diameter needle. Each sample was then left at least 3 h at room temperature and equilibrated at 23 °C and 50% RH for at least 24 h (TAPPI T 402 sp-03) before weighing. The uptake (UP) was calculated as follows (Eq. 1):
where wb and wa are the paper weight before and after the treatment, respectively.
All the samples were treated to a similar uptake (between 6 and 9%) for a relevant comparison of the data. In the following, the term “treatment’’ will refer exclusively to the application of AAASs on the paper, whereas the term “step” will refer to the pre- or post-treatment optimization phases.
After the treatment, some of the papers underwent a thermal step at 50 °C or 70 °C in an oven for 7 days. RH values in the oven were 7 ± 2% and 0.9 ± 0.3%, respectively, as measured by a humidity and temperature datalogger (Ibutton®, Hygrochron, Measurement Systems Ltd).
Physico-chemical characterizations
The amount of mineral fillers in paper was determined by measuring the ash content (TAPPI T 211 om-02) (two repeat measurements).
The determination of the Equilibrium Moisture Content (EMC) at 23 °C and 50% RH was performed according to TAPPI T 550 om-08, adapted to a sample mass of 500 mg. The cold extract pH was measured with a pH-meter (Mettler Toledo MA235) according to TAPPI T 509 om-02, adapted to a sample mass of 100 mg (mass/volume ratio). Two to three repeat measurements were carried out per paper.
The copper number N(Cu) (g Cu2O) is defined as the number of grams of metallic copper resulting from the reduction of CuSO4 by 100 g of the pulp of paper fibers. It provides an indication of the amount of carbonyls in paper. It was determined according to TAPPI standard T 430 cm-99, with a reduced mass of paper of 300 mg. Two repeat measurements were done per paper.
Color and opacity measurements were performed with a hand-held spectrophotometer CM-26dG (Konica Minolta) equipped with an integrating sphere. The configuration adopted was in reflectance mode (spectral range 360–740 nm in 10 nm steps), with the D65 Standard Illuminant and 10° Standard Observer (ISO 5631-2 standard), specular component included (SPIN). The 3 mm diameter measurement aperture was used. The colorimetric coordinates values L*, a* and b* were calculated in the CIELAB 1976 color space. The opacity values were measured with Standard Illuminant C and 10° Standard Observer (ISO 2471 standard). The measurements were done in five to ten different locations on the samples’ surface and averaged. The averages are reported with the standard deviations. The color difference was calculated as \({\Delta \mathrm{E}}^{*} = \sqrt{{{\Delta \mathrm{L}}^{*}}^{2}+ {\Delta \mathrm{a}}^{{*}^{2}}+{\Delta \mathrm{b}}^{{*}^{2}}}\).
Tensile Strength (TS) was measured with an Adamel Lhomargy tensile instrument (DY-20 N) according to TAPPI T 494 om-01. Elongation at Break (EB), which provides information about the material’s ductility, and Young modulus (Y), which is an indicator of the paper stiffness and elasticity, were calculated. Folding endurance (FE) was determined with a Tinius Olsen instrument (applied load: 500 g) according to TAPPI T 511 om-02 standard. FE is reported in terms of number of double folds before breaking (N(FE)). For tensile and fold tests, ten measurements were done per sample in the machine direction of the paper. Prior to the measurements, the samples were equilibrated in adsorption mode at 23 °C and 50% RH according to TAPPI T 402 sp-03. The average values are reported with the standard deviations.
FTIR was carried out on the papers and on the AM/H2O solutions with a Nicolet 6700 spectrometer, equipped with a diamond Smart Endurance ATR macro-system scanning from 500 to 4 000 cm−1. For each spectrum, 64 scans were recorded, with a resolution of 4 cm−1.
The 1H → 29Si and 1H → 13C cross-polarization (CP) under magic-angle spinning (MAS) NMR spectra were recorded on a NEO Bruker 500 WB NMR spectrometer (11.7 T). The papers were cut in small pieces and introduced in 4 mm outer diameter zirconia rotors. All samples were spun at 10 kHz MAS frequency. The 29Si spectra (respectively, 13C spectra) were recorded by using Larmor frequency at 99.4 MHz (respectively, 125.8 MHz), and the radiofrequency fields applied on 1H and 29Si (respectively, 1H and 13C) channels were 50 kHz and 60 kHz (respectively, 60 kHz and 50 kHz), respectively. The contact time was set to 4 ms (respectively, 3 ms), and the recycle delay to 4 s in all cases. 65 058 transients were accumulated for the 29Si spectrum (about 55 h experimental time), and between 1 592 and 1 708 transients for the 13C spectra (about 2 h experimental time). The 29Si and 13C chemical shifts were externally referenced to tetramethylsilane (TMS) at 0 ppm. The deconvolution of the spectra was performed with the Dmfit software (Massiot et al. 2002), using simple Lorentzian/Gaussian line shapes.
Results and discussion
Improving the polymerization of AAAS
The aim of the optimization was twofold: to speed up and to enhance the polycondensation of AAAS (i.e., form longer polysiloxane chains). The first study was carried out in liquid phase where three catalysts (NaOH, Ph2ICl and Al(acac)3) were added in AM/H2O solutions at different temperatures. The second study was carried out in solid phase, in-situ with the treated paper.
Impact of catalysts and temperature on AM reactions in solution
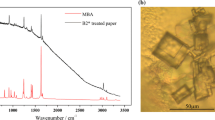
The hydrolysis and polycondensation of AM were monitored in solution using FTIR spectroscopy. The spectra of the AM/H2O solution recorded at (t0) and 1, 2, 5 and 7 days later are shown in Fig. 1. As expected, the absorbance of Si–O–Et band decreased, whereas the intensity of Si–O–Si band progressively increased until (t0 + 5 d), and then stabilized. Simultaneously, the bands related to the silanol groups (Si–OH) and to ethanol (Et-OH) appeared from (t0 + 1 d), which was congruent with the hydrolysis of the ethoxysilane groups (Eq. 2).
