Abstract
The combination of egg white proteins (W) and hypophosphorous acid (HA) was used for developing a flame retardant coating for cotton fabrics by adopting the layer-by-layer assembly technique. This has resulted in a novel phosphorous-nitrogen-based assembly due to strong electrostatic interactions between W and HA. Coated cotton fabrics were characterized by using a variety of microscopic, analytical and thermal/combustion tests. Flammability properties of the fabrics were evaluated as per widely recognized standards like BS EN ISO 15025:2016 for protective clothing and ASTM D6413 for flame resistance of textiles. Coated cotton fabric showed self-extinguishing property with significant but fragile residual char structure, whereas uncoated fabric completely burnt with a little ash as residue. Thermogravimetric analysis revealed that decomposition temperature of coated fabric was lowered compared to control fabric, but char residue increased at 800 °C. Forced combustion cone calorimeter tests on the coated and uncoated fabrics showed that peak heat release rate, total heat released, and average rate of heat emission values reduced by 80%, 38%, and 40%, respectively, for coated fabrics as compared to control fabric. Even the mass loss rate value of the coated fabric has reduced significantly (from 514 g/m2.s for the uncoated fabric to 2 g/m2.s for coated fabric). Wash durability of the coated fabrics, though showed a slight reduction in flame-retardant properties as a consequence of ~ 9.7 wt% coating loss during soaking, yet there was no after-glow.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clothes are made up of mainly cotton, which protects the human body from the harsh environment and gives comfort. Cotton clothes are used by common people, military, medical staff, and firefighters. Further, cotton is commonly used in home furnishings and various industrial items due to its softness, respiration, and moisture absorption characteristics (Rehan et al. 2018). Unfortunately, cotton is flammable with a low limiting oxygen index value and ignites even at temperatures in the range of 360–425 °C, leading to fire hazards that can result in loss of lives and severe damage to property (Shariatinia et al. 2015). During fire hazards, flammability is enhanced due to the combustion of cotton as it is rich in hydrocarbons (Wang et al. 2018). Thus, imparting resistance to burning cotton fabric is a challenge for textile industries (Liu et al. 2020). According to a report from the National Fire Protection Association, USA, a total of 1.31 million fire accidents had occurred in 2018 in which nearly 3655 people lost their lives, 15,000 civilians were injured, and more than $25.6 billion worth of property damage has occurred. Among the total fire accidents, ~ 74% of total fire accidents/deaths occurred in homes, and this was 3% higher than the previous year (2017) (Evarts 2019).
There are different approaches available for improving the flame retardancy performance of cotton fabrics, and the LbL assembly technique is one such approach. Using the LbL assembly technique, nanostructured surfaces for fire-proofing on cotton fabrics can be easily produced (Malucelli et al. 2014). During the flame exposure and combustion processes, these nanostructures control the heat transfer by creating a thermal barrier between the gas and condensed phases. This barrier further minimizes the intensity of heat experienced by the underneath cotton substrate (Decher and Hong 1991). LbL assembly method has gained significant attention and has been a promising method to prepare efficient flame retardant coatings (Li et al. 2010). This method is economically viable for the production of thin, multilayer films over complex substrates such as textiles through electrostatic attraction between cations and anions or nanoparticles (Qiu et al. 2018). Other approaches utilize attractive forces like, e.g., hydrogen bonds (Bergbreiter et al. 2001), donor/acceptor (Shimazaki et al. 1997) and covalent bonds (Sun et al. 2000) to form LbL assembly. Priolo et al.used the LbL method for the first time to make fire-resistant fabric in 2010 using branched polyethylenimine (PEI) and sodium montmorillonite (MMT) (Priolo et al. 2010).
During the last decade, the most commonly used flame retardants were mainly halogen-containing compounds such as pentabromodiphenyl ether, decabromodiphenyl ether and polychlorinated biphenyls (van der Veen and de Boer 2012). Halogen-based flame retardants are not ecologically safe as toxic gases, and halogen compounds are released in case of fire that may cause endocrine interference (Rahman et al. 2001; Legler and Brouwer 2003) along with polluting the environment (Carosio et al. 2015). Recently, some halogen-based flame retardants have been banned in the United States and European countries (Abou-Okeil et al. 2013). Instead, environmentally benign flame retardants are preferred (Pan et al. 2014, 2019; Liu et al. 2017), which do not release toxic gases during combustion. As a result, many phosphorus-based fire retardants were used, including ammonium polyphosphate-based salts (Wu and Wang 2008; Vakhitova et al. 2016) and phosphorus derivative phenyl dichlorophosphate (PDCP) (Yuan et al. 2012). Some phosphorous compounds such as tetrakis (hydroxymethyl) phosphonium chloride (THPC) containing hydroxymethyl groups may emit formaldehyde during the coating process, which is carcinogenic (Nielsen and Wolkoff 2010). Therefore, there is an urgent need to develop novel, non-toxic (i.e., halogen-free and formaldehyde-free) and economically feasible flame retardants.