FTIR spectra of AM/H2O (9/1) recorded at (t0), (t0 + 1 d), (t0 + 2 d), (t0 + 5 d) and (t0 + 7 d). Absorption bands used for the calculation of the relative intensity (Ir) are in bold.  : bands related to Si–O–Et bond (1 165, 1 100, 1 080 and 945 cm−1).
: bands related to Si–O–Et bond (1 165, 1 100, 1 080 and 945 cm−1).  : bands related to ethanol (1 050 and 880 cm−1) (Socrates 2004)
: bands related to ethanol (1 050 and 880 cm−1) (Socrates 2004)


The relative intensity (Ir) of the absorption bands Si–O–Si (1 005 cm−1, Si–O stretching), Si–O–Et (945 cm−1, Si–O symmetric stretching) and Si–OH (910 cm−1, Si–O stretching) was calculated with respect to the Si-CH3 band as follows (Eq. 4) (Zhang and Sakka 1999; Socrates 2004; Daher et al. 2018):
where Abs(X) is the absorbance of the band considered and Abs(Si–CH3) the absorbance of Si-CH3 band (1 255 cm−1).
The relative intensity of the four bands of interest monitored as a function of time for the solutions that contain a catalyst (NaOH, Ph2ICl or Al(acac)3), and those heated at 40 °C, 50 °C, 60 °C and 70 °C, are shown in Fig. 2. The choice of temperatures was based on the assumption that when used on a short timeframe (a few days), they would not impact paper mechanical strength to any significant extent (Graminski et al. 1979; Havlínová et al. 2009). At (t0 + 1 d), for all the solutions, Ir(Si–O–Et) was lower than for the control solution (black dots) (Fig. 2a and e), whereas Ir(Si–O–Si) was higher (Fig. 2b and f). This evidenced that using catalysts and heat allowed a faster hydrolysis of the ethoxysilane groups and a faster polycondensation.
Among the catalysts, the fastest kinetics was obtained with NaOH (purple crosses), where both hydrolysis and polycondensation of AM occurred as soon as they were added in the solution (t0) (Fig. 2a and b). The higher hydrolysis rate in the condition of the alkaline catalysis was corroborated through the instant formation of Si–OH bonds (Fig. 2c) and ethanol (Fig. 2d).
Silanols are intermediate products of the hydrolysis of the ethoxysilane groups (Eq. 2). They react to form siloxane bonds (Eq. 3). If hydrolysis is faster than condensation, Ir(Si–OH) is expected to first increase and then decrease. This behavior was observed for the solutions with Ph2ICl and Al(acac)3, but not with the alkaline catalyst NaOH (Fig. 2c), for which there was no initial increase but a continuous decrease starting from a high value at (t0) (highest value of all samples). This evidenced the immediate and total hydrolysis at (t0), producing silanol groups, which afterwards accumulated as they reacted more slowly to form siloxane bonds (on the scale of days). These observations corroborated that hydrolysis was always faster than polycondensation and, therefore, that hydrolysis was not a rate-limiting step.
After seven days, the highest proportion of Si–O-Si bonds was found in the heated samples (Fig. 2f), all the more at the highest temperatures, concomitantly with the smallest Ir(Si–O–Et) (Fig. 2e). Higher temperature was also correlated to a lower proportion of silanols during the kinetics monitoring (Fig. 2g). For T ≥ 60 °C, from (t0 + 1 d), Ir(Si–O–Et) and Ir(Si–OH) were the lowest, whereas Ir(Si–O–Si) was the highest, which can be attributed to longer polysiloxane chains. Thus, the increase in temperature not only enabled to increase the polycondensation rate, but also led to a higher degree of polymerization (DP). This was facilitated by the removal of ethanol from the solution upon heating, which circumvented the alcoholysis of the siloxane bonds and favored the polycondensation reaction. After 2 days, the relative intensity of Si–O–Et and Si–O–Si bands reached a plateau, which was thus identified as the end of hydrolysis and polycondensation reactions of AM.
In order to more closely examine the kinetics of the reactions taking place, the impact of temperature was studied on a shorter time frame, by recording the FTIR spectra of the solutions heated at 50 °C, 60 °C and 70 °C, 1 h, 13 h and 23 h after (t0) (Fig. 2e–h, and Figure S1 in the Supporting Information). On the day scale, a sharp increase in the proportion of silanol groups and ethanol at (t0 + 1 h) was observed (Fig. 2g and h), preceding the decrease of Ir(Si–O–Et) (Fig. 2e) and the increase of Ir(Si–O–Si) (Fig. 2f). The most significant variations were recorded at the highest temperatures, 60 °C and 70 °C. This observation confirmed that the hydrolysis of the ethoxysilane groups of AM was fast and was followed by a much slower polycondensation.
Based on these first results, the cumulative effect of catalysts (NaOH or Al(acac)3) and temperature was studied (Fig. 3). The use of Ph2ICl was discarded as it led to similar variations of Ir than Al(acac)3 (Fig. 2a–d), yet, unlike the latter, it did not dissolve completely in the AM/H2O mixture and turned the solution yellow. At 40 °C, the variations of Ir(Si–O–Et) and Ir(Si–O–Si) after (t0 + 1 d) were similar, in the solutions with catalysts (purple and blue crosses) and without catalysts (red crosses). Meanwhile, as described above, heating (T ≥ 40 °C) was the best way to form longer polysiloxane chains. When used in conjunction with a thermal step, the catalysts were therefore superfluous.
Impact of a heating step on AAAS treated papers
Heating the AM solutions was shown to speed up and increase the polycondensation (higher DP). To verify if this happens in-situ in the paper as well, 29Si CP-MAS NMR was performed on C3a paper treated with DIA and subsequently heated during 7 days at 70 °C (Fig. 4b). This temperature was chosen as it led to the highest DP of AM in solution ("Impact of catalysts and temperature on AM in solution" section). The overlapping peaks at about − 16 ppm and − 22 ppm correspond to the D1 (silicon atom bonded to one –O–Si group) and D2 (silicon atom bonded to two –O–Si groups) signals, respectively (Souguir et al. 2012; Pellizzi et al. 2012). The third intense signal at about − 91 ppm is related to kaolinite (Rocha and Klinowski 1990), the mineral filler in C3a (Table 1). There was no signal in the [ − 3; − 7] ppm range, characteristic of D0 of DIA monomer (Monredon-Senani 2004), indicating that all the free monomers reacted. This absence of D0 had been observed before for treated papers, with and without heating (Souguir et al. 2011, 2012; Ferrandin-Schoffel et al. 2021). CP-MAS was used semi-quantitatively by integrating the deconvoluted D1 and D2 peaks, which yielded an approximate number-average degree of polymerization (\(\overline{{DP }_{n}}\)) of 53. This DP value is an achievement: it is higher than the value determined for the same paper treated with DIA at a similar uptake and stored under ambient conditions, as measured several weeks after treatment (\(\overline{{DP }_{n}}\) ≈ 13) (Fig. 4a) and even as measured six years later (\(\overline{{DP }_{n}}\) ≈ 36) (Ferrandin-Schoffel et al. 2021). This result corroborated the main conclusions of the kinetics monitoring of the AM/H2O solutions, namely that the heating step speeds up and favors the polycondensation.
It was noted that after the treatment and before heating, C3a turned highly hydrophilic as it readily absorbed a drop of water deposited on its surface. This had been observed previously on paper treated with a difunctional AAAS (AM and DIA) and with AAAS mixtures (Ferrandin-Schoffel et al. 2021). However, after the thermal step, C3a became again as hydrophobic as before the treatment. Regaining a hydrophobic character is consistent with a lower amount of hydrophilic silanol groups and consequent to the formation of longer polysiloxane chains.
To evaluate the impact of the thermal step on the folding endurance and the tensile strength, several papers treated with AP/DIA 50/50 were heated. This formulation has been used previously (Ferrandin-Schoffel et al. 2021). Here again, the papers, which had become hydrophilic immediately after treatment, recovered a hydrophobic character after heating at T ≥ 50 °C for 7 days (water drop test). This corroborated that the polysiloxane chains formed were long enough to provide hydrophobicity. Table 2 gathers the folding endurance (FE) of the treated model papers C4 and C3a heated at 50 °C and 70 °C. Measuring FE of a C4 sample immediately after heating to 70 °C for the same duration (N(FE) = 44 ± 10) allowed to confirm that the thermal step did not affect this mechanical property.
Without heating, the AAAS treatment led to a twofold increase of N(FE) of C4. The thermal step improved FE even further. Nonetheless, at 70 °C, the spread of the data was large, which could be explained by a heterogeneous polycondensation in the paper, leading to large polymer dispersity. Conversely, after treatment, N(FE) of C3a decreased, and did so similarly with and without the thermal step. This recalcitrance to the treatment was not unexpected as a previous study had evidenced that a GWP-rich paper with N(FE) < 20 such as C3a could not be strengthened by any of the AAASs formulations developed (Ferrandin-Schoffel et al. 2021). To explain this result, it is thought that at the same time as favoring the polycondensation, heat may also hamper the reinforcement, by promoting side reactions between the AAASs and the oxidized moieties in paper (Ferrandin-Schoffel et al. 2020). For this type of paper, the solution to improve FE was therefore sought in avoiding these counterproductive side-reactions.
In addition, Elongation at Break (EB), Young modulus (Y) and Tensile Strength (TS) of the newsprint papers J1, J2 and J3 treated with AP/DIA 50/50 heated at 50 °C for 7 days were measured (Fig. 5). Without the thermal step (green bars), the treatment led to an increase in TS and Y, as observed previously (Ferrandin-Schoffel et al. 2021). However, FE of the papers was not significantly improved.
The thermal step (orange bars) significantly improved the FE achieved after treatment (green bars) for J1 (twofold increase), but not for J2 and J3. Unlike J1, both J2 and J3 are lignin-rich papers since they contain a significant proportion of GWP (Table 1). As hypothesized above, the side reactions that are likely to occur between the AAASs and lignin in J2 and J3 could have been favored by the heating, thereby hindering the FE strengthening. In parallel, EB and TS of J3 were the highest after treatment and heating, whereas the thermal step had a lower impact on J1 and J2. Young modulus (Y) of J2 was lower with the thermal step, but for J1 and J3 it did not significantly change.
The improvement of some of the mechanical properties of J1 and J3 after treatment with AP/DIA 50/50 and heating at T = 50 °C for 7 days was attributed to the increase in the DP. However, if the thermal step was not detrimental to the mechanical properties of C3a and J2, it did not enable better folding endurance either. The high degradation state and the presence of groundwood pulp in these papers are still a barrier to the strengthening. Despite the better polycondensation, the side reactions that occur in the paper between AAAS and carbonyl groups are most likely a cause (Ferrandin-Schoffel et al. 2020).
Reduction of the carbonyls in paper
The reduction of the carbonyl groups in paper before the AAAS treatment was thought to possibly help overcome the obstacle to FE strengthening.
NaBH4 is a well-known reduction agent. It is specific for carbonyls, as it does not react with alkenes and carboxyls (Brown et al. 1960; Walker 1976; Bruice 2009). It is a source of hydride ions, which act as nucleophiles that react with aldehydes and ketones to form alkoxide ions. The latter are subsequently protonated to yield alcohols. As the hydride ion is a very strong base (pKa of H2 is 35 (Bruice 2009)), NaBH4 aqueous solutions are highly alkaline. For instance, in the concentration range of 0.1 wt% and 1 wt% (mass/volume ratio) recommended in conservation for the stabilization and the bleaching of paper (Hey 1977; Burgess 1988), the pH of the solution is between 9 and 10 (Tang 1986; Burgess 1988). The reduction is always followed by a thorough washing in water to avoid residual alkaline boron salts, boric acid and sodium hydroxide to remain in the paper, and to convert the alkoxides resulting from the reduction into their alcohol counterparts. Tang (1986) showed that washing with a slightly alkaline or neutral aqueous solution, after the use of NaBH4, increased the paper’s permanence (i.e., its long-term stability) (Tang 1986).