Biomolecules such as proteins (Liu et al. 2020), chitosan (Xiong et al. 2019), starch (Carosio et al. 2015), hydrophobins (Alongi et al. 2014), phytic acid (Cheng et al. 2019) and DNA (Carosio et al. 2013) were also explored as alternatives to halogen/phosphorous based flame retardants. Alongi et al. applied casein (a phosphorus-based compound found in milk protein) and hydrophobins (sulfur-rich proteins obtained from filamentous fungi) to improve flame retardancy and thermal stability of cotton (Alongi et al. 2014). The extraction process of proteins from their sources is tedious, time-consuming and cost-intensive. These limitations restrict large-scale protein production. Recently, a coating made from a combination of egg white protein and phytic acid was developed to impart flame retardancy to cotton fabrics (Liu et al. 2020). An egg white protein is rich in various amino acids along with an abundant amount of calcium, ferric and phosphorous compounds and is cost-effective. On the other hand, phytic acid is a natural compound extracted from plant seeds, and its extraction also involves multiple steps making it expensive (Oatway et al. 2001). Despite the positive effect on flame retardant properties, the highly acidic nature of phytic acid (containing six phosphate compounds) affected the mechanical and chemical properties of cotton fabrics.
Hypophosphite is another effective flame retardant for cotton textiles. Braun et al. used two different metal phosphinates with and without melamine cyanide on glass-fiber reinforced poly (butylene terephthalate) (PBT / GF) and measured their thermal decomposition and combustion behavior (Braun et al. 2008). Results showed that the gas-phase mechanism was dominant for both the phosphinate-containing materials. For example, aluminum diethylphosphinate (AlPi) works by releasing diethylphosphic acid, which inhibits flame (Gallo et al. 2009). Yang et al. used the pad-dry-cure method to prepare sodium hypophosphite and maleic acid crosslinked coating over cotton fabric. Flame retardancy of coated fabric was increased due to the presence of phosphorus-containing groups (Yang et al. 2010). Recently, Liu et al. investigated flame retardancy of hypophosphorous acid-modified chitosan along with polyethylenimine (PEI) on polyester-cotton (PTCO) fabric by LbL assembly (Liu et al. 2017). Results revealed that gaseous pyrolysis products of coated samples were reduced compared to those of uncoated specimens.
In this report, a combination of amino acids from chicken egg white protein (W) and hypophosphorous acid (HA) was used for the development of a cost-effective flame retardant coating. The flame retardant coating was formed through electrostatic interaction between the nitrogen-phosphorus compound and cellulose structure. Pre and post-burning surface morphology, structure, thermal and flammability behavior of treated cotton were observed to assess the efficiency of the coating.
Materials and methods
Materials
Cotton fabric (150 GSM, 49 EPI and 54 PPI) was sourced from a local market in Saharanpur, India. Eggs and yolk separator was sourced from a supermarket. Hypophosphorous acid (HA, 50 wt% aqueous solution) was procured from Avra Synthesis Private Limited, India. A 2 wt% aqueous HA solution was prepared and used in this work.
Application of fire retardant coating over the cotton fabric using egg white protein and hypophosphorous acid
Firstly, cotton fabrics were dipped in distilled water at 80 °C for 1 and then dried in a vacuum oven overnight at 40 °C. Egg white protein was removed from eggs with the help of a yolk separator. The separated protein was then dissolved in distilled water (to make 30 wt% of protein) using a magnetic stirrer at 400 rpm for 90 min at 30 °C (pH = 8.5). The vacuum oven dried fabrics were dipped in egg white protein solution for 5 min, and then the excess solution was removed by squeezing and rinsing with water for 2 min. Protein-coated fabrics were then dipped for 2 min in 2% HA solution, and excess of HA solution was removed by squeezing and rinsing with water for 2 min. The process of applying a bilayer is schematically shown in Scheme 1a. The coated fabrics were dried overnight at 90 °C. This process was used for making four samples (egg white protein-coated cotton sample (CTW), hypophosphorous acid-coated cotton sample (CTHA), CTHA+W and CTW+HA). The control sample without any coating is designated as CT. These samples were named according to the order of coating, i.e., CTHA+W represents cotton first coated by hypophosphorous acid as primary layer followed by egg white protein as secondary layer coating, whereas CTW+HA represented cotton fabric coated first by egg white protein and then hypophosphorous acid.
Characterization
Functional groups and nature of bonds present in CT, CTW, CTHA and CTW+HA were analyzed with Fourier transformation infrared (FTIR) spectrophotometer (PerkinElmer, USA) in ATR mode. The samples were scanned over the wavenumber range 4000–500 cm−1.