A concentration above 1.0 wt% is considered harmful for fragile cellulosic materials (Burgess 1988), due to possible blistering in solution (Müller et al. 2019). This is due to the very high pH and the hydrogen gas produced during the decomposition of NaBH4, which is more pronounced in aqueous solutions than in alcoholic solutions (Burgess 1988; Ďurovič and Zelinger 1993). Alternative reduction chemicals, amine-borane complexes, soluble in both organic and aqueous solvents that do not produce H2 in solution can be used for the bleaching of paper (Bicchieri and Brusa 1997; Bicchieri et al. 1999, 2000). Among them, tert-butylamine borane complex (TBAB) was proven to be an efficient bleaching agent, which reduces carbonyls and alkenes (Brown et al. 1960; Walker 1976). However, it was found less efficient than NaBH4 in decreasing the carbonyl content in paper (Henniges and Potthast 2009). Moreover, the reduction of carbonyl groups with amine-borane complexes is said to produce borinates through hydroboration (Brown et al. 1960; Walker 1976), which can be eliminated afterwards by oxidation, usually with hydrogen peroxide (Brown et al. 1986). To our knowledge, this oxidation step has not been discussed or reported in the paper conservation literature, which raises the question of whether residual borinate anions remain in the paper after bleaching with TBAB.
As our aim was to specifically reduce the carbonyls of the aged papers, sodium borohydride was preferred over TBAB. To limit the evolving of hydrogen gas, it was dissolved in ethanol. They were characterized after drying at ambient temperature, heating and moisture equilibration ("Reduction step" section).
NMR 13C CP-MAS and FTIR were used to evaluate the efficiency of the reduction and the presence of possible by-products (Figures S2 and S3 in the Supporting Information). The spectra of paper C3a before and after reduction were similar. However, in the [185–220] ppm range, CP-MAS is not sensitive enough to detect carbonyl and carboxyl groups in paper (Hemmingson and Morgan 1990). Moreover, the newly formed alcohol groups are most likely hidden in the intense signals of the alcohol groups of cellulose in the [60–110] ppm range (Heinze et al. 2018). The FTIR band at 1 725 cm−1 corresponding to C=O bond stretching (Socrates 2004) was still visible after the reduction. This was attributed to the fact that this band also includes the signals of other characteristic groups in paper (carboxyls, acetyls) (Calvini and Gorassini 2002; Heinze et al. 2018). No conclusion could thus be drawn about the level of residual oxidation of the reduced papers.
As an alternative to quantify the amount of carbonyls in C3a and J1, N(Cu) was determined (Table 3). It has to be noted that N(Cu) was used only as an indication of the degree of carbonylation, and hence of the reduction efficacy. Indeed, the TAPPI standard applies to paper that contains no groundwood pulp or unbleached chemical pulp. As shown by the decrease in N(Cu) for both papers, NaBH4 allowed to reduce a significant portion of carbonyls, which is consistent with literature data (Henniges and Potthast 2009).
Hydrophilicity of the reduced papers
Although the papers C3a, J1, J2 and J3 are sized with alum-rosin and have a hydrophobic character, they became highly hydrophilic after reduction. The reduced samples immediately absorbed the drop of water deposited on their surface, as opposed to their non-reduced counterparts. To our knowledge, this phenomenon has not been described in the literature on paper bleaching.
After reduction, EMC of C3a and J1 were 18% and 8% higher, respectively (Table 3). This indicates a higher affinity to water molecules and can be partly explained by the increase of accessible hydroxyl groups in paper. However, it is unlikely that the conversion of carbonyls into hydroxyls would be the only cause of the higher EMC. All the papers reduced had been subsequently gently heated, so their subsequent equilibration at 23 °C and 50% RH could always take place in the adsorption regime ("Reduction step" section). Therefore, the increase was not due to the hysteresis effect in the moisture sorption of cellulose either, where EMC is higher in desorption mode (Moropoulou and Zervos 2003). Moreover, since the ash content of C3a was unchanged after reduction (13.4 ± 0.1 wt%), it seems that no newly formed inorganic salt after the immersion in NaBH4 could be a cause. Furthermore, the alum-rosin sizing in C3a was not extracted during the reduction, or at least to any significant extent, as indicated by the microchemical test for rosin performed on the sample (Browning 1977).
Additional tests were thus carried out to understand the cause of the papers’ hydrophilicity (Table S1 in the Supporting Information). The hydrophobic to hydrophilic conversion was not due to the solvent, but only to NaBH4. Further investigation is necessary to better identify the cause of the change of behavior towards moisture, since a change in the hydrophobic nature of a sized paper after a treatment involving NaBH4 is potentially a problem in terms of conservation of artworks.
Mechanical properties changes
With the reduction step, elongation at break EB improved, which indicates a larger plastic deformation (Table 4). The values of Y and TS, on the other hand, decreased, which is a sign of a higher elasticity (Y), but also a weakening (TS). These results are consistent with the increase in EMC (ΔEMC = + 18%) that followed the reduction (Table 3). This was not unexpected since water acts as a lubricant of the cellulosic fibers in paper, but also weakens inter- and intrafibers bonds (Casey 1961; Banik and Bruckle 2011).
The reduced samples C3a-r, whether untreated or treated with AAASs (AP/AM or AP/DIA 50/50), showed an improvement in FE (N(FE) increased from 12 to 16/19) (Table 4). Their folding endurance was significantly higher than for the non-reduced/treated C3a (N(FE) = 4). Therefore, it was not the AAASs treatment that was responsible for the improvement in the folding endurance of C3a-r. The same observation was made with EB, where higher values would most likely be attributable to the reduction (Table 4). TS and Y decreased after reduction and increased again upon treatment (C3a-r). The AAAS treatment was able to counteract the decrease in Y and TS induced by the reduction.