The field emission scanning electron microscope (FESEM) was used to capture surface morphology of all samples and residue of char produced after combustion using MIRA3 TESCAN FESEM equipped with Energy Dispersive X-ray spectroscopy (EDAX) from AMETEK. The elemental composition of coated fabric with egg white protein and hypophosphorous acid was determined with the help of EDAX.
Weight loss% of cotton fabric and all coated samples with temperature and thereby their thermal stability were measured using a thermogravimetric analyzer (TGA 55, TA Instruments, USA). The sample was placed in a platinum pan, which was kept inside the furnace, maintained in nitrogen and air atmosphere. A constant heating rate of 10 °C min−1 was employed, and the temperature range was taken from 30 to 800 °C.
Tensile strength of control and coated fabrics was measured using a universal testing machine (UTM, model No. 3365, Instron Co., Macclesfield, U.K.) as per ISO 527-5A. A load cell of 5 kN and cross-head speed of 5 mm min−1 was used.
A limiting oxygen index (LOI) test was performed using an oxygen index test apparatus according to ASTM D2863. The vertical flame tests were performed for control and coated fabrics using a methane gas flame for 12 s as per the ASTM D6413-08 standard. Total char length was measured after burning. The sample size taken for the test was 76 × 300 mm2.
Flammability parameters of control and coated fabrics were examined using a cone calorimeter (DnG Technologies Pvt. Ltd., India) as per the ISO 5660-1 standard. Fabrics (10 cm × 10 cm) were exposed to 35 kW/m2 irradiative heat flux, and various parameters such as peak heat release rate (PHRR), total heat release rate (THR), average rate of heat emission (ARHE) and mass loss rate (MLR) were obtained.
Water soak test was performed according to standard ISO 5651-1978, where coated samples were soaked in distilled water (1: 20 w / v) with a non-ionic wetting agent (0.5 g / 1000 ml) for 30 min at room temperature. Weight loss was measured after the water soak test.
Results and discussion
Coating growth with layer deposition
Flame retardancy of coated fabric depends on the gain in weight percent (Wg%) due to the bilayer deposition compared to the control fabric. The gain in weight percent (Wg%) of coated fabric is expressed as:
where Wt and Wc indicate the weight of coated and control samples, respectively.
The increased weight of the control fabric after treatment with flame-retardants is presented in Fig. 1. As evident, percent weight gain is dependent on the sequence of application of coatings. Wg% of CTW was 8.40%, which is higher than that of CTHA (4.3%). Moreover, different coating sequences considerably influenced Wg% in the control fabric. If HA was used as a primary layer of coating followed by egg white as secondary, Wg% was found to be higher at 19.8% and was highest among all other samples. This is because hypophosphorous acid destroyed fabric structure (acid affects the cellulosic structure), and this might have resulted in greater deposition of egg white protein resulting in increased weight gain. In contrast, when egg white protein was used as the primary layer and HA as secondary (CTW+HA), Wg% was only 16.3%. In fact, the mechanical properties of the coated fabrics are also in line with the above explanations. That is, hypophosphorous acid affects the cellulosic structure and reduces the mechanical properties of coated fabric (CTHA) when HA was used as the primary layer. After HA treatment, CTHA fabric became so fragile (drop in mechanical strength) that for subsequent egg white protein application, it needed careful handling to obtain CTHA+W fabric. Thus, egg white protein was selected as the primary coating, which no doubt increased the percentage weight gain over the control sample. Egg white protein derived from chicken egg showed alkaline behavior (pH = 8.5), possibly because of amino acids. Electrostatic and hydrogen bonding interactions between the coating and functional groups (-OH groups) of cellulose facilitated easy adherence to the fabric (Scheme 1b).
ATR-FTIR analysis of control and coated cotton fabric
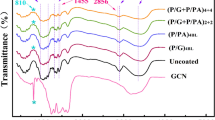
IR spectra of control fabric and coated fabrics are presented in Fig. 2. In the spectrum of control fabric, characteristic peaks corresponding to H bonded OH stretch at 3320 cm−1, symmetric CH2 stretch at 2910 cm−1, absorbed H2O at 1645 cm−1, CH wagging at 1432 cm−1, CH bending at 1370 cm−1, CH wagging at 1313 cm−1, CH deformation stretch at 1282 cm−1, C–O stretch at 1024 cm−1 and 1000 cm−1 and OH stretch at 895 cm−1 (Chung et al. 2004). CTW spectra showed two new peaks at 1630 cm−1 and 1525 cm−1, indicating amide-I and amide-II from egg white protein (Bosco et al. 2013). In the case of CTHA, a new peak appeared at 980 cm−1 for P = O from hypophosphorous acid (Yu et al. 2017). In the spectrum corresponding to CTW+HA, all these three peaks characteristic of amide-I, amide-II and P=O are seen at 1630 cm−1, 1525 cm−1 and 980 cm−1, respectively. Therefore, these results indicate that hypophosphorous acid binds protein through electrostatic interactions.
Thermal stability of control and coated cotton fabrics
Thermogravimetric analysis (TGA) and derivative thermogravimetric analysis (DTG) were carried out to understand the thermal stability of all samples. TGA was conducted both in the presence of nitrogen and air atmosphere. TGA and DTG thermograms obtained for CT, CTHA, CTW, CTW+HA, and CTHA+W samples performed in nitrogen environment are shown in Fig. 3a, b and the thermograms obtained with samples under air environment are shown in Fig. 3c, d. Their quantitative data are reported in Tables 1 and 2, respectively. Under a nitrogen atmosphere, a significant weight loss between 200 and 800 °C was noticed for all fabrics. TGA analysis of control fabric showed two steps of weight loss: (i) cotton was dehydrated and carbonized, producing CO2, H2O, and char, and (ii) cellulose was depolymerized to levoglucosan, which was further converted into low molecular weight compounds and secondary char (Lin et al. 2019). In the case of cotton coated with egg white (CTW), initial decomposition started at a lower temperature, i.e., 260 °C, compared to control fabric, 294 °C. However, the rate of weight loss with control fabric beyond 350 °C was much higher than that of CTW. At 450 °C, the residual weight % with control fabric was found to be 12 wt%, whereas it was 39 wt% with CTW. These results indicated that cotton coated with egg white (CTW) could effectively reduce the rate of cotton decomposition even at higher temperatures. CTHA showed decomposition at lower temperatures, i.e., 252 °C, relative to CT and CTW. At higher temperatures, the rate of weight loss with CTHA was lowered, possibly due to the presence of phosphoric acid groups of HA, which strongly inhibited the depolymerization process of cotton fabric facilitating char formation. With the CTHA+W sample, primary degradation temperature was found to be 230 °C, which is lower than CTHA (252 °C), but carbon residue obtained was higher at 800 °C (7.8 wt%) compared to CTHA (4.9 wt%). As discussed in the above section, the strong acidity of HA damages the cotton fibers, thereby adversely affecting the softness and mechanical properties of the fabric. Instead, if HA is applied as a secondary layer keeping the egg white protein as the primary layer in CTW+HA, it could improve the fabric performance. TGA analysis of the CTW+HA sample showed initiation of decomposition at a relatively lower temperature of 153 °C compared to all other samples. However, the rate of weight loss was lowered beyond 350 °C. Char residue leftover at 700 °C and 800 °C were 18.8 wt% and 11.6 wt%, respectively. These values are much higher than control fabric, i.e., 0.64 wt% and 0.28 wt% at 700 °C and 800 °C, respectively. Therefore, CTW+HA is expected to show appreciable flame retardancy due to considerably higher char formation compared to other samples.
a, c TGA and b, d DTG of CT (Control fabric), CTW (Egg white protein-coated cotton fabric), CTHA (Hypophosphorous acid-coated cotton fabric), CTW+HA (Egg white protein with hypophosphorous acid-coated cotton fabric in sequence) and CTHA+W (Hypophosphorous acid with egg white protein-coated cotton fabric in sequence)
In the presence of air, the control fabric produced 0.2 wt% char residue at 800 °C, while other coated fabrics, i.e., CTW, CTHA, CTW+HA and CTHA+W, produced 3.8 wt%, 2.9 wt%, 11.4 wt% and 7.4 wt% of char, respectively, at 800 °C. The levoglucosan chains of cotton continued to oxidize in the presence of oxygen, generating exothermic heat that facilitated further degradation of cellulose. Thus, all fabric samples showed lower char residues compared to those obtained with an inert nitrogen atmosphere (Lin et al. 2019). Alongi et al. separately applied non-toxic/eco-friendly coating materials like caseins and hydrophobins over cotton, and char residues were 18 wt% and 19 wt%, respectively, at 600 °C under nitrogen atmosphere, while in the presence of air it was reduced to 2 wt% and 4 wt%, respectively (Alongi et al. 2014). Annalisa et al. tried deposition of DNA derived from herring sperm as coating, which yielded char residue of 23 wt% and 6.9 wt%, respectively, in nitrogen and air atmosphere (Annalisa et al. 2016). Bosco et al.also reported 1.5 wt% and 2.5 wt% of char formation under air atmosphere, when cotton fabric was separately coated with folded and unfolded whey proteins, respectively (Bosco et al. 2013). In the present investigation, coating sequence of egg white protein followed by hypophosphorous acid layer (single bilayer over cotton fabric, CTW+HA) resulted in 37.3 wt% and 11.5 wt% of char residue under nitrogen atmosphere at 600 °C and 800 °C, which was reduced to 27.6 wt% and 11.4 wt% of char under an air atmosphere at 600 °C and 800 °C, respectively. Therefore, layer by layer coating of egg white protein and HA, i.e., CTW+HA, showed relatively better performance compared to earlier reports.