Ultimately, N(FE), EB and TS of the reduced samples C3a-r treated with AP/AM and AP/DIA (50/50) were all higher than those of the non-reduced untreated C3a. The type of difunctional AAAS (AM or DIA) in the AAAS mixture did not impact the extent of the mechanical strengthening (Table 4). Therefore, in the examination of the impact of the reduction on the newsprint papers, it seemed more relevant to modify the proportion rather than the type of difunctional AAAS. To this purpose, J1, J2 and J3 were reduced with NaBH4, and/or treated with AP/DIA in two different proportions: 50/50 and 5/95 (wt%).
J1 and J1-r had the same EB of 0.9% before AAASs treatment (Fig. 6). The higher EMC after reduction (Table 3) did not impact the plastic deformation of J1, as it did with C3a. This is consistent with the fact that the increase in EMC after reduction was significantly smaller for J1-r (+ 8%) than for C3a-r (+ 18%), and therefore may not have impacted these mechanical properties. The Y and TS values of J1-r were lower than for the non-reduced counterpart J1, which was consistent with the weakening of the inter- and intrafiber bonds, also related to a higher moisture content.
Elongation at Break (EB), Young modulus (Y) and Tensile Strength (TS) of the newsprint papers J1 and J3 untreated and treated with AP/DIA 50/50 and 5/95 (UP = 6–9%), with and without reduction with NaBH4. Paper J3, reduced and treated with AP/DIA 50/50, was also heated at 50 °C for 7 days (yellow bar)
The application of AP/DIA 50/50 and 5/95 both increased TS of J1 and J3, with and without reduction (Fig. 6). Ultimately, as for C3a, the AAASs treatments induced a higher mechanical strength, the highest values of EB being those of J1-r and J3-r, and the highest values of Y and TS being those of the non-reduced J1 and J3. The decrease in TS after the reduction was balanced by its increase due to AAASs, which confirms previous findings showing that the latter always lead to higher TS values (Ferrandin-Schoffel et al. 2021). For instance, for J1-r treated with AP/DIA 50/50, TS was higher than for the non-reduced untreated J1 (TS = 27 and 20 MPa, respectively). The 50/50 proportion led to higher TS than 5/95, which was attributed to the larger proportion of trifunctional AP in the mixture.
Similarly, as with C3a, FE of the three newsprint papers J1, J2 and J3 after treatment with AP/DIA 50/50 improved only when they had been previously reduced (Fig. 7). Achieving some level of FE strengthening with J2, the most recalcitrant paper, even modest (N(FE) = 9), is noteworthy since J2 gathers the two challenging factors, namely the presence of groundwood pulp and a critical degradation state (Piovesan et al. 2017, 2018b; Ferrandin-Schoffel et al. 2021). The twofold increase in N(FE) is unprecedented. The results of the treatments with the proportion 5/95 were less uniform. J1-r had lower FE than J1 (yet still higher than for the untreated sample). For J3, FE with AP/DIA 5/95 was the same with and without reduction, thus weaker than when treated with AP/DIA 50/50. For J2, the proportion 5/95 did not fare as well as 50/50 either. Thus, regarding the FE strengthening, the pre-reduction step with NaBH4 was useful especially in conjunction with the 50/50 proportion in AAASs treatment, and less so, or even somewhat detrimental with the 5/95 proportion.
For J1, FE was similar whether treated with AP/DIA 5/95 without pre-reduction or treated with AP/DIA 50/50 with pre-reduction (J1-r) (N(FE) = 27 and 22, respectively) (Fig. 7). As, unlike J2 and J3, J1 does not contain GWP (Table 1), this tends to indicate that the use of NaBH4 is not always beneficial to further improve FE of lignin-free papers (such as bleached woodpulp) with AAAS mixtures. For such papers, the mere use of a formulation with a dominant proportion of difunctional AAAS would be better suited. The type and location of carbonyls in the paper and their reactivity towards AAAS could explain this, since carbonyls in lignin-free papers (such as J1) are largely located on cellulose and hemicelluloses, whereas in lignocellulosic papers (such as J2 and J3), they are also in the lignin.
To this date, all the attempts to improve the folding endurance of the most recalcitrant papers, i.e., lignin-rich and highly degraded, using AAAS, had been unfruitful. The reduction step prior to a AAAS treatment allowed to improve the treatment efficacy towards this type of paper. The significant FE increase of J1 and J3, as well as the twofold strengthening of J2, the most brittle sample, is unprecedented. When treated with AP/DIA 50/50, all the papers that had been pre-reduced were reinforced in their folding properties. Several assumptions can be made to explain this. First, the carbonyl groups in paper being a cause of resistance to treatment, as shown in our previous research, reducing them would solve the problem. Moreover, to account for the considerable increase in hydrophilicity of sized paper after reduction ("Hydrophilicity of the reduced papers" section), the hypothesis proposed, related to a possible disruption of the sizing layer on the fibers, would also speak in favor of a better access of the AAASs to the fibers (Table S1 in the Supporting Information). Further investigation is needed to support this hypothesis. Additionally, as acid–base reactions between carboxylic acids in the paper and AAAS have also been shown to hamper the strengthening efficacy (Ferrandin-Schoffel et al. 2020), NaBH4 has the additional benefit of being alkaline. The acidity of paper is neutralized, as confirmed by pH measurements (Table 4 and "pH" section), and the corollary hampering thereby minimized.
An experiment was carried out by applying the full sequence of pre-reduction, treatment and post-heating (50 °C for 7 days) to J3-r (AP/DIA 50/50) (yellow bars in Figs. 6 and 7). The results showed that the thermal step did not allow to improve the mechanical properties further. Moreover, for the three newsprint papers treated with AP/DIA 50/50, the pre-reduction was more efficient than the post-thermal step (Fig. 5). At this stage of the research, applying a pre-reduction step with NaBH4 seems the most sustainable way of optimizing the strengthening of paper with AAASs. Nevertheless, as shown previously, heating helps the paper regain a hydrophobic character through the formation of longer polysiloxane chains ("Impact of a heating step on AAAS treated papers" section). Since the application of AAASs and NaBH4 both lead to an increase in hydrophilicity, the thermal step could then be considered as an interesting way to compensate such changes.
pH
The use of NaBH4 led to a significant increase in pH for paper C3a, from 5.4 to 7.8 (Table 4). Since the ash content of paper C3a was the same before and after reduction ("Hydrophilicity of the reduced papers" section), the formation of an inorganic alkaline salt that could explain this result was excluded.