Surface morphology of control and coated cotton fabrics
Surface morphology analysis of coated and control fabrics using FESEM is presented in Fig. 4. The control fabric (CT) showed a smooth surface without any defect, whereas the HA-coated fabric (CTHA) showed the formation of surface cracks. This is possibly due to the destruction of certain cellulose fibers, possibly because of the corrosive nature of HA. On the other hand, the thickness of egg white layer coated fabric (CTW) was higher compared to HA-coated fabric (CTHA). Coating thickness is expected to protect the fiber from corrosive HA during its application as a second layer.
Egg white protein and hypophosphorous acid are electrostatically linked through amino acid and phosphorus group of HA. The surface of fabric CTW+HA appeared smooth without any defect. Egg white protein as the first layer protected cotton fabric from damage due to highly acidic HA. HA application resulted in the formation of electrostatic linkages between amino acid groups and HA.
Elemental composition and distribution over the surface of coated cotton fabric
The elemental composition and distribution over the surface of fabric were estimated through elemental dispersive X-ray analysis (EDAX) coupled with SEM. Figure 5 and Table 3 showed the elemental composition of CTW+HA. The main elements which impart flame retardancy were nitrogen (N), phosphorus (P), and sulfur (S) and their content on the surface of CTW+HA were found to be 16.03%, 8.09% and 2.33%, respectively.
Tensile strength of control and coated fabric
Tensile properties of control and coated fabric were evaluated to understand the influence on mechanical properties, and the data obtained are reported in Table 4. The control fabric (CT) showed maximum tensile stress of 11.5 MPa. The sample coated with egg white protein (CTW) showed a 7.6% increase in tensile stress compared to the control sample, possibly because of electrostatic interactions between yarn and egg white protein. CTHA showed the lowest tensile stress compare to all coated samples due to the high acidity of hypophosphorous acid, which destroyed the structure of the fabric, thereby reducing its strength. Interestingly, the tensile stress of CTW+HA was 217% higher than CTHA and 80% lower than control.
Flame retardancy behavior of control and coated cotton fabrics
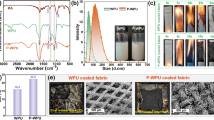
Flame retardancy of control and coated fabrics were examined using LOI tests. The control sample was easily ignited and had an index of 18%, while CTW and CTHA had LOI values of 22% and 23%, respectively. Interestingly, CTW+HA showed a higher LOI value (26%) compared to all other samples. Vertical Fire Test (VFT) for 12 s and BS EN ISO 15025 standard test of 10 s were also carried out to establish the susceptibility to ignition and sustained flaming of CTW+HA sample and control fabric (CT). Figures 6 and 7 show the images of control fabric and coated fabric before and after burning through VFT and BS EN ISO 15025 tests, and related data on flammability are presented in Table 5 and 6. The control fabric did not produce any char, and after-glow time was 26 ± 5 s in VFT and 130 ± 5 s in BS EN ISO 15025 test. CTW+HA sample resulted in a fragile char, and no after-glow phenomenon was observed. Coating played a crucial role in promoting dehydration reaction and char formation during burning.
After the VFT test, morphologies of control fabric (CT) and coated cotton fabric (CTW+HA) were separately tested using FESEM (Fig. 8). The control fabric generated ash with no char, but a continuous char layer appeared with CTW+HA. The char formation occurred due to dehydration of cellulose, which was catalyzed due to the presence of hypophosphorous acid. FESEM images of CTW+HA interestingly showed the appearance of numerous bubbles due to the escape of volatiles from the surface and thus resulting in swelling of the fibrous structure of char. This swollen (intumescent) structure of char prevented the interaction of combustible mass from arial oxygen and external heat source (Liu et al. 2017; Zhang et al. 2018). These bubbles also confirmed the intumescent behavior of coating. Results indicated that the synergistic effect between nitrogen (egg white protein) and phosphorus (hypophosphorous acid) leads to the formation of a char network.
Cone calorimeter test of control and coated fabric
Flammability behavior of control and coated fabric in terms of mass loss rate (MLR), total heat release rate (THR), peak heat release rate (PHRR), and average rate of heat emission (ARHE) was also measured using a cone calorimeter. The data trends are shown in Fig. 9 and Table 7. CTW+HA showed a much lower heat release rate than control fabric. Control fabric burnt rapidly with higher MLR (514 g/m2.s), PHRR (77 kW/m2), THR (2.8 MJ/m2) and ARHE (11.7 kW/m2). However, coated sample (CTW+HA) showed excellent results with significantly lower MLR (2.1 g/m2.s). PHRR, THR, and ARHE were reduced by 80%, 38% and 40%, respectively, as compared to the control fabric. These results showed that coated sample CTW+HA had better flame retardancy even if compared to previously reported studies on cotton coated with different proteins such as caseins, DNA and egg white protein. In previous studies, Alongi et al. (Alongi et al. 2014) showed that treated cotton with caseins had reduced PHRR value by 27% (Bosco et al. 2015) reported a 22% reduction in PHRR value with DNA (obtained from herring sperm) coated fabric. When egg white protein with phytic acid to treat the cotton fabric by Liu et al., a reduction of 23% in PHRR and 67% reduction in THR value (Liu et al. 2020) was noted as compared to control. Therefore, in contrast to literature reports, the protective layer formed on the fabric surface due to electrostatic interactions of egg white protein and hypophosphorous acid has shown a better flame retardancy performance.