The pH of C3a-r treated with AP/AM and AP/DIA (50/50) was alkaline, which is consistent with the pKas of the AAASs. The pKa of AM (pKa = 10.4) being higher than the two pKas of DIA (pKa1 = 10.0; pKa2 = 7.0) (Ferrandin-Schoffel et al. 2021), AP/AM yielded slightly higher pH than AP/DIA, irrespective of whether C3a was or was not reduced prior to the treatment. After treatment, the reduced papers had a significantly higher pH (9–9.6) than the unreduced papers (8.0–8.3). Indeed, as mentioned above, the immersion in the solution enables to neutralize and/or extract the acids in paper. The AAASs are then able to contribute to a larger alkaline reserve as they are not consumed by the initial acidity. It was shown that in some papers the alkaline reserve was consumed within a few years after treatment, especially in the most degraded papers sized with alum-rosin (Ferrandin-Schoffel et al. 2021). Therefore, the use of NaBH4 could lead to a more durable alkaline reserve by preventing its fast consumption.
It is believed that ß-alkoxy-elimination reactions, which happen under more drastic alkaline conditions and lead to the degradation of paper, would not occur at these pH values (9.2–9.6) (Ahn et al. 2012, 2013). Moreover, it has been shown that NaBH4 1 wt% (pH between 9 and 10) does not induce a decrease in DP of cellulose in papers, including historic ones (Henniges and Potthast 2009).
Color and opacity changes
After the reduction, all the papers were bleached (ΔE* = 8.2), as shown by the photographs before and after reduction (Figures S4 and S5 in the Supporting Information). This was corroborated by the variations of L*, a* and b* of C3a (Table 4). L* increased (ΔL* = + 5.2), whereas a* and b* decreased (Δa* = − 1.6; Δb* = − 5.8), which corresponds to higher lightness, less redness and less yellowness, respectively (Sève 1997). On the other hand, the reduction induced a noticeable opacity loss (− 3.5 points for C3a). As far as we know, such modification of the opacity following the bleaching step is not described in the literature.
The treatment with AP/AM and AP/DIA (50/50) of non-reduced paper C3a led to optical changes: b* increased (+ 2.5 points) and opacity decreased (− 2 points) (Table 4). L* and a* values varied less significantly (± 1.7 points at most). This confirms previous results where the AAASs treatments have been shown to induce some yellowing of the paper, and sometimes also a decrease of the opacity (Ferrandin-Schoffel et al. 2021). For AP/AM, ΔE* = 9.4, and for AP/DIA, ΔE* = 9.7. These chromatic changes are perceptible with the naked eye, considering that the Just Noticeable Difference (JND) in the 1976 CIELAB color space can be defined as ΔE* \(\approx\) 2 (Sève 1997; Richardson and Saunders 2007). For the reduced paper C3a-r, all b* values were lower than for the non-reduced counterpart C3a. The bleaching associated with the reduction with NaBH4 counteracted the yellowing induced by the AAASs.
Conclusion
Two procedures aiming at a better strengthening of paper with AAAS were studied. First, a thermal step when applied after the AAAS mixtures treatment on several papers was found efficient to favor their polycondensation in-situ by producing longer polysiloxane chains, as evidenced by 29Si CP-MAS NMR. The monitoring of the reaction kinetics in AM/H2O solutions with FTIR showed that heating at T ≥ 50 °C for 7 days led to higher DP of the AAAS than using catalysts such as NaOH, Ph2ICl or Al(acac)3. With the post-treatment thermal step, the folding endurance and the tensile strength of some of the AAASs treated papers were improved further. On the other hand, the oxidized moieties in paper being an obstacle to the strengthening, the reduction of the carbonyls with sodium borohydride prior to a treatment with AP/DIA 50/50 enabled a better strengthening of all the papers. In particular, the reinforcement of the highly degraded lignocellulosic newsprint paper J2 is an unprecedented achievement. Moreover, because of its bleaching properties, the use of NaBH4 counteracted the yellowing induced by the AAAS treatments, and thanks to its alkalinity, it neutralized acids in the paper, leaving the amines of the AAAS free to build a larger and hence more durable alkaline reserve.
In the future, it will be interesting to monitor the folding endurance and the tensile strength of the heated and pre-reduced AAASs treated papers. The choice of the temperature and the duration of the thermal step, as well as the concentration in solution and the solvent used with the reducing agent, can also be refined.
References
Ahn K, Henniges U, Banik G, Potthast A (2012) Is cellulose degradation due to β-elimination processes a threat in mass deacidification of library books? Cellulose 19:1149–1159. https://doi.org/10.1007/s10570-012-9723-3
Ahn K, Rosenau T, Potthast A (2013) The influence of alkaline reserve on the aging behavior of book papers. Cellulose 20:1989–2001. https://doi.org/10.1007/s10570-013-9978-3
Area MC, Cheradame H (2011) Paper aging and degradation: recent findings and research methods. Bio Resour 6:5307–5337. https://doi.org/10.15376/biores.6.4.5307-5337
Banik G, Bruckle I (2011) Paper and water: a guide for conservators, 1st edn. Butterworth-Heinemann, Amsterdam, New York
Bennevault-Celton V, Maciejak O, Desmazières B, Cheradame H (2010) Condensation of alkoxysilanes in alcoholic media: I oligomerization of dimethyldiethoxysilane. Polym Int 59:43–54. https://doi.org/10.1002/pi.2687
Bicchieri M, Brusa P (1997) The bleaching of paper by reduction with the borane tert-butylamine complex. Restaurator 18:1–11. https://doi.org/10.1515/rest.1997.18.1.1
Bicchieri M, Bella M, Semetilli F (1999) A quantitative measure of borane tert-butylamine complex effectiveness in carbonyl reduction of aged papers. Restaurator 20:22–29. https://doi.org/10.1515/rest.1999.20.1.22
Bicchieri M, Sementilli FM, Sodo A (2000) Application of seven borane complexes in paper conservation. Restaurator 21:213–228. https://doi.org/10.1515/REST.2000.213
Brochier Salon M-C, Bayle P-A, Abdelmouleh M et al (2008) Kinetics of hydrolysis and self condensation reactions of silanes by NMR spectroscopy. Colloids Surf A 312:83–91. https://doi.org/10.1016/j.colsurfa.2007.06.028
Brown HC, Murray KJ, Murray LJ et al (1960) Hydroboration. V. A study of convenient new preparative procedures for the hydroboration of olefins. J Am Chem Soc 82:4233–4241. https://doi.org/10.1021/ja01501a030
Brown HC, Snyder C, Rao BCS, Zweifel G (1986) Organoboranes for synthesis. 2. Oxidation of organoboranes with alkaline hydrogen peroxide as a convenient route for the cis-hydration of alkenes via hydroboration. Tetrahedron 42:5505–5510
Browning BL (1977) Analysis of paper, Enlarged 2. M. Dekker, New York
Bruice PY (2009) Essential organic chemistry, 2nd edn. Prentice Hall, Upper Saddle River, N.J.
Burgess HD (1988) Practical considerations for conservation bleaching. JIIC-CG 13:11–26
Calvini P, Gorassini A (2002) FTIR: deconvolution spectra of paper documents. Restaurator 23:48–66. https://doi.org/10.1515/REST.2002.48
Casey JP (1961) Pulp and paper: chemistry and chemical technology III: paper testing and converting, 2nd edn. Interscience Publishers Ltd., New York
Chojnowski J, Cypryk M (2000) Synthesis of linear polysiloxanes. In: Jones RG, Ando W, Chojnowski J (eds) Silicon-containing polymers: the science and technology of their synthesis and applications. Springer, Netherlands, Dordrecht, pp 3–41
Colby MW, Osaka A, Mackenzie JD (1988) Temperature dependence of the gelation of silicon alkoxides. J Non-Cryst Solids 99:129–139. https://doi.org/10.1016/0022-3093(88)90465-6
Daher C, Fabre-Francke I, Balcar N et al (2018) Consolidation of degraded polyurethane foams by means of polysiloxane mixtures: polycondensation study and application treatment. Polym Degrad Stab 158:92–101. https://doi.org/10.1016/j.polymdegradstab.2018.10.029
Dupont A-L, Egasse C, Morin A, Vasseur F (2007) Comprehensive characterisation of cellulose- and lignocellulose-degradation products in aged papers: capillary zone electrophoresis of low-molar mass organic acids, carbohydrates, and aromatic lignin derivatives. Carbohydr Polym 68:1–16. https://doi.org/10.1016/j.carbpol.2006.07.005
Dupont A-L, Lavédrine B, Cheradame H (2010) Mass deacidification and reinforcement of papers and books VI–Study of aminopropylmethyldiethoxysilane treated papers. Polym Degrad Stab 95:2300–2308
Ďurovič M, Zelinger J (1993) Chemical processes in the bleaching of paper in library and archival collections. Restaurator 14:78–101. https://doi.org/10.1515/rest.1993.14.2.78
Ferrandin-Schoffel N, Haouas M, Martineau-Corcos C et al (2020) Modeling the reactivity of aged paper with aminoalkylalkoxysilanes as strengthening and deacidification agents. ACS Appl Polym Mater 2:1943–1953. https://doi.org/10.1021/acsapm.0c00132
Ferrandin-Schoffel N, Martineau-Corcos C, Piovesan C et al (2021) Stability of lignocellulosic papers strengthened and deacidified with aminoalkylalkoxysilanes. Polym Degrad Stab 183:109413. https://doi.org/10.1016/j.polymdegradstab.2020.109413
Fox FJ, Noren RW, Krankkala GE (1978) Catalyst for condensation of hydrolyzable silanes and storage stable compositions thereof. United States Patent 4,101,513
Graminski EL, Parks EJ, Toth EE (1979) The effects of temperature and moisture on the accelerated aging of paper. Durability of macromolecular materials. American Chemical Society, pp 341–355
Havlínová B, Katuščák S, Petrovičová M et al (2009) A study of mechanical properties of papers exposed to various methods of accelerated ageing. Part I. The effect of heat and humidity on original wood-pulp papers. J Cult Herit 10:222–231. https://doi.org/10.1016/j.culher.2008.07.009
Heinze T, El Seoud OA, Koschella A (2018) Structure and properties of cellulose and its derivatives. Cellulose derivatives. Springer International Publishing, Cham, pp 39–172
Hemmingson JA, Morgan KR (1990) ACP/MAS carbon-13 NMR study of photodegraded newsprint. Holzforschung 44:127–131. https://doi.org/10.1515/hfsg.1990.44.2.127
Henniges U, Potthast A (2009) Bleaching revisited: impact of oxidative and reductive bleaching treatments on cellulose and paper. Restaurator 30:294–320. https://doi.org/10.1515/rest.017
Hey M (1977) Paper bleaching: its simple chemistry and working procedures. Pap Conserv 2:10–23. https://doi.org/10.1080/03094227.1977.9638494
Lienardy A, Van Damme P (1988) A bibliographical survey of the bleaching of paper. Restaurator 9:178–198. https://doi.org/10.1515/rest.1988.9.4.178
Massiot D, Fayon F, Capron M et al (2002) Modelling one- and two-dimensional solid-state NMR spectra: modelling 1D and 2D solid-state NMR spectra. Magn Reson Chem 40:70–76. https://doi.org/10.1002/mrc.984
Monredon-Senani S (2004) Interaction organosilanes/silice de précipitation du milieu hydro-alcoolique au milieu aqueux. Thesis, Paris, p 6
Moropoulou A, Zervos S (2003) The immediate impact of aqueous treatments on the strength of paper. Restaurator 24:160–177. https://doi.org/10.1515/REST.2003.160
Müller EMK, Henniges U, Brückle I (2019) Retreatment of a print damaged by excessive sodium borohydride bleaching. Restaur Int J Preserv Libr Arch Mater 40:123–137. https://doi.org/10.1515/res-2019-0001
Pellizzi E, Lattuati-Derieux A, de d’EspinoseLacaillerie J-B et al (2012) Reinforcement properties of 3-aminopropylmethyldiethoxysilane and N-(2-Aminoethyl)-3-aminopropylmethyldimethoxysilane on polyurethane ester foam. Polym Degrad Stab 97:2340–2346. https://doi.org/10.1016/j.polymdegradstab.2012.07.031
Piovesan C, Dupont A-L, Fabre-Francke I et al (2014) Paper strengthening by polyaminoalkylalkoxysilane copolymer networks applied by spray or immersion: a model study. Cellulose 21:705–715. https://doi.org/10.1007/s10570-013-0151-9