Water soak test of coated fabric
A water soak test was performed to know the wash stability of the coating with water. For this purpose, a preweighted CTW+HA was dipped in water for 30 min at room temperature. Subsequently, the wet sample was dried and weighed again. It was observed that the weight of the coated fabric was reduced by 9.67 wt%. As mentioned earlier, the combination of egg white protein and hypophosphorous acid bind to each other through electrostatic interaction that opposed the dissolution of coating in water during the water soak test. In addition, LOI and vertical flame tests were performed on the coated fabric (CTW+HA) after the water soak test. The LOI value was reduced by 2% after the water soak test. In the vertical flame test, after-flame was observed for 30 ± 5 s, and the after-glow was not seen (Fig. 10). This could be attributed to phosphorus-nitrogen synergism formed due to interactions between egg white protein and HA.
Based on the obtained results, it is clear that a primary layer coating of egg white protein and a secondary layer of hypophosphorous acid will give the best flame protection to the fabric. The egg white protein initiated decomposition at higher temperatures and produced less-flammable substances (Liu et al. 2020), while phosphorous acid promoted dehydration of cellulose. The combined effect of egg white protein and hypophosphorous acid resulted in swelling of the fibrous structure. This swollen structure worked as a thermal barrier to protect the underlying fabric from heat and oxygen.
Conclusions
The layer-by-layer flame retardant coating was applied on cotton fabrics by adopting a water-based method that combined egg white protein and hypophosphorous acid.
-
1.
The gain in weight percentage with CTW, CTHA, CTW+HA, and CTHA+W were 8.4%, 4.3%, 16.3%, and 19.8%, respectively. A remarkable lowering in Wg% with the HA layer was attributed to the acidic nature of HA. Hence, the egg white layer was preferred as the primary layer.
-
2.
ATR-FTIR spectra data on CTW+HA has confirmed the electrostatic interactions (nitro-phospho) between egg white protein and HA, which resulted in P = O, amide-I, and amide-II peaks.
-
3.
TGA analysis under nitrogen and air environment showed that most of the mass loss occurred between 200–800 °C. CTW+HA showed minimum weight loss compared to all other samples, and char residues were 37.3 wt% and 11.6 wt% at 600 °C and 800 °C in nitrogen and 27.6 wt% and 11.4 wt% in an air environment at 600 °C and 800 °C, respectively.
-
4.
EDAX analysis of the surface of CTW+HA coating showed the presence of nitrogen, phosphorus, and sulfur as 16.03%, 8.09%, and 2.33%, respectively.
-
5.
CTW+HA fabric exhibited improved flame retardancy with 26% LOI value and self-extinguishing properties as estimated through VFT and BS EN ISO 15025 tests. The control fabric did not produce any char. The after-glow time was 26 ± 5 s and 130 ± 5 s, respectively, with VFT and BS EN ISO 15025 tests. But, CTW+HA fabric resulted in significant char formation and showed no after-glow phenomenon.
-
6.
In cone calorimeter test, PHRR, THR, and ARHE values of CTW+HA fabric were reduced by 80%, 38%, and 40%, respectively, while MLR value was reduced from 513.6 to 2.1 g/m2.s compared to control fabric.
-
7.
The evolution of volatile gases during forced combustion tests resulted in the formation of numerous bubbles and swelling, as shown from the SEM images of the char of CTW+HA. This would prevent the interaction of combustible mass from oxygen and heat.
-
8.
Vertical flame test after water soak sample CTW+HA exhibited lower fire retardancy but showed no after-glow.
Therefore, a coating made with egg white protein (as a primary layer) followed by HA has resulted in an excellent performance as a flame retardant layer on cotton fabrics.