Piovesan C, Fabre-Francke I, Dupont A-L et al (2017) The impact of paper constituents on the efficiency of mechanical strengthening by polyaminoalkylalkoxysilanes. Cellulose 24:5671–5684. https://doi.org/10.1007/s10570-017-1513-5
Piovesan C, Fabre-Francke I, Nguyen T-P et al (2018a) Application de traitements à base de polyaminoalkylalcoxysilanes pour la désacidification et le renforcement simultanés de documents anciens sur papier. Support/Tracé 18:106–116
Piovesan C, Fabre-Francke I, Paris-Lacombe S et al (2018b) Strengthening naturally and artificially aged paper using polyaminoalkylalkoxysilane copolymer networks. Cellulose 25:6071–6082. https://doi.org/10.1007/s10570-018-1955-4
Piovesan C (2016) Elaboration de traitements de conservation à base de réseaux de polysiloxanes pour le renforcement et la désacidification simultanés adaptables à différents papiers : exemple des papiers de presse. Cergy-Pontoise
Richardson C, Saunders D (2007) Acceptable light damage: a preliminary investigation. Stud Conserv 52:177–187. https://doi.org/10.1179/sic.2007.52.3.177
Rocha J, Klinowski J (1990) 29Si and 27Al magic-angle-spinning NMR studies of the thermal transformation of kaolinite. Phys Chem Minerals 17:179–186. https://doi.org/10.1007/BF00199671
Sève R (1997) Physique de la couleur: de l’apparence colorée à la technique colorimétrique. Dunod, Paris
Socrates G (2004) Infrared and Raman characteristic group frequencies: tables and charts, 3rd edn. Wiley-Blackwell, Chichester
Souguir Z, Dupont A-L, d’Espinose de Lacaillerie J-B et al (2011) Chemical and physicochemical investigation of an aminoalkylalkoxysilane as strengthening agent for cellulosic materials. Biomacromolecules 12:2082–2091. https://doi.org/10.1021/bm200371u
Souguir Z, Dupont A-L, Fatyeyeva K et al (2012) Strengthening of degraded cellulosic material using a diamine alkylalkoxysilane. RSC Adv 2:7470–7478. https://doi.org/10.1039/c2ra20957h
Tang LC (1986) Stabilization of paper through sodium borohydride treatment. In: historic textile and paper materials, pp 427–441
Torry SA, Campbell A, Cunliffe AV, Tod DA (2006) Kinetic analysis of organosilane hydrolysis and condensation. Int J Adhes Adhes 26:40–49. https://doi.org/10.1016/j.ijadhadh.2005.03.008
Walker ERH (1976) The functional group selectivity of complex hydride reducing agents. Chem Soc Rev 5:23–50. https://doi.org/10.1039/cs9760500023
Whitmore PM, Bogaard J (1994) Determination of the cellulose scission route in the hydrolytic and oxidative degradation of paper. Restaurator 15:26–45. https://doi.org/10.1515/rest.1994.15.1.26
Williams JC (1981) A review of paper quality and paper chemistry. Libr Trends 30(2):203–224
Zhang Z (1997) Catalytic effect of aluminum acetylacetonate on hydrolysis and polymerization of methyltrimethoxysilane. Langmuir 13:473–476. https://doi.org/10.1021/la960771+
Zhang Z, Sakka S (1999) Hydrolysis and polymerization of dimethyldiethoxysilane, methyltrimethoxysilane and tetramethoxysilane in presence of aluminum acetylacetonate. A complex catalyst for the formation of siloxanes. J Sol-Gel Sci Technol 16:209–220. https://doi.org/10.1023/A:1008761002205
Zhang Z, Tanigami Y, Terai R (1995) Catalytic effect of acetylacetonates on gel formation of CH3SiO32. J Non-Cryst Solids 191:304–310. https://doi.org/10.1016/0022-3093(95)00316-9
Acknowledgments
The participation of the Bibliothèque Nationale de France is acknowledged. Sabrina Paris-Lacombe, Camille Piovesan, Alice Gimat and Oulfa Belhadj (CRC) are warmly thanked for their contribution and for fruitful discussions.
Funding
This research was supported by the Paris Seine Graduate School Humanities, Creation, Heritage, Investissements d’Avenir ANR-17-EURE-0021—Foundation for Cultural Heritage Science. Charlotte Martineau-Corcos has received financial support from the Paris Ile-de-France Region—DIM “Respore”.
Author information
Authors and Affiliations
Contributions
NF-S: Conceptualization, Investigation, Validation, Visualization, Writing—original draft. A-LD: Funding acquisition, Project administration, Resources, Conceptualization, Investigation, Supervision, Writing—review and editing. CM-C: Investigation. OF: Funding acquisition, Project administration, Conceptualization, Investigation, Supervision, Writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest and no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferrandin-Schoffel, N., Dupont, AL., Martineau-Corcos, C. et al. Routes to improve the strengthening of paper with aminoalkylalkoxysilanes. Cellulose 30, 539–556 (2023). https://doi.org/10.1007/s10570-022-04906-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04906-x





 ) was monitored at room temperature without catalyst/heat
) was monitored at room temperature without catalyst/heat
 ) was monitored at room temperature without catalyst
) was monitored at room temperature without catalyst