References
Abou-Okeil A, El-Sawy SM, Abdel-Mohdy FA (2013) Flame retardant cotton fabrics treated with organophosphorus polymer. Carbohydr Polym 92:2293–2298. https://doi.org/10.1016/j.carbpol.2012.12.008
Alongi J, Carletto RA, Bosco F et al (2014) Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym Degrad Stab 99:111–117. https://doi.org/10.1016/j.polymdegradstab.2013.11.016
Annalisa C, Francesca B, Giulio M et al (2016) DNA-chitosan crosslinking and photografting to cotton fabrics to improve washing fastness of the fire-resistant finishing. Cellulose 23:3963–3984. https://doi.org/10.1007/s10570-016-1067-y
Bergbreiter DE, Tao G, Franchina JG, Sussman L (2001) Polyvalent hydrogen-bonding functionalization of ultrathin hyperbranched films on polyethylene and gold. Macromolecules 34:3018–3023. https://doi.org/10.1021/ma000873d
Bosco F, Andrea R, Alongi J et al (2013) Thermal stability and flame resistance of cotton fabrics treated with whey proteins. Carbohydr Polym 94:372–377. https://doi.org/10.1016/j.carbpol.2012.12.075
Bosco F, Casale A, Mollea C et al (2015) DNA coatings on cotton fabrics: effect of molecular size and pH on flame retardancy. Surf Coatings Technol 272:86–95. https://doi.org/10.1016/j.surfcoat.2015.04.019
Braun U, Bahr H, Sturm H, Schartel B (2008) Flame retardancy mechanisms of metal phosphinates and metal phosphinates in combination with melamine cyanurate in glass-fiber reinforced poly (1, 4-butylene terephthalate ): the influence of metal cation. Polym Adv Technol. https://doi.org/10.1002/pat.1147
Carosio F, Di Blasio A, Alongi J, Malucelli G (2013) Green DNA-based flame retardant coatings assembled through layer by layer. Polymer (guildf) 54:5148–5153. https://doi.org/10.1016/j.polymer.2013.07.029
Carosio F, Fontaine G, Alongi J, Bourbigot S (2015) Starch-based layer by layer assembly: efficient and sustainable approach to cotton fire protection. ACS Appl Mater Interfaces 7:12158–12167. https://doi.org/10.1021/acsami.5b02507
Cheng XW, Guan JP, Yang XH et al (2019) A bio-resourced phytic acid/chitosan polyelectrolyte complex for the flame retardant treatment of wool fabric. J Clean Prod 223:342–349. https://doi.org/10.1016/j.jclepro.2019.03.157
Chung C, Lee M, Choe EK (2004) Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr Polym 58:417–420. https://doi.org/10.1016/j.carbpol.2004.08.005
Decher G, Hong JD (1991) Buildup of ultrathin multilayer films by a self-assembly process: ii. consecutive adsorption of anionic and cationic bipolar amphiphiles and polyelectrolytes on charged surfaces. Berichte Der Bunsengesellschaft Für Phys Chemie 95:1430–1434. https://doi.org/10.1002/bbpc.19910951122
Evarts B (2019) Fire loss in the United States during 2018. Natl Fire Prot Assoc USA October 2019
Gallo E, Braun U, Schartel B et al (2009) Halogen-free flame retarded poly ( butylene terephthalate) (PBT) using metal oxides/PBT nanocomposites in combination with aluminium phosphinate. Polym Degrad Stab 94:1245–1253. https://doi.org/10.1016/j.polymdegradstab.2009.04.014
Legler J, Brouwer A (2003) Are brominated flame retardants endocrine disruptors? Environ Int 29:879–885. https://doi.org/10.1016/S0160-4120(03)00104-1
Li Y, Schulz J, Mannen S et al (2010) Flame retardant behavior of polyelectrolyte—clay thin film assemblies on cotton fabric. ACS Nano 4:3325–3337. https://doi.org/10.1021/nn100467e
Lin D, Zeng X, Li H et al (2019) One-pot fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via sol-gel reaction. J Colloid Interface Sci 533:198–206. https://doi.org/10.1016/j.jcis.2018.08.060
Liu L, Pan Y, Wang Z et al (2017) Layer-by-layer assembly of hypophosphorous acid-modified chitosan based coating for flame-retardant polyester-cotton blends. Ind Eng Chem Res 56:9429–9436. https://doi.org/10.1021/acs.iecr.7b02303
Liu X, Zhang Q, Peng B et al (2020) Flame retardant cellulosic fabrics via layer-by-layer self-assembly double coating with egg white protein and phytic acid. J Clean Prod 243:118641. https://doi.org/10.1016/j.jclepro.2019.118641
Malucelli G, Carosio F, Alongi J et al (2014) Materials engineering for surface-confined flame retardancy. Mater Sci Eng R Rep 84:1–20. https://doi.org/10.1016/j.mser.2014.08.001
Nielsen GD, Wolkoff P (2010) Cancer effects of formaldehyde: a proposal for an indoor air guideline value. Arch Toxicol 84:423–446. https://doi.org/10.1007/s00204-010-0549-1
Oatway L, Vasanthan T, Helm JH (2001) Phytic acid. Food Rev Int 17:419–431. https://doi.org/10.1081/FRI-100108531
Pan H, Song L, Ma L et al (2014) Layer-by-layer assembled thin films based on fully biobased polysaccharides: Chitosan and phosphorylated cellulose for flame-retardant cotton fabric. Cellulose 21:2995–3006. https://doi.org/10.1007/s10570-014-0276-5
Pan Y, Liu L, Zhang Y et al (2019) Effect of genipin crosslinked layer-by-layer self-assembled coating on the thermal stability, flammability and wash durability of cotton fabric. Carbohydr Polym 206:396–402. https://doi.org/10.1016/j.carbpol.2018.11.037
Priolo MA, Gamboa D, Grunlan JC (2010) Transparent clay-polymer nano brick wall assemblies with tailorable oxygen barrier. ACS Appl Mater Interfaces 2:312–320. https://doi.org/10.1021/am900820k
Qiu X, Li Z, Li X, Zhang Z (2018) Flame retardant coatings prepared using layer by layer assembly: a review. Chem Eng J 334:108–122. https://doi.org/10.1016/j.cej.2017.09.194
Rahman F, Langford KH, Scrimshaw MD, Lester JN (2001) Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ 275:1–17. https://doi.org/10.1016/S0048-9697(01)00852-X
Rehan M, El-Naggar ME, Mashaly HM, Wilken R (2018) Nanocomposites based on chitosan/silver/clay for durable multi-functional properties of cotton fabrics. Carbohydr Polym 182:29–41. https://doi.org/10.1016/j.carbpol.2017.11.007
Shariatinia Z, Javeri N, Shekarriz S (2015) Flame retardant cotton fibers produced using novel synthesized halogen-free phosphoramide nanoparticles. Carbohydr Polym 118:183–198. https://doi.org/10.1016/j.carbpol.2014.11.039
Shimazaki Y, Mitsuishi M, Ito S, Yamamoto M (1997) Preparation of the layer-by-layer deposited ultrathin film based on the charge-transfer interaction. Langmuir 13:1385–1387. https://doi.org/10.1021/la9609579
Sun J, Wu T, Liu F et al (2000) Covalently attached multilayer assemblies by sequential adsorption of polycationic diazo-resins and polyanionic poly(acrylic acid). Langmuir 16:4620–4624. https://doi.org/10.1021/la991482z
Vakhitova L, Drizhd V, Taran N et al (2016) The effect of organoclays on the fire-proof efficiency of intumescent coatings. Eastern-Eur J Enterp Technol 6:10–16. https://doi.org/10.15587/1729-4061.2016.84391
van der Veen I, de Boer J (2012) Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153. https://doi.org/10.1016/j.chemosphere.2012.03.067
Wang YW, Shen R, Wang Q, Vasquez Y (2018) ZnO microstructures as flame-retardant coatings on cotton fabrics. ACS Omega 3:6330–6338. https://doi.org/10.1021/acsomega.8b00371
Wu K, Wang Z (2008) Intumescent flame retardation of EVA using microencapsulated ammonium polyphosphate and pentaerythritols. Polym Plast Technol Eng 47:247–254. https://doi.org/10.1080/03602550701866733
Xiong Z, Zhang Y, Du X et al (2019) Green and Scalable fabrication of core-shell biobased flame retardants for reducing flammability of polylactic acid. ACS Sustain Chem Eng 7:8954–8963. https://doi.org/10.1021/acssuschemeng.9b01016
Yang CQ, Chen D, Guan J, He Q (2010) Crosslinking cotton cellulose by the combination of maleic acid and sodium hypophosphite. 1. Fabric wrinkle resistance. Ind Eng Chem Res 49:8325–8332. https://doi.org/10.1021/ie1007294
Yu X, Pan Y, Wang D et al (2017) Fabrication and properties of biobased layer-by-layer coated ramie fabric-reinforced unsaturated polyester resin composites. Ind Eng Chem Res 56:4758–4767. https://doi.org/10.1021/acs.iecr.7b00101
Yuan H, Xing W, Zhang P et al (2012) Functionalization of cotton with UV-cured flame retardant coatings. Ind Eng Chem Res 51:5394–5401. https://doi.org/10.1021/ie202468u
Zhang XLY, Ren BCY, Chen QZ et al (2018) Preparation of durable and flame retardant lyocell fibers by a one-pot chemical treatment. Cellulose 25:6745–6758. https://doi.org/10.1007/s10570-018-2005-y
Acknowledgments
Financial support to execute the experimental work through MHRD (Ministry of Human Resources Development) Plan grant (2019-20) and IIT Roorkee (Indian Institute of Technology Roorkee (No. IITR/SRIC/OH-35-71-142), India. is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Human and or animal rights
No animal/human studies were carried out by authors in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vishwakarma, A., Reddy, V.J., Kandola, B.K. et al. Egg white protein-hypophosphorous acid-based fire retardant single bilayer coating assembly for cotton fabrics. Cellulose 28, 10689–10705 (2021). https://doi.org/10.1007/s10570-021-04208-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04208-8